Abstract
Objective
The aim of this study was to analyse wound biofilm from a clinical perspective. Research has shown that biofilm is the preferred microbial phenotype in health and disease and is present in a majority of chronic wounds. Biofilm has been linked to chronic wound inflammation, impairment in granulation tissue and epithelial migration, yet there lacks the ability to confirm the clinical presence of biofilm. This study links the clinical setting with microscopic laboratory confirmation of the presence of biofilm in carefully selected wound debridement samples.
Method
Human wound debridement samples were collected from adult patients with chronic non-healing wounds who presented at the wound care centre. Sample choice was guided by an algorithm that was developed based on what is known about the characteristics of wound biofilm. The samples were then evaluated by light microscopy and scanning electron microscopy for the presence of biofilm. Details about subject history and treatment were recorded. Adherence to biofilm-based wound care (BBWC) strategies was inconsistent. Other standard antimicrobial dressings were used and no modern antiseptic wound dressings with the addition of proven antibiofilm agents were available for use.
Results
Of the patients recruited, 75% of the macroscopic samples contained biofilm despite the prior use of modern antiseptic wound dressings and in some cases, systemic antibiotics. Wounds found to contain biofilm were not all acutely infected but biofilm was present when infection was noted. The clinical histories associated with positive samples were consistent with ideas presented in the algorithm used to guide sample selection.
Conclusion
Visual cues can be used by the clinician to guide suspicion of the presence of wound biofilm. This suspicion can be further enhanced with the use of a clinical algorithm. Standard antiseptic wound dressings used in this study demonstrated limited antibiofilm efficacy. This study also highlighted a need for the clinical team to focus on expiration of dressing action and consistent practice of BBWC strategies which includes the use of proven antibiofilm agents.
Declaration of interest
This work was supported by the Department of Veterans Affairs Career Development Award CDA-2 1IK2BX001701 to J.A.G. Microscopical experiments were performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126).
Keywords: biofilm, wound, non-healing, clinical
The incidence of chronic non-healing wound (CNHW) infections has been shown to be as high as 53%1 and wound infection is linked to the presence of biofilm but the diagnostic relationship remains to be clarified.2,3 Introduction of biofilm into in vivo animal models markedly impairs healing but use of multimodal strategies to remove and suppress biofilm reverses this delay.4 Evidence indicates that bacterial biofilms can chronically infect wounds and that they are present in the majority of CNHWs.5,6
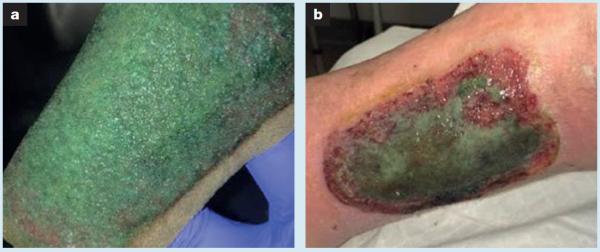
Biofilms are tertiary architectural structures of living microbial cells surrounded by a protective extracellular polymeric substance (EPS).7,8 Biofilm protects microbes from host immunity as well as renders them significantly more tolerant than planktonic microbes to topical and systemic antimicrobials.9 Persistence of biofilms in CNHWs has been shown to delay healing trajectory by promoting a chronic inflammatory response as well as impairing granulation formation and epithelial migration.2,10,11 It is logical and has been shown that sharp debridement of biofilm temporarily increases bacterial susceptibility to antimicrobial onslaught by exposing them in the planktonic state. Yet, after sharp disruption, biofilm will reform to maturity in 48–72 hours.12 Biofilm-based wound care (BBWC) is a treatment algorithm that is based on what is currently known about biofilm.13 This algorithm has been proposed to address the resistant nature of biofilm by highlighting the need for several consecutive and concurrent treatment interventions including regular physical disruption with sharp debridement, the use of antibiofilm agents along with topical antiseptic dressings and the use of systemic antibiotics for signs/symptoms of infection. In addition, although in vitro studies have shown that both silver and iodine wound dressings have some ability to kill bacteria in biofilm, the body of evidence is inconsistent.14–19 It is increasingly recognised that there remains a pressing need to reliably translate laboratory data into the clinical setting due to the potential impact of many clinical variables on sustained dressing antimicrobial action and availability. Fig 1a shows what is most likely to be the green pigment indicative of pyocyanin produced within a Pseudomonas aeruginosa biofilm growing on a metallic silver foam saturated with wound exudate.20 This same green film was present on the bed of this significantly ischaemic wound after dressing removal (Fig 1b). This supports the concern that clinical antibiofilm action of our current formulary of antimicrobial wound dressings may be inadequate to address the challenges found in some CNHWs. Since clinicians still lack a point-of-care tool to diagnose the presence of biofilm on a wound, the risk is that wound care protocols may be promoting routine use of aggressive and costly, yet unreliable, strategies when treating CNHWs.
Fig 1.
Significantly ischemic left lateral leg traumatic ulcer. The chronic non-healing wound was treated with a polyurethane foam dressing containing silver nanoparticles that are purported to have antimicrobial activity. After removal of polyurethane foam dressing observed in (a), green film was present on the bed of this significantly ischaemic wound (b)
While the concept of wound biofilm is widely accepted, there remains debate about whether or not it is possible for biofilm to be seen with the naked eye on a particular wound.9,21,22 To enhance cost-effectiveness of care delivery, it is crucial that research begin to identify ways of making a clinical confirmation. Visual evidence of biofilm formation has been proposed but not yet confirmed.23,24
The aim of the current study was to confirm the presence or absence of wound biofilm in a visible, adherent, gel-like wound bed film found on a series of CNHWs using advanced microscopy techniques. The goal was to extend our understanding of the potential to visualise biofilm on a wound. Information about patient history, clinical culture results, and wound treatment strategy data was provided to support a link between theoretical understanding of biofilm and clinical implications.
Materials and methods
Human subjects and ethics statement
Human tissues in this study were obtained from subjects enrolled according to protocols approved by the Institutional Review Board of University of Tennessee Health Science Center (IRB #14-02997) and the Institutional Review Board of Vanderbilt University Medical Center (IRB #140848). Briefly, wound debridement samples were collected from adult patients with CNHWs who presented to the Wound Care Center. Written informed consent was obtained from all subjects (≥18 years old) included in the study. CNHWs were defined as being ≥30 days old with failure to progress through normal wound-healing trajectories as previously defined.25 Subjects were chosen for this study by using an evidence-based but not yet validated clinical algorithm for wound biofilm identification.23 CNHWs were sampled if they were either partially or wholly covered with a firmly adherent, gel-like wound bed film which had reformed under antiseptic wound dressings and sometimes despite guided systemic antibiotics.
The substance that was identified for this evaluation was intentionally distinguished from slough. Slough is defined by consensus as necrotic (dead) host protein with a stringy or fibrous texture that is intimately attached to the underlying, viable wound bed tissue.25 This intimate attachment requires cutting into viable tissue for complete removal.24 This distinction was made in effort to advance cost-effective wound care delivery. The goal was to separate the characteristics of a living biofilm and dead host cells or extracellular proteins, the latter of which can be rapidly and selectively cleared with a proteolytic enzyme.26 An enzyme designed to denature devitalised protein is not a cost-effective option for treatment of bacteria protected in a predominantly polysaccharide biofilm.
The characteristics of the substance chosen for this study were based on descriptions of biofilm which report a gel-like substance strongly anchored with an adhesion pedestal to the extracellular matrix.27 Although anchored, this substance was not intimately attached to the underlying viable wound bed therefore allowing atraumatic removal. In this study, the substance considered potential biofilm could be sharply removed without the need for deep sharp debridement. However, this wound film was also adequately anchored to the wound bed to distinguish it from what could be considered to be a fibrinous wound exudate.
The samples were collected by sharply lifting the film off of the wound bed with a #5 disposable curette. Topical 4% lidocaine HCL spray was applied before this procedure to control the risk of any related discomfort. This sharp debridement process was performed on these subjects per normal standard of care for their wound management. Clinical pictures, labelled `macroscopic view', of each wound before film removal are shown in Figs 2–5, samples 1–16. The debrided film was used for various assays as described below. Some wounds were also subjected to Levine's culture technique and clinical microbiological laboratory analyses to determine the identity of microbes associated with the patient's wound.
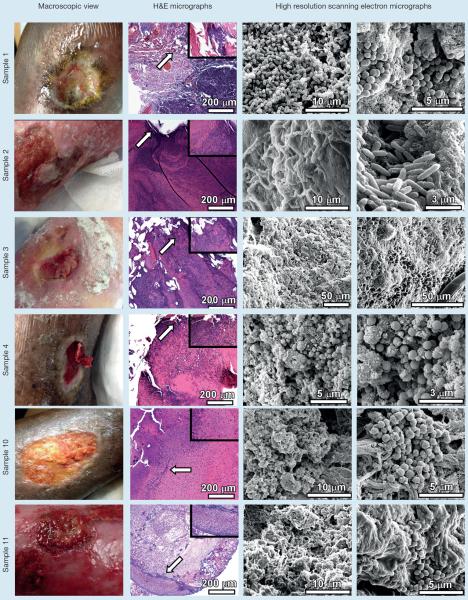
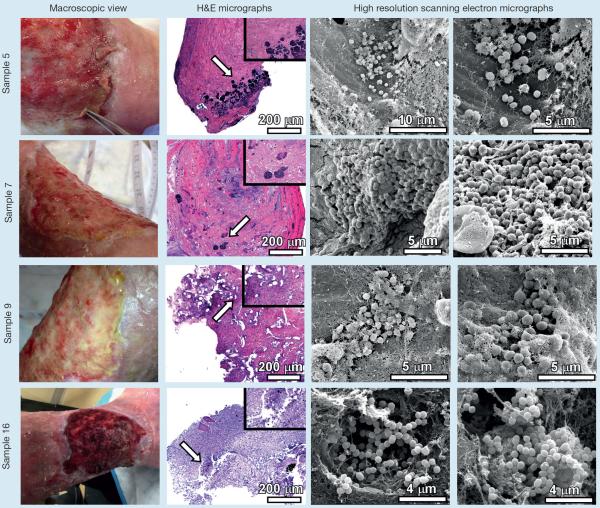
Fig 2.
Analyses of chronic non-healing wound samples by microscopical techniques. Chronic non-healing wounds (macroscopic view) were subjected to sharp debridement. The tissue removed from debridement was fixed, sectioned and analyzed by either hematoxylin and eosin stain and light microscopy (H&E micrographs), or by high resolution field-emission gun scanning electron microscopy (high resolution scanning electron micrographs). H&E micrographs were collected at 100X and inset panels were collected at 400X total magnification. Arrows indicate region magnified in inset panel. High resolution scanning electron micrographs were collected between 2,000X and 20,000X
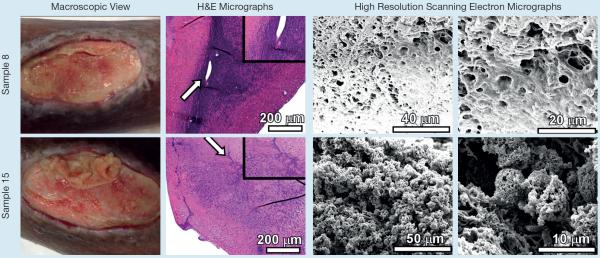
Fig 5.
Analyses of chronic non-healing wound samples by microscopical techniques. Samples 8 and 15 are taken from one patient over a number of weeks and following different treatments. Chronic non-healing wounds (macroscopic view) were subjected to sharp debridement. The tissue removed from debridement was fixed, sectioned and analysed by either hematoxylin and eosin stain and light microscopy (H&E micrographs), or by high resolution field-emission gun scanning electron microscopy (high resolution scanning electron micrographs). H&E micrographs were collected at 100X and inset panels were collected at 400X total magnification. Arrows indicate region magnified in inset panel. High resolution scanning electron micrographs were collected between 2,000X and 20,000X
Histological examination
We collected 16 samples from wounds on 11 patients. In those occurrences where a patient provided more than one sample, the samples were provided from the same wound but on a different day. Samples were fixed with 2.0% paraformaldehyde, 2.5% glutaraldehyde in 0.05M sodium cacodylate buffer at room temperature overnight before being embedded in paraffin. Tissue was cut into 5 μm sections and multiple sections were placed on each slide for analysis. The sections were stained with haematoxylin and eosin (H&E), rinsed, dehydrated and mounted with Cytoseal XYL before light microscopy analysis was performed.
Bacterial strains and biofilm propogation in vitro
In vitro bacterial biofilms were grown on polystyrene coverslips as previously described.28 Bacterial strains used in this study included meticillin-resistant Staphylococcus aureus (MRSA) USA300, Acinetobacter baumannii 19606T, Pseudomonas aeruginosa PA01, and Streptococcus agalactiae 2603V/R (Group B Streptococcus) (ATCC Global Bioresource Center). Bacteria were grown individually in shaking cultures of Luria-Bertani broth (LBB) overnight at 37°C. The following day, overnight cultures were diluted 1:100 in 1 ml of fresh LBB and vortexed vigorously. A polystyrene coverslip was added to the culture tube as a substrate for biofilm propogation. Biofilms were allowed to mature for 24 hours and were processed for high-resolution microscopy.
Scanning electron microscopy (SEM)
Field-emission gun scanning electron microscopy (FEG-SEM) sample preparation was performed as previously described.28 Briefly, samples were fixed with 2.0% paraformaldehyde, 2.5% glutaraldehyde in 0.05 M sodium cacodylate buffer (pH7.4) at room temperature overnight. Samples were washed three times with 0.05 M sodium cacodylate buffer before secondary fixation with 1% osmium tetroxide. Samples were sequentially dehydrated by washing with increasing concentrations of ethanol before being dried at the critical point, mounted onto SEM stubs and sputter-coated with 20 nm of gold-palladium. A thin line of colloidal silver paint was applied at the sample edge to facilitate grounding of the coverslip and prevent charging during FEG-SEM imaging. Samples were visualised with an FEI Quanta 250 FEG-SEM at high vacuum and micrographs were analysed with Image J software to generate cellular measurements as previously described.29
Results
In this study, we found that 75% of the described macroscopic wound bed films contained microbial biofilms despite the use of currently available antiseptic wound dressings and an effort to provide BBWC (Table1). Biofilms were confirmed by the presence of microbial cells adhering to the wound surface and forming tertiary architectural structures of cells with three or more cells interacting in this architecture. None of the wounds in these cases received the frequency of sharp biofilm disruption/debridement consistent with BBWC recommendations. This was in part due to the limitations in local nursing practice, as many practitioners are not allowed to perform any type of sharp debridement.30 Frequency of sharp biofilm disruption is most effective if it coincides with speed of biofilm maturation, every 2–3 days. This can be a challenge in the outpatient setting and in the case of weekly dressing changes. Many of the wounds were treated with an antiseptic cleanser to attack newly exposed planktonic microbes. Of note is that an antiseptic wound cleanser without regular debridement did not appear to control biofilm development. This supports a model in which biofilm must be addressed with a concurrent constellation of strategies.
Table 1.
| Sample No. | Subject/wound history | Biofilm found |
|---|---|---|
| 1 | 50-year-old obese, nondiabetic female with recurrent right lateral leg venous stasis ulcer. Antibiotics were initiated to treat the methicillin-resistant staphylococcus aureus (MRSA) noted on Levine culture. Current treatment: Cadexomer iodine gel, compression stockings with daily dressing changes |
Yes |
| 2 | 56-year-old female with Lupus, osteomalacia, and non-healing venous stasis ulcers, 5 years chronicity, copious exudate. Arterial studies within normal limits (WNL), on IV antibiotics for recalcitrant Pseudomonas aeruginosa (PA) wound infection. Current treatment: Intermittent sharp debridement, antiseptic cleanser at all dressing changes, silver gelling fiber dressing covered by absorptive non-gelling fibre dressing, then absorptive cotton padding secured with 4 layer compression |
Yes |
| 3 | 79-year-old female with a traumatic ankle ulcer reoccurring over past 6 years. History of chronic obstrutive pulmonary disease (COPD), congestive heart failure (CHF), peripheral arterial disease (PAD), and numerous rounds of oral and IV antibiotics for wound infection. Current treatment: Intermittent sharp debridement, silver gelling fiber dressing secured with very light compression to control peri-ulcer dependent oedema |
Yes |
| 4 | 68-year-old diabetic female with history of poor glucose management and a recurrent venous ulcer left lateral ankle. Ankle brachial index (ABI) WNL, Levine swab revealed MRSA which was treated based on sensitivity for 10 days. Current treatment: Intermittent sharp debridement, cellular and tissue based product (CTP) covered with silver gelling fibre dressing, secured with layered compression |
Yes |
| 5 | 66-year-old female with right lateral calf venous ulcer, 2 years chronicity. Unable to elevate leg without transfer to supine. Current treatment: Intermittent sharp debridement, antiseptic impregnated PVA foam and gentle compression. Treatment strategy was altered. Return to clinic (RTC) one month |
No |
| 6 | 63-year-old, poorly managed diabetic, paraplegic, crusted lesions over L shin of edematous L leg. Bilateral ABI of WNL. Wound culture revealed MRSA and PA, treated based on sensitivity. Current treatment: Cadexomer iodine gel, wrap compression and elevation |
Yes |
| 7 | See history under #5. Current treatment: Intermittent sharp debridement, antiseptic cleanser at all dressing changes, collagen dressing covered with antiseptic impregnated PU foam dressing then pneumatic compression. Treatment strategy was altered. RTC one month |
Yes |
| 8 | 69-year-old female with moderately draining traumatic ulcers over right leg. Easily palpable pedal pulses. On chemotherapy for advanced ovarian cancer. Current treatment: Silver gelling fibre dressing and leg elevation |
No |
| 9 | See history under #5. Current treatment: Intermittent sharp debridement, regular use of antiseptic wound cleanser, fenestrated bioelectric dressing, pneumatic compression strategy. Treatment strategy altered. RTC one month |
Yes |
| 10 | 54-year-old male, with recurrent venous ulcer right medial calf. Presented on empiric penicillin. Levine culture revealed MRSA, revised treatment based on sensitivity. Current treatment: Weekly sharp debridement, antiseptic cleanser with dressing changes, silver gelling fiber dressing secured with 4 layer compression |
Yes |
| 11 | 69-year-old female presented with 7-month old recurrent venous stasis ulcers. History of osteoporosis and bilateral hip replacements. ABI WNL. Levine swab revealed MRSA and PA. Current treatment: Intermittent sharp debridement, antiseptic cleanser with all dressing changes, silver polyurethane foam then layered compression. Underwent 3 PICC placements for IV antibiotics. |
Yes |
| 12 | See history under # 6. Current treatment: Cadexomer iodine gel, wrap compression and elevation |
Yes |
| 13 | 65-year-old female with left medial ankle wound, within peri-ulcer scarring related failed surgical closure 1 year prior followed by 10 subsequent failed site revisions. ABI WNL. Current treatment: Cadexomer iodine gel, wrap compression. |
No |
| 14 | 94-year-old female with recurrent venous ulcers of 18 months chronicity. History of CHF, PAD, OA, DVT/PE and recurrent wound infection. Current treatment: Inconsistent sharp debridement, antiseptic impregnated PVA foam and three layer compression system |
Yes |
| 15 | See history under # 8. Current treatment: Inconsistent sharp debridement, antiseptic cleanser with daily dressing changes, collagen covered with antiseptic impregnated PVA foam, then non-gelling fibre dressing secured with gauze wrap |
No |
| 16 | See history under #5. Current treatment: Inconsistent sharp debridement, antiseptic cleanser with all dressing changes, antiseptic impregnated PU foam dressing and Polysporin powder. |
Yes |
Light microscopy analyses of H&E stained slides of all CNHW samples reveal darkly stained putative clusters of microbial cells (Figs 2–5). H&E stain is commonly used for histopathological examination of tissue to analyse architecture, cell proliferation, and immune cell infiltrate. However, haematoxylin is basic and can stain acidic molecules such as DNA or RNA, which can be useful to identify potential microbes infecting tissues. We used the H&E stained sections as landmarks for possible areas of microbial colonisation and refined our imaging techniques further by employing high resolution FEG-SEM as has been previously described.
Upon FEG-SEM imaging of samples 1–6, (Fig 2 and 3) all contained microcolonies consistent in size and shape with microbial cells. However, in sample 5, these cells did not form the tertiary architectural structure formally recognised as a biofilm.
Fig 3.
Analyses of chronic non-healing wound samples by microscopical techniques. Chronic non-healing wounds (macroscopic view) were subjected to sharp debridement. The tissue removed from debridement was fixed, sectioned and analysed by either hematoxylin and eosin stain and light microscopy (H&E micrographs), or by high resolution field-emission gun scanning electron microscopy (high resolution scanning electron micrographs). Samples 5, 7, 9,16 are taken from one patient over a number of weeks and following different treatments. H&E micrographs were collected at 100X and inset panels were collected at 400X total magnification. Arrows indicate region magnified in inset panel. High resolution scanning electron micrographs were collected between 2,000X and 20,000X
Sample 1 revealed a large mixed species biofilm despite use of a product shown to prevent and disrupt mature biofilms in vitro.17,31,32 This product is recommended for use on highly exudating wounds because it is purported to absorb exudate. However, in this clinical setting it did not demonstrate length of absorptive or antibiofilm action within a routine of daily dressing changes. Variables in the clinical setting can impact dressing action leading to outcomes that differ from those obtained in the laboratory setting. Sample 1 harboured numerous cocci embedded in a webbed and globular matrix. These spherical cells exhibited a mean diameter of 656±39 nm, morphologically similar to Staphylococcus aureus. This result is consistent with the Levine's culture results (Table 1). Sample 1 also contained large colonies of bacilli encased in a fibrous matrix. These rod-shaped cells measured 496±68 nm in width and 1371±427 nm in length, indicating this CNHW had at least two distinct populations of biofilm-forming bacteria.
Sample 2 revealed a putative Pseudomonas biofilm on the wound of a patient who was on guided intravenous (IV) antibiotics chosen to address these particular bacteria. This provides clinical support for what is understood to be the protective action of the biofilm phenotype. Further, despite use of several layers, absorptive wound dressings wound exudate was difficult to manage. Expiration of dressing absorptive and/or antiseptic action may promote biofilm maturation, despite concominant guided IV antibiotics. Sample 2 harboured numerous rod-shaped bacteria measuring 829±82 nm in width and 3868±333 nm in length, morphologically similar to Pseudomonas aeruginosa and consistent with patient history of recalcitrant Pseudomonas aeruginosa wound infection (Table 1).
Sample 3 was collected from a CNHW with history of underlying peripheral arterial disease (PAD) along with history of repeated oral and IV antibiotics. Often PAD will limit patient's tolerance of elevation and compression potentially leading to difficulty managing wound exudate, promoting rapid expiration of dressing action. Interestingly, the biofilm found on this wound's bed contained cells with an average size that was consistent with fungal rather than bacterial cells. Sample 3 harboured numerous ovoid-shaped cells that measured 3317±590 nm by 4695±320 nm in diameter, sizes that were consistent with fungal cells such as Candida albicans. It is interesting to note that this patient received numerous rounds of oral and IV antibiotics for putative wound infection, a therapeutic strategy that could have predisposed this patient to fungal colonisation.
Sample 4 was a thick red film that reformed weekly over a venous stasis ulcer on a patient with poorly controlled diabetes. Layered compression strategies have been designed to support prolonged wear time. This challenges the ability to debride every 2–3 days as recommended by the biofilm maturation research. The thick red film was confirmed to be a putative bacterial biofilm which ranged in thickness from 1 mm to 2.8 mm. This is particularly significant because it developed shortly after completion of 10 days of oral antibiotics chosen based on culture to control meticillin-resistant Staphylococcus aureus (MRSA). Further, this film developed weekly underneath a costly cellular/tissue based product (CTP) chosen to enhance healing in the face of wound recalcitrance. This macroscopic biofilm developed despite the use of an antiseptic dressing used for secondary coverage. Sample 4 contained numerous cocci measuring 642±98 nm in diameter dominating the sample. Consecutive cross-sectional measurements at low magnification revealed a biofilm measuring 1–2.8 mm in thickness. These bacterial cells were embedded in a globular matrix. The size, shape and arrangement of cells were consistent with the Levine's swab results, which revealed Staphylococcus aureus within the wound (Table 1).
Sample 10 was collected from a recurrent venous stasis ulcer. A large putative staphylococcal biofilm was seen with advanced microscopy (Fig 2). The patient presented from a primary care provider who had prescribed empiric antibiotics, which were not adequate to address the MRSA noted by culture. Sample 10 was observed to have large microcolonies containing cocci, measuring 663±81 nm, embedded in a globular and fibrous matrix.
Sample 11 was collected from an acutely infected CNHW with venous aetiology. Again, moisture management was a significant challenge. This patient exhibited noticeable improvement in signs of infection while on IV antibiotics but wounds slowly deteriorated within weeks of completing antibiotic therapy. This may have been due to residual bacteria protected in biofilm, which survived antibiotic therapy only to again proliferate in this moist, nutritious wound environment. Sample 11 contained clusters of cocci, including diplococci, measuring 912±125 nm and a few rod-shaped cells (observed at lower magnification), measuring 716±108 nm in width and 3124±399 nm in length (Fig 2). These cellular dimensions and morphology are consistent with the clinical culture results indicating Pseudomonas aeruginosa and/or MRSA are present (Table 1).
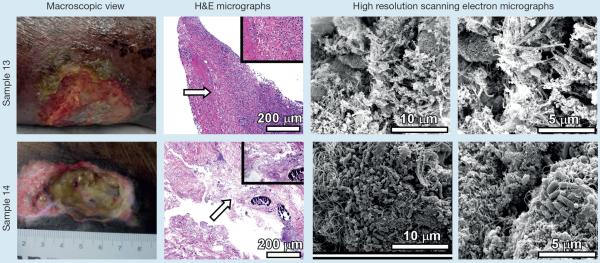
Sample 13 was collected from a well-perfused chronic, non-healing surgical wound on a patient with well-controlled diabetes. The wound bed consisted of scar tissue resulting from repeated efforts to repair and wound moisture was easily managed. Treatment was carried out similarly to sample 1; daily wound care with a product shown to prevent and disrupt mature biofilms in vitro. Sample 13 had few scattered bacilli with no appreciable biofilm observed (Fig 2).
Sample 14 was collected from a CNHW with underlying venous and arterial insufficiency. Mixed aetiology provokes challenges to adequately compress and elevate resulting in inadequately managed wound exudate, which was a constant challenge with this patient. Antiseptic wound cleanser was diligently used during all dressing changes by home health nursing care and wound bed film was debrided at 2–3 week wound center visits. The film sample collected from this wound was found to contain biofilm, despite the use of a highly absorbent antiseptic PVA foam primary dressing. Sample 14 contained numerous bacilli, measuring 518±102 nm in width and 1202±225 nm in length, decorated with both flagella and pili adhering to tissue substrate and forming large dome-shaped biofilms in which bacteria were encased in a fibrous and globular matrix (Fig 2).
Samples 5, 7, 9, and 16 were collected from the same patient several weeks apart (Fig 3), of a film present under several topical products ranging from antiseptic polyvinyl alcohol (PVA) foam to collagen with antiseptic polyurethane (PU) foam to a bioelectric dressing to antiseptic PU foam and antibiotic powder. Wound exudate management was an ongoing challenge. As previously mentioned, bacteria were seen in sample 5 but the tertiary structure of a biofilm was not visualised. One potential factor that may have impacted this finding is that a PVA foam holds more exudate than a PU foam, promoting a prolonged time until dressing action expiration. It also remains plausible that an inadequate sample was collected for sample 5. Sample 5 contained numerous clustered cocci embedded in a fibrous matrix, sparsely decorating the wound section. These cocci measured 555±48 nm in diameter and were not observed forming any appreciable tertiary structure indicative of biofilm formation at this time point. High-resolution imaging of sample 7 revealed large clusters of cocci-shaped cells, measuring 627±80 nm, embedded in a sheet-like matrix populated with numerous fibrous structures. Sample 9 contained clusters of cocci, measuring 712±48 nm, encased in a fibrous, web-like matrix. Sample 16 harboured numerous cocci in diplo- or strepto- configurations as well as clusters held together with fibrous and web-like matrices. These cells measured 497±73 nm in diameter. It is interesting to note that this sample exhibited tunnel-like destruction beneath bacterial biofilm microcolonies, an observation that could be associated with tissue destruction and/or invasion by these microbes.
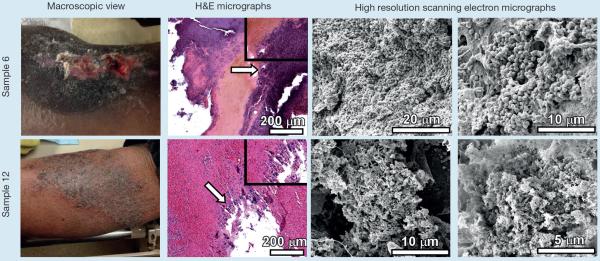
Samples 6 and 12 (Fig 4) consisted of scabs from relatively dry wounds with an initial stasis aetiology that was subsequently well controlled. Scab is defined by the Association for the Advancement of Wound Care (AAWC) Quality of Care Wound Glossary25 as `dried secretions, exudates, and dead cells covering a wound'. In both samples biofilm was present when evaluated. These results are consistent with Zhao et al.33 who found a high biofilm bacterial load in the scab covering a chronic wound. This fact supports the idea that a scab, the result of dry wound healing strategies, may not be a reliably protective cover against wound infection, especially when located over joints where movement can cause cracking and remoistening of the scab. In sample 6, the entirety of the cross-section of the wound debridement sample was populated with cocci-shaped cells, measuring 597±74 nm in diameter, consistent with culture results indicating patient is chronically colonised with MRSA (Table 1). Cells formed a tenacious biofilm and were tightly embedded in a thick matrix that is both fibrous, and sheet-like. Consecutive cross-sectional measurements at low magnification reveal this biofilm is 1.5–3.4 mm in thickness. SEM of sample 12 reveals few bacilli and coccobacilli, measuring 496±71 nm across and 764±51 nm in length, and coccoid cells, measuring 512±67 nm in diameter, forming large clusters encased in a globular and fibrous matrix.
Fig 4.
Analyses of chronic non-healing wound samples by microscopical techniques. Samples 6 and 12 are taken from one patient over a number of weeks and following different treatments. Chronic non-healing wounds (macroscopic view) were subjected to sharp debridement. The tissue removed from debridement was fixed, sectioned and analysed by either hematoxylin and eosin stain and light microscopy (H&E micrographs), or by high resolution field-emission gun scanning electron microscopy (high resolution scanning electron micrographs). H&E micrographs were collected at 100X and inset panels were collected at 400X total magnification. Arrows indicate region magnified in inset panel. High resolution scanning electron micrographs were collected between 2,000X and 20,000X
Samples 8 and 15 (Fig 5) were of a thick, opaque white, reforming film on the wound of a patient who was undergoing chemotherapy for ovarian cancer. A silver-impregnated fibre dressing was used for primary dressing for this wound with an active, watery exudate. This patient was seen in the wound centre every 2–3 weeks, these being the only times the wounds' bed film was debrided. Sample 8 was observed to have a thick matrix coating all surfaces of the sample, which ultimately confounded imaging strategies and no microbial cells were visualised. Advanced microscopy identified host tissue without evidence of bacterial biofilm. These results indicate that concurrent chemotherapy could play a role in modulating biofilm formation in CNHWs. High resolution imaging of sample 15 did not reveal intact biofilms, but instead clusters of fibrous spherical objects were observed. These objects were consistent in size and shape with microbial cells, measuring 4713±500 nm in diameter, indicating these could represent lysed microbial cells.
In vitro grown Biofilm
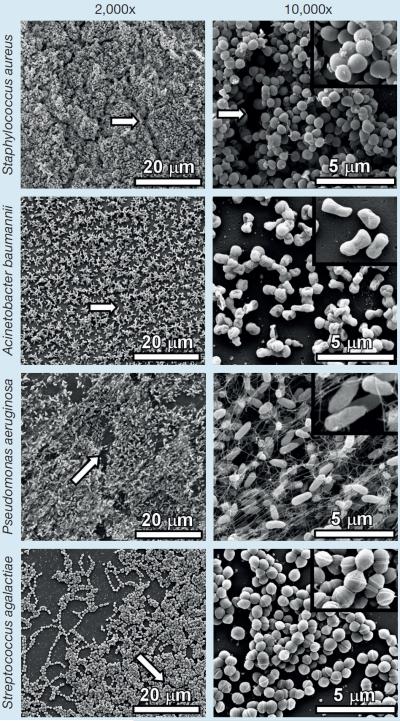
Fig 6 demonstrates typical in vitro laboratory biofilm formation by numerous relevant human pathogens similar to those potentially observed in wound biofilms imaged. High-resolution imaging of MRSA USA300 reveals cocci measuring 529±86 nm forming large clusters of cells with defined tertiary structure, size and arrangements that are comparable with those cells observed in wound samples 1, 4, 5, 6, 7, 9, 10, 11. Biofilms formed by Acinetobacter baumannii 19606T contain cocco-bacilli measuring 721±120 nm in width and 1103±225 nm in length. Pseudomonas aeruginosa PA01 forms biofilms containing bacilli measuring 503±96 nm in width and 1419±363 nm in length, encased in a fibrous matrix that is likely comprised of flagella and somatic pili; results that are similar to those observed in wound samples 2, and 14. Streptococcus agalactiae 2603V/R (Group B Streptococcus) biofilms harbor diplo- and strepto- arrangements of cells packed tightly together and measuring 488±36 nm in diameter.
Fig 6.
Evaluation of in vitro biofilm formation. High resolution scanning electron micrographs were collected between 2,000X and 10,000X total magnification (inset panel 20,000X) for comparison of in vitro biofilm with in vivo biofilm found within wound samples. Magnification bars indicate scale for each micrograph. White arrows indicate channels formed between clusters of cells that are likely used for diffusion of nutrients within the biofilm and dispersal of metabolic waste products out
Discussion
Results revealed that 10 of the 16 samples of recurring wound bed film found on a CNHW were a macroscopic bacterial biofilm. In two other samples of wound scab/crust, bacteria in biofilm were located within the crust, a finding consistent with what has been previously reported by Zhao and Park.33,34 Also, important to note is that two of the four samples which were found to not contain biofilm, did contain bacteria but not in a tertiary architectural structure. In some cases, the putative bacteria identified in the biofilm were consistent in cell morphology and arrangement with the infection identified by Levine's swab. Further, in some cases these bacteria existed despite ongoing or recently completed guided systemic antibiotic therapy. This supports what is understood about the protective properties of the EPS matrix to antimicrobial chemotherapeutic onslaught.31,32 Interestingly, one case revealed a putative yeast biofilm in a sample collected from a CNHW on a patient with history of recurrent antibiotics.
In most of these cases, management of wound exudate was difficult and was noted to have led to a rapid, sometimes unexpected expiration of dressing action; including both absorptive and antiseptic action. This highlights how important it is for the wound clinician to evaluate wound dressings at the time of removal from the wound to assess effectiveness of current wound care strategy. In all cases, there was an inability to maintain BBWC recommendation of regular sharp debridement of wound bed film.30 And although all wounds were treated with currently available antiseptic dressings, no antiseptic dressings containing proven antibiofilm agents were available for use.
The challenged management of patient comorbid conditions was recognised as a contributor to wound chronicity in all the samples that contained biofilm. These comorbid conditions included PAD, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), obesity, impaired mobility related to severe osteoarthritis, inadequately controlled diabetes and immune dysfunction. There were two samples of recurring film on CNHWs found not to contain biofilm collected from a wound on a patient undergoing chemotherapy. Of these samples one contained a large amount of putative lysed bacterial cells. It is uncertain if this finding is related to chemotherapy.
Biofilm on a wound can indeed develop to a clearly macroscopic state, as most remarkably evidenced by sample 4. This advanced biofilm maturation was linked to chronicity but not to clinical evidence of acute wound infection. Conversely, the visible biofilm in sample 11 was linked with clinical evidence of acute infection. This evidence resolved with administration of IV antibiotics yet reappeared 2–3weeks after the antibiotic regimen was completed. No sample was collected during episodes of treatment to objectively confirm extent of bacterial suppression. Biofilm may be linked to infection but at this time it remains unclear whether it can be either predictive of impending infection or diagnostic of current infection.
The characteristics of the samples confirmed to contain biofilm, 75% of the samples collected, coincided with the descriptions included on the evidence-based clinical biofilm algorithm that was chosen to guide sample selection. The surface substance evaluated detached atraumatically, reformed quickly, demonstrated recalcitrance to standard systemic antimicrobial dosing, and responded poorly to standard antiseptic dressings reported to control biofilm in vitro. This paper does provide validation for the algorithm used, which outlines characteristics of sample choice to enrich for potential biofilm presence.
The fact that the antiseptic dressings chosen to treat these wounds were ineffective in fully controlling biofilm is not solely related to shortcomings in the dressings. Based on BBWC recommendations, no single strategy is adequate to manage wound biofilm and antiseptic dressings can only be considered as potentially efficacious as an adjunct treatment to be considered along with regular biofilm disruption and the use of proven antibiofilm agents.30 Debridement, as a key component of antibiofilm recommendations requires a more thorough investigation of practicality. As previously stated, many nurses are not allowed to sharp debride and nurses compose a significant percentage of the wound care delivery team. Also, since biofilms can mature in vitro or ex vivo within 2–3 days.12,31 any effective debridement would need to be performed at this regular interval; a strategy which could be difficult to implement. Our results point to a need to reconsider practice paradigms and to consider the addition of proven antibiofilm agents into current antiseptic wound dressings. Though this remains to be confirmed, it may be that the addition of an antibiofilm agent may limit the need for regular sharp debridement. This could enhance the cost-effectiveness of any chosen antimicrobial treatment strategy and support sustained efficacy between dressing changes.
Limitations
This study involved a small number of samples and, thus, is limited in its prediction strength. Some of these samples were taken from the same patient. This repeat sampling cannot provide a representative sample of the likely prevalence of biofilm in CNHWs. However, expert work on this topic has previously been completed. Repeat sampling can, however, serve as confirmation of earlier results and provide some evaluation of the efficacy of products chosen as a change in treatment strategy. This was particularly the case for samples 5, 7, 9, 16. It is also true that we included no mechanism to ensure that adequate sampling was obtained to rule out that biofilm was not present in macroscopic film in other locations on the wound bed.
Conclusion
This study has allowed wound care science to begin to bridge a gap between the wound clinic and the microbiology laboratory. In this study a particular visible wound bed substance was chosen for evaluation and, in the majority of cases, this substance was revealed to contain bacteria in biofilm which were consistent in size, arrangement and architecture with biofilms formed by relevant human pathogens in vitro. The SEM results clearly demonstrate cellular aggregates indicative of microbial biofilm formation in human chronic non-healing wounds, a result that is largely in agreement with the clinical algorithm used. This result provides beginning foundation for clinical suspicion of the presence of biofilm which then can be used to justify more aggressive and potentially more costly antibiofilm strategies which can achieve an overall more cost-effective healing outcome.
Acknowledgements
This work was funded primarily by a Career Development Award IK2BX001701 (to J.A.G.) from the Office of Medical Research, Department of Veterans Affairs. Core services, including use of the Cell Imaging Shared Resource, were performed through both Vanderbilt University Medical Center's Digestive Disease Research Center, supported by NIH grant P30DK058404 Core Scholarship, and the Vanderbilt Institute for Clinical and Translational Research program, supported by the National Center for Research Resources, grant UL1 RR024975-01, and the National Center for Advancing Translational Sciences, grant 2 UL1 TR000445-06.
References
- 1.Reddy M, Gill SS, Wu W, et al. Does this patient have an infection of a chronic wound? JAMA. 2012;307(6):605–611. doi: 10.1001/jama.2012.98. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf DG, Bowler PG. Biofilm delays wound healing: a review of the evidence. Burns & Trauma. 2013;1(1):5–12. doi: 10.4103/2321-3868.113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurlow J, Couch K, Laforet K, et al. Clinical Biofilms: A Challenging Frontier in Wound Care. Adv Wound Care. 2015;4(5):295–301. doi: 10.1089/wound.2014.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seth AK, Geringer MR, Gurjala AN, et al. Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: a quantitative study using an in vivo rabbit ear model. Plast Reconstr Surg. 2012;129:262e–274e. doi: 10.1097/PRS.0b013e31823aeb3b. [DOI] [PubMed] [Google Scholar]
- 5.James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wounds Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 6.Kirketerp-Møller K, Jenson PO, Fazli M, et al. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol. 2008;46:2712–2722. doi: 10.1128/JCM.00501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 8.Branda SS, Vik A, Friedman L, Kolter R. Biofilms: The matrix revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Watters C, DeLeon K, Trivedi U, et al. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med Microbio. Immunol. 2013;202(2):131–141. doi: 10.1007/s00430-012-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen KT, et al. Deficient cytokine expression and neutrophil oxidative burst contribute to impaired cutaneous wound healing in diabetic, biofilm-containing chronic wounds. Wound Repair Regen. 2013;21(6):833–841. doi: 10.1111/wrr.12109. [DOI] [PubMed] [Google Scholar]
- 11.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time dependent therapeutic window. J Wound Care. 2010;19(8):320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
- 13.Rhoads DD, Wolcott RD, Percival S. Biofilms in Wounds: Management Strategies. J Wound Care. 2008;17(11):502–508. doi: 10.12968/jowc.2008.17.11.31479. [DOI] [PubMed] [Google Scholar]
- 14.Oduwole KO, Glynn AA, Molony DC, et al. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J Orthop Res. 2010;28(9):1252–1256. doi: 10.1002/jor.21110. [DOI] [PubMed] [Google Scholar]
- 15.Kalishwaralal K, Barath Mani Kanth S, Pandian SR, et al. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf Biointerfaces. 2010;79(2):340–344. doi: 10.1016/j.colsurfb.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Kostenko V, Lyczak J, Turner K, Martinuzzi RJ. Impact of Silver-Containing Wound Dressings on Bacterial Biofilm Viability and Susceptibility to Antibiotics during Prolonged Treatment. Antimicrob. Agents Chemother. 2010;54:125120–125131. doi: 10.1128/AAC.00825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips P, Yang Q, Davis S, et al. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm in porcine skin explants. Int Wound J. 2015;12(4):469–483. doi: 10.1111/iwj.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Percival S, McCarty S. Silver and alginates: Role in wound healing and biofilm control. Adv Wound Care. 2015;4(7):407–414. doi: 10.1089/wound.2014.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourdillon K. Dressings and biofilms: interpreting evidence from in vitro biofilm models. Wounds International. 2016;7(1):9–14. [Google Scholar]
- 20.Larko E, Persson A, Blom K. Effect of superabsorbent dressings in a 3D acellular tissue model of Pseudomonas aeruginosa biofilm. J Wound Care. 2015;24(5):204–210. doi: 10.12968/jowc.2015.24.5.204. [DOI] [PubMed] [Google Scholar]
- 21.Walker M, Metcalf D, Parsons D, Bowler P. A real-life clinical evaluation of a next-generation antimicrobial dressing on acute and chronic wounds. J. Wound Care. 2015;24(1):11–22. doi: 10.12968/jowc.2015.24.1.11. [DOI] [PubMed] [Google Scholar]
- 22.Cowan T. Visible biofilms – a controversial issue! J Wound Care. 2012;21:106. doi: 10.12968/jowc.2012.21.3.106. [DOI] [PubMed] [Google Scholar]
- 23.Metcalf DG, Bowler PG, Hurlow J. A clinical algorithm for wound biofilm identification. J. Wound Care. 2014;23(3):137–142. doi: 10.12968/jowc.2014.23.3.137. [DOI] [PubMed] [Google Scholar]
- 24.Hurlow J, Bowler PG. Clinical experience with wound biofilm and management: a case series. Ostomy Wound Manage. 2009;55(4):38–49. [PubMed] [Google Scholar]
- 25.Association for Advanced Wound Care (AAWC) [accessed 15 August 2016];Wound Glossary. Released to membership April 2012. http://bit.ly/1TwjxmX.
- 26.Milne C, Ciccarelli A, Lassy M. Comparison of collagenase to hydrogel dressing in wound debridement. Wounds. 2010;22(11):270–274. [PubMed] [Google Scholar]
- 27.Wolcott RD, Rhoads DD, Dowd SE. Biofilms and Chronic Wound Inflammation. J Wound Care. 2008;17(8):333–341. doi: 10.12968/jowc.2008.17.8.30796. [DOI] [PubMed] [Google Scholar]
- 28.Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun. 2009;77(8):3150–3160. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaffer C, et al. Helicobacter pylori exploits a unique repertoire of type IV secretion system components for pilus assembly at the bacteria-host cell interface. PLoS Pathog. 2011;7(9):e1002237. doi: 10.1371/journal.ppat.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [accessed 15 August 2016];State by State Summary of Nurses Allowed to Perform Conservative Sharp Debridemen. http://bit.ly/2b4XBxJ.
- 31.Yang Q, et al. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen. 2013;21(5):704–14. doi: 10.1111/wrr.12074. [DOI] [PubMed] [Google Scholar]
- 32.Phillips PL, Yang Q, Sampson E, Schultz G. Effects of antimicrobial agents on an (in-vitro) Biofilm Model of Skin Wounds. Advances in Wound Care. 2010;1:299–304. [Google Scholar]
- 33.Zhao G, Usui ML, Lippman SI, et al. Biofilms and Inflammation in Chronic Wounds. Adv Wound Care. 2013;2(7):389–399. doi: 10.1089/wound.2012.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park E, Long SA, Seth AK, et al. The use of desiccation to treat Staphylococcus aureus biofilm infected wounds. Wound Repair Regen. 2016;24(2):394–401. doi: 10.1111/wrr.12379. [DOI] [PubMed] [Google Scholar]