Abstract
Patient: Male, 34
Final Diagnosis: ME/CFS
Symptoms: Exertion intolerance • loss of functional capacity • pain • severe fatigue
Medication: —
Clinical Procedure: Cardiopulmonary exercise test
Specialty: Sports Medicine
Objective:
Unknown ethiology
Background:
Patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) present with profound fatigue, flu-like symptoms, pain, cognitive impairment, orthostatic intolerance, post-exertional malaise (PEM), and exacerbation of some or all of the baseline symptoms.
Case Report:
We report on a pair of 34-year-old monozygotic twins discordant for ME/CFS, with WELL, the non-affected twin, and ILL, the affected twin. Both twins performed a two-day cardiopulmonary exercise test (CPET), preand post-exercise blood samples were drawn, and both provided stool samples for biochemical and molecular analyses. At peak exertion for both CPETs, ILL presented lower VO2peak and peak workload compared to WELL. WELL demonstrated normal reproducibility of VO2@ventilatory/anaerobic threshold (VAT) during CPET2, whereas ILL experienced an abnormal reduction of 13% in VAT during CPET2. A normal rise in lactate dehydrogenase (LDH), creatine kinase (CK), adrenocorticotropic hormone (ACTH), cortisol, creatinine, and ferritin content was observed following exercise for both WELL and ILL at each CPET. ILL showed higher increases of resistin, soluble CD40 ligand (sCD40L), and soluble Fas ligand (sFasL) after exercise compared to WELL. The gut bacterial microbiome and virome were examined and revealed a lower microbial diversity in ILL compared to WELL, with fewer beneficial bacteria such as Faecalibacterium and Bifidobacterium, and an expansion of bacteriophages belonging to the tailed dsDNA Caudovirales order.
Conclusions:
Results suggest dysfunctional immune activation in ILL following exercise and that prokaryotic viruses may contribute to mucosal inflammation and bacterial dysbiosis. Therefore, a two-day CPET and molecular analyses of blood and microbiomes could provide valuable information about ME/CFS, particularly if applied to a larger cohort of monozygotic twins.
MeSH Keywords: Cytokines; Exercise Test; Microbiota; Plasma; Twins, Monozygotic
Background
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), also termed Systemic Exertion Intolerance Disease, is characterized by profound fatigue, a lack of energy, pain, cognitive impairment, orthostatic intolerance, and especially a phenomenon termed post-exertional malaise (PEM), which is brought about by physical, cognitive, or emotional stress [1]. PEM symptoms often include flu-like symptoms and exacerbations in pain, gastrointestinal (GI) symptoms, unrefreshing sleep, and other symptoms of the disease [2].
Analysis of several small cohorts of ME/CFS patients and controls has demonstrated abnormalities in successive cardiopulmonary exercise tests (CPETs), in which ME/CFS patients did not reproduce parameters measured on the first day of testing, despite the fact that such tests are highly reproducible when performed by individuals with heart conditions or other diseases [3,4]. Altered gene expression following exercise challenge has also been reported [5].
In addition to abnormal responses to exercise, ME/CFS biomarkers across a number of the body’s core regulatory systems have been detected [6]. For example, several reports have detected altered NK cell activity or other changes in immune function, including altered levels of cytokines and peroxides [6–10]. Brain imaging has also detected distinct differences between ME/CFS patients and healthy controls [11–15].
A confounding factor in examining ME/CFS patients versus controls is the possible contribution of genetic differences to symptom constellation and severity. Variation among ME/CFS cohorts has led to the concept of sub-groups with regard to the type of biological abnormalities, impact of particular symptoms, and response to drugs. Analysis of monozygotic twins provides an opportunity to reduce variability introduced by genetic background, and most such twins also have experienced similar childhood environments. We report here the analysis of peak exertion, ventilatory/anaerobic threshold (VAT), biochemical parameters, and the gut microbiome in a pair of monozygotic twins discordant for ME/CFS.
Case Report
We describe a pair of Caucasian male monozygotic twins discordant for ME/CFS assessed at age 34.3 years. The study was approved by the Ithaca College Institutional Review Board. Both twins completed a two-day CPET protocol to determine functional capacity (CPET1) and assess effects of PEM on functional capacity (CPET2). Two CPETs separated by 24 hours were completed by each twin. Pre- and post-exercise blood samples were obtained for each CPET. Fecal samples were obtained prior to the CPETs for study of the gut microbiome.
The twins were similar in height, weight, and BMI (Table 1), and had a shared family history significant only for cancer in a maternal grandfather, type 1 diabetes (maternal aunt), type 2 diabetes (paternal uncle), and hypertension (father). However, the affected twin (ILL) had sudden onset of chronic fatigue 3.5 years prior to this study (August 2010) following an influenza vaccine, and over the next several months the subject noted exercise recovery taking much longer than usual. In February 2011, a sleep study showed no evidence of sleep apnea, despite difficulty sleeping. During a weekend vacation with friends in July 2011, he was unable to participate in activities due to extreme exhaustion accompanied by mild to moderate pain. Throughout the development of his fatiguing illness, he also experienced cognitive dysfunction (brain fog) that he considered to be a primary symptom, in addition to fatigue. Prior full-time employment was as a project support manager at a large equipment company that required high-level organizational skills, multi-tasking, and ability to perform in the midst of multiple interruptions. ILL twin met the Fukuda definition for CFS at the time of evaluation [16]. The subject continues to be unable to work and relies on family/friend support for some transportation and assistance with some activities of daily living such as grocery shopping and medical appointments, but he is not confined to the house. He is unable to perform on-going physical work or desk work due to significant impairment of cognitive skills and inability to use a computer for more than one hour without causing debilitating fatigue and exacerbation of cognitive impairment. Activity prior to his illness was at a high level on a daily basis in his job. He also did weight training, running, and cycling every other day. Current consumption of alcohol is 1 serving of beer per month. The subject reported intestinal disturbances such as diarrhea or constipation. Current medications or supplements are Benicar 20 mg qd for hypertension, Soma 3×350 mg qd, Cymbalta prn and hydrocodone prn for pain, and Adderall 3×20 mg qd.
Table 1.
Characteristics of WELL and ILL.
| Characteristic | WELL | ILL |
|---|---|---|
| Age (years) | 34.3 | |
| Race/ethnicity | White/not hispanic | |
| Time with CFS (years) | NA | 2.5 |
| BMI | 25.4 | 25.5 |
| HRrest | 59 | 112 |
| BPrest* | 140/88 | 136/84 |
| Onset of illness | NA | Sudden |
| Tilt test | Not performed | (–) |
| Gastrointestinal symptoms | None | Yes |
| Post-exertional malaise | NA | Yes |
| Chalder Fatigue Scale score** | 0 | 10 |
| Bell Disability Scale score*** | 100 | 40 |
| Physical Component Score SF-36 | 55.6 | 21.7 |
| Mental Component Score SF-36 | 92 | 56 |
Both medicated for hypertension;
0–11, 0=no fatigue;
100=no impairment.
The unaffected twin (WELL) was apparently healthy and employed at the time of evaluation. Present symptoms included seasonal and soy allergies, and he did not report gastrointestinal disturbances. Medical history included hypertension, occasional low back pain, and seasonal and soy allergies. Current employment was a full-time mid-level management position in a large equipment company, characterized as high stress but predominantly sedentary. Activity history included occasional low-intensity fitness walking and yoga/stretching three times per week. Consumption of 12–14 servings of beer/wine per week was reported. Sleep was described as sound, 7–8 hours per night. Current medication was losartan potassium 25 mg qd for hypertension.
Both twins were medicated for familial hypertension and had similar high resting blood pressures (BPs); however, ILL’s resting heart rate (HR) of 112 bpm was markedly higher than that of WELL (59 bpm). Both WELL and ILL completed the Chalder Fatigue Scale, the Bell Disability Scale [17], and the Medical Outcome Survey Short Form 36 (SF-36; https://www.rand.org/health/surveys_tools/mos/mos_core_36item_terms.html). As expected, ILL’s scores were lower for all three scales (Table 1). For the SF-36 form, the eight subscales were aggregated into two summary measures: the Physical (PCS) and Mental (MCS) Component Summary scores [18]. PCS and MCS scores range from 0 to 100, with higher scores representing better self-reported health. PCS encompasses physical functioning, role-physical, and bodily pain, whereas MCS includes social functioning, role-emotional, and mental health. ILL’s PCS score (21.7) was below the expected value (>50) in the US normative population [18], while the MCS score (56) was above. However, compared to WELL, ILL’s MCS and PCS scores were substantially lower (Table 1).
Peak exertion
Even though both twins characterized ILL as highly physically active and more physically active compared to WELL prior to the onset of illness, ILL’s VO2peak from CPET1 was now 7.5% lower than WELL’s based on CPET1 (Table 2). Both ILL and WELL reproduced VO2peak normally during CPET2, although WELL reached VO2peak during CPET2 with a borderline abnormal increase of 8%. WELL completed a workload of 240 W at peak exercise during both tests in contrast to a much lower peak workload of 165 W for ILL. Both twins met criteria for maximal exertion during both CPETs as evidenced by respiratory exchange ratio (RER) >1.1, HRpeak >90% age predicted maximum heart rate (167+ bpm), and peak exertion blood lactate values of greater than 8 mmol/L (Table 2).
Table 2.
Cardiopulmonary exercise test results at peak exercise and ventilatory/anaerobic threshold.
| Test | Peak exercise | Ventilatory/anaerobic threshold | |||||
|---|---|---|---|---|---|---|---|
| WELL | ILL | % Difference | WELL | ILL | % Difference | ||
| VO2 | CPET1 | 36.95 | 34.37 | −7.5 | 17.3 | 14.9 | −16.1 |
| CPET2 | 40 | 34.5 | −15.9 | 16.8 | 12.9 | −30.2 | |
| Work | CPET1 | 240 | 165 | −45.5 | 90 | 60 | −50 |
| CPET2 | 240 | 165 | −45.5 | 90 | 45 | −100 | |
| HR | CPET1 | 192 | 200 | 4.0 | 136 | 136 | 0 |
| CPET2 | 194 | 201 | 3.5 | 132 | 146 | 9.6 | |
| RER | CPET1 | 1.34 | 1.39 | 3.6 | NA | ||
| CPET2 | 1.37 | 1.34 | −2.2 | ||||
| LA | CPET1 | 9.5 | 11.1 | 14.4 | NA | ||
| CPET2 | 10.1 | 9.3 | −8.6 | ||||
| % VO2 | CPET1 | NA | 47 | 42 | |||
| CPET2 | 42 | 37 | |||||
Ventilatory/anaerobic threshold (VAT)
Table 2 shows results related to VAT for both CPETs. WELL demonstrated normal reproducibility of VO2@VAT during CPET2 with less than a 3% change in VAT. In contrast, ILL experienced an abnormal reduction of 13% in VAT during CPET2. Compared to his twin, ILL’s VO2@VAT from CPET1 was 16% lower than that of WELL; however, it was 30% lower based on CPET2 (Table 2). Not surprisingly, work produced by WELL at VAT was 50% (CPET1) to 100% (CPET2) higher compared to work produced by ILL. Interestingly, VAT occurred at a relatively low percentage of VO2peak for both WELL and ILL. For WELL, VAT@ VO2peak was normal (47%, CPET1) to borderline low (42%, CPET2), and decreased on CPET2 due to the 8% increase in VO2peak on that test. Despite the 8% increase in VO2peak, unexpectedly, WELL’s VAT did not also increase during CPET2.
Biochemistry
Plasma values of lactate dehydrogenase (LDH), creatine kinase (CK), creatinine, magnesium, adrenocorticotropic hormone (ACTH), cortisol, C-reactive protein (CRP), and ferritin were measured before and after each CPET and processed at the Human Metabolic Research Unit, Division of Nutritional Sciences at Cornell University. LDH, CK, creatinine, and magnesium were measured by colorimetric assay on a Dimension XPand Plus chemistry analyzer (Siemens Medical Solutions Diagnostics, Deerfield, Illinois). ACTH, cortisol, C-reactive protein, and ferritin were quantified using a chemiluminescence immunoassay on an Immulite 2000 (Siemens Medical Solutions Diagnostics, Deerfield, Illinois). Levels of soluble CD40 ligand (sCD40L), resistin, and soluble Fas ligand (sFasL) in plasma were determined before and after the first CPET by a customized Bio-Plex multiplex cytokine assay system on a Luminex MAGPIX Multiplex Reader (Biorad, Hercules, California).
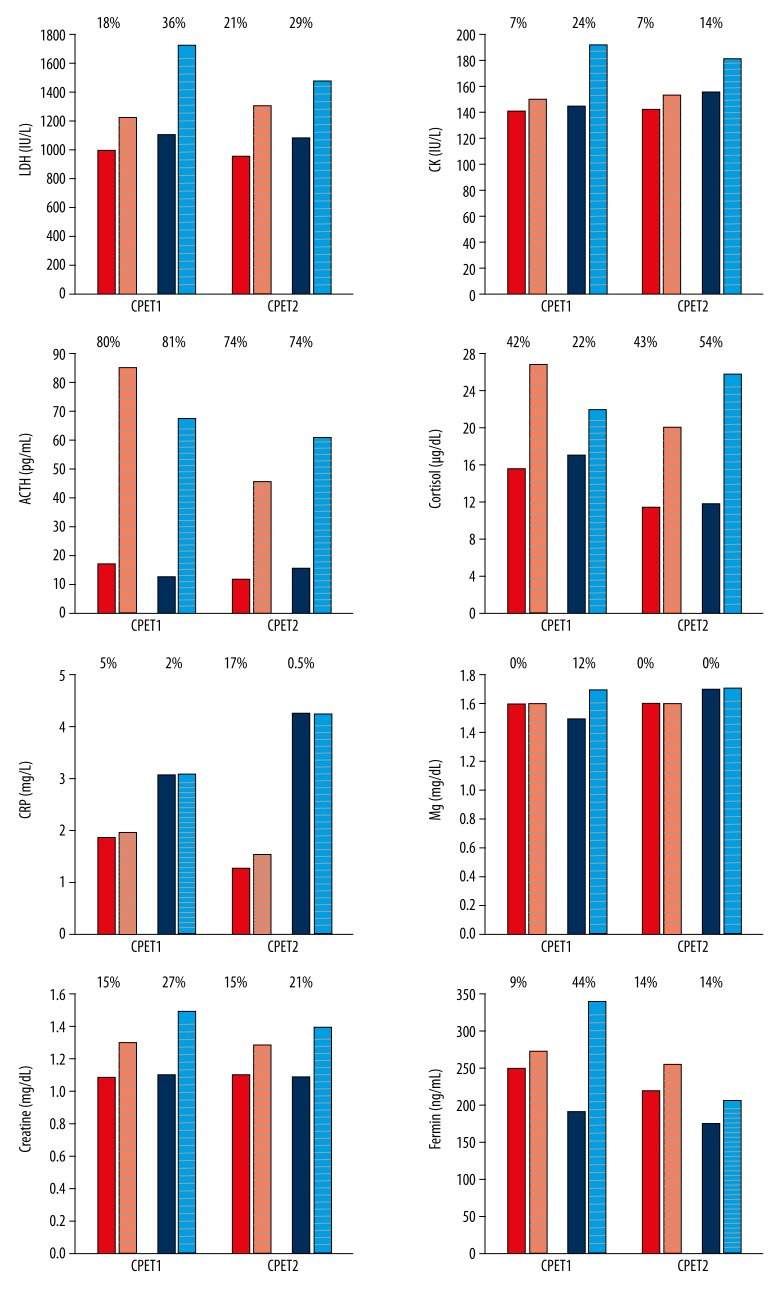
Figures 1 and 2 present the results related to the measurements of plasma analytes. Both WELL and ILL showed LDH values 5 to 13 times higher than the normal range of 85–227 IU/L before each CPET (Figure 1; 986.1 and 1093.7 IU/L at CPET1 and 950.7 and 1077.0 IU/L at CPET2 for WELL and ILL, respectively). ILL presented higher levels of high-sensitivity C-reactive protein (hsCRP) (3.05 and 4.27 mg/L before CPET1 and CPET2, respectively) in comparison to WELL (1.86 and 1.28 mg/L before CPET1 and CPET2, respectively). These values are above the average risk (>1 mg/L) of developing cardiovascular disease. For all the other analytes, results constantly fell within normal ranges.
Figure 1.
Measurements of plasma analytes at CPET1 and CPET2. Plain bars are pre-exercise measurements and striped bars are post-exercise. Red for WELL and blue for ILL. % above each pair of bars is % difference between pre- and post-CPET measure.
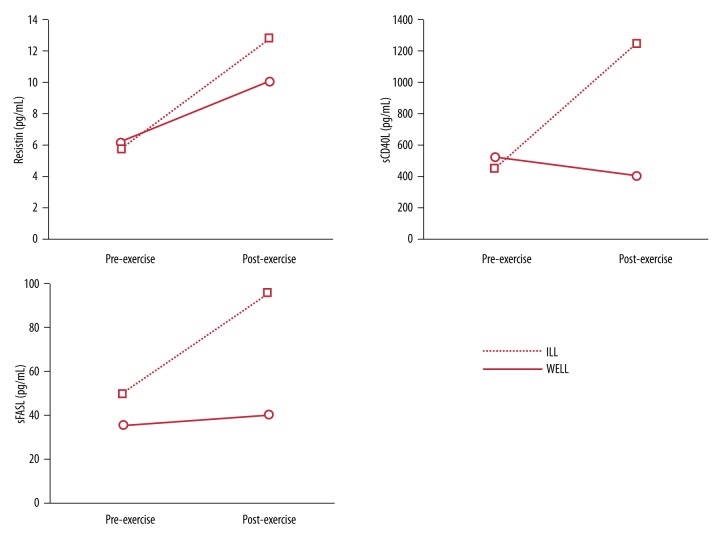
Figure 2.
Measurement of plasma cytokines before and after CPET1.
A rise in the plasma LDH, CK, ACTH, cortisol, creatinine, and ferritin content was noted in association with exercise for both WELL and ILL and at each CPET. ACTH levels exceeded the upper normal range value of 52 pg/mL after CPET1 for both WELL and ILL (85.7 and 68.0 pg/mL, respectively). Exercise testing resulted in a greater increase in ILL in comparison to WELL for the majority of the analytes tested (Figure 1; percentages above bars). For example, CPET1 resulted in a 36% versus 18% increase for LDH and a 24% versus 7% increase for CK in ILL and WELL, respectively (Figure 1).
Magnesium levels fell within normal range, and no increase was observed after exercise at both CPET2s for both subjects. Creatinine levels were similar for both WELL and ILL before exercise at CPET1 and CPET2, and fell within normal ranges. Increased levels of creatinine were observed for both subjects after exercise with a higher increase for ILL (27% vs. 15% and 21% vs. 15% at CPET1 and CPET2, respectively). Resistin, sCD40L, and sFasL were only quantified before and after CPET1 due to sample availability (Figure 2). Exercise testing resulted in increases in plasma resistin and sFasL levels in both WELL and ILL (resistin: 6.22 to 10.08 pg/mL and 5.77 to 12.80 pg/mL; sFasL: 35.1 to 40.1 pg/mL and 49.5 to 95.3 pg/mL for WELL and ILL, respectively).
ILL showed higher increases of the 3 analytes after exercise in comparison to WELL. The only decrease observed was in the levels of sCD40L for WELL after exercise (from 525.2 to 403.9 pg/mL) (Figure 2).
Gut microbiome
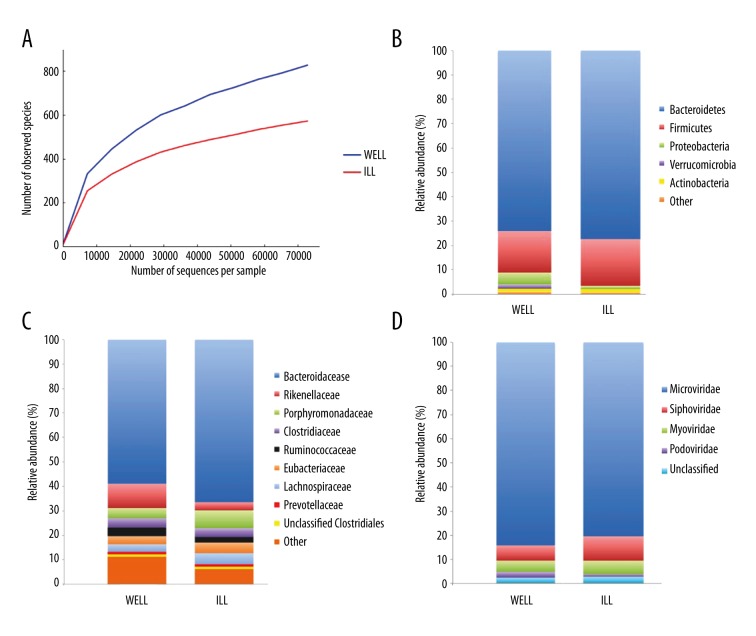
DNA was extracted from stool samples, and we used 16S rRNA sequencing to determine the compositions of microbial communities in feces samples collected from WELL and ILL, as previously described [19]. The sequence-based rarefaction curves based on the number of observed species metric demonstrated a lower microbial diversity in ILL compared to WELL (Figure 3A). Even if more sequences had been obtained and therefore greater diversity observed in both twins, ILL’s microbial diversity would have continued to be less than WELL’s. In both subjects, bacteria from the Firmicutes, Bacteroidetes, Proteobacteria, Verrucomcrobia, and Actinobacteria phyla represented the vast majority of sequences identified (Figure 3B). Sequences representative of the Bacteroidetes and Firmicutes were most abundant in ILL compared to WELL (77 and 19% vs. 74 and 17% for ILL and WELL, respectively), whereas Proteobacteria were substantially more abundant in WELL (5% vs. 1.5%) (Figure 3B). At the family level, Rikenellaceae, Ruminococcaceae, and Prevotellaceae were substantially more abundant in WELL in comparison to ILL, whereas Bacteroidaceae were depleted in WELL (Figure 3C). In ILL, several genera of key beneficial bacteria were decreased, including Faecalibacterium and Bifidobacterium (a member of the Actinobacteria phylum) (data not shown).
Figure 3.
Gut microbiome composition in WELL and ILL. Rarefaction curves of bacterial richness rarefied at 72,936 sequences (A). Plots of the relative abundance of bacterial phyla (B), bacterial families (C), and viral taxa (D).
We also defined the enteric virome of both WELL and ILL stool samples, using the MiSeq platform and the MetaGenome Rapid
Annotation using Subsystem Technology (MG-RAST) server (http://metagenomics.nmpdr.org). We obtained 13,554,793 and 13,726,232 sequences for WELL and ILL, respectively. MG-RAST was able to assign viral taxonomy to 22,821 and 18,979 sequences for WELL and ILL respectively (∼0.15% of the total sequences). Bacteriophages of the tailed, dsDNA Caudovirales order (Siphoviridae, Myoviridae, and Podoviridae) and the non-tailed, ssDNA Microviridae family were the most abundant viral taxa identified in both ILL and WELL (Figure 3D). Interestingly, we observed an increase in the richness of bacteriophages, specifically Siphoviridae and Myoviridae, members of the Caudovirales, in ILL in comparison to WELL (Figure 3D). The predicted hosts of the identifiable phages and prophages were members of the Firmicutes and Bacteroidetes; these phyla, and in particular the families Ruminococcaceae, Lachnospiraceae, and Bacteroidaceae, constituted the most abundant bacterial families in the sampled fecal microbial communities, as defined by 16S rRNA gene analysis.
Discussion
VO2peak represents the maximum energy-producing capacity, which is a level of energy production that one can sustain only briefly for less than a minute. It is also referred to as functional capacity because it represents the highest level of energy production one can sustain to do work. However, most activities of daily living are performed at intensities well below that of VO2peak, and typically work-related or ongoing types of activities are performed at intensities that correspond to about 33–50% of VO2peak or functional capacity [20,21]. Compared to age/sex matched normal values [22], VO2peak for WELL was 13% lower and VO2peak for ILL was 20% lower than that for sedentary adults. Compared to active adults, WELL had a 24% lower and ILL had a 30% lower VO2peak.
Ventilatory/anaerobic threshold (VAT) is the point during incremental exercise when production of energy is derived increasingly through anaerobic (vs. aerobic) metabolism. Energy production from anaerobic processes is limited and ultimately requires rest or low-intensity activity to recover. Unlike aerobic energy production, anaerobic energy production cannot be maintained for long durations without causing fatigue and/ or necessitating recovery. When VO2 at anaerobic threshold is identified using expired ventilation, anaerobic threshold is referred to as VAT. VAT typically occurs between 45% and 65% of VO2peak [23], and work at intensities below VAT can be sustained for prolonged periods of time without undue fatigue. In contrast, work at or above VAT will provoke fatigue due to anaerobic energy production that begins to predominate at these intensities. From a functional perspective, the reduction in VAT observed in ILL indicates that the threshold workload or intensity that ILL could tolerate (remain “aerobic”) decreased 13% due to his abnormal response and recovery following exertion. Also, the low VAT@ VO2peak observed in ILL and the further decrease at CPET2 explains that, when experiencing PEM, even work characterized as low intensity will exceed WELL’s VAT and exacerbate illness symptoms.
Indicators of (1) health status, (2) metabolic adaptation to physical training of skeletal muscles, and (3) inflammatory response were measured in plasma samples from WELL and ILL. LDH and CK give an indication of the degree of metabolic adaptation to physical training of skeletal muscles. The reduced VAT and increased LDH values observed in ILL following exercise suggest a disproportionate dependence upon anaerobic as opposed to aerobic metabolism. As a consequence, greater acid generation within the muscle due to over-utilization of the LDH pathway suggests an overall dysfunction of the pyruvate dehydrogenase complex in the muscle of ILL. This has implications for premature expression of fatigue.
Cortisol is a steroid hormone that is regulated by ACTH, which is secreted by the pituitary gland. Cortisol affects blood pressure and cardiovascular function, slows the immune system’s inflammatory response, and balances the effects of insulin in breaking down glucose for energy. It has been shown that an insufficient production of adrenal hormones results in impaired physical capacities. Baschetti [24] proposed that a reduction in exercise capacity in ME/CFS was primarily due to adrenal insufficiency. Neither WELL nor ILL showed hypocortisolism, and no adrenal insufficiency could be found because of the normal ACTH and cortisol values measured. A very rapid and very intensive increase in ACTH levels has been previously reported after high-intensity exercise and seems to be the primary mechanism for inducing an increase in cortisol [25,26].
ILL showed higher levels of hsCRP than WELL at baseline for both CPETs. hsCRP is often used as a biochemical marker for chronic inflammation, and high levels can indicate conditions such as cancer, infection, lupus, fever, and tuberculosis [27], which are associated with muscle aches and pains as well as many other symptoms commonly found in ME/CFS. Several studies reported increased levels of hsCRP in people with ME/CFS [28–30].
Even though both WELL and ILL had similar levels of resistin at baseline, ILL showed greater levels compared to WELL after exercise. Increased levels of serum resistin in males with type II diabetes were observed after one single bout of cycling [31]. We found a similar result for both ILL and WELL in response to exercise. This may be attributed to the role of resistin in defending the body against oxidation. It has been previously hypothesized that resistin is secreted in response to a chronic low-grade inflammation and has antioxidant properties in response to inflammatory stimuli [32]. sCD40L, a TNF superfamily transmembrane protein found on endothelial and smooth muscle cells, is essential for regulation of B cell maturation and mediates a wide variety of inflammatory responses [33,34]. Only ILL’s sCD40L levels were increased by exercise, suggesting again immune dysfunction.
The soluble apoptosis inducer sFasL is an important cytokine involved in inflammation that regulates immune responses, and its insoluble form, FasL, is predominantly expressed in activated T cells and natural killer cells [35,36]. Levels of sFasL at baseline were higher for ILL, which has been observed previously [8], and showed a greater increase after exercise. It has been shown that physical training has a beneficial effect by decreasing levels of sFasL in patients with chronic heart failure [37]. This result indicates a persistent immune activation, possibly contributing to the impaired exercise capacity of the ILL twin.
With regard to the gut microbiome, ILL exhibited a reduction in bacterial diversity, a finding also seen in our recent study of a larger cohort of ME/CFS patients and controls [19] and also observed in inflammatory bowel disease [38]. We observed that Ruminococcaceae were less abundant in ILL, consistent with other reports on subjects with Crohn’s disease (CD) [39] and ulcerative colitis [40]. The role of the enteric microbiota in driving the phenotype of CD or ulcerative colitis was illustrated recently in 40 twin pairs divergent or concordant for these diseases. Specific species of bacteria were associated with ileal CD [38]. We noted a decrease of core bacteria such as Faecalibacterium and Bifidobacterium in ILL, bacteria known to be beneficial in healthy subjects. Members of the Faecalibacterium genus have been shown to be consistently decreased in inflammatory bowel disease (IBD) [41] and CD [39].
Faecalibacterium is a prominent producer of short-chain fatty acids (especially butyrate), which play a role in protecting the intestine and regulating hormone and cytokine secretion such as leptin and IL-10 [42,43]. We also found that the number of Bifidobacteria, a group of bacteria that have been shown to improve the mucosal barrier functions [44], was significantly reduced in feces from ILL as previously reported [19]. Whether or not probiotics could improve the gut microbial composition and gastrointestinal function of individuals with ME/CFS is not known and would require clinical studies.
In both WELL and ILL, the gut virome was dominated by prokaryotic viruses that prey on gut bacteria. ILL had many fewer Microviridae and more Caudovirales than WELL, indicating that the viromes were different between the two disease states. We observed a higher abundance of Myoviridae and Siphoviridae members of the Caudovirales in ILL in comparison to WELL. Our data are consistent with reports that detected more Caudovirales bacteriophage sequences in CD patients compared to non-inflammatory controls [45,46], reflecting an expansion of infectious bacteriophages in inflammatory states. Bacteriophages drive bacterial diversity in the gut by transferring genetic material among communities [47] and leading to the lysis of their bacterial hosts, which could trigger an inflammatory response such as cytokine release. Taken together, these findings suggest how bacteriophages may contribute to mucosal inflammation and bacterial dysbiosis.
Conclusions
Prior studies of identical twins discordant for ME/CFS have not yielded useful biomarkers and have been limited in their value for identifying the physiological basis of the disease [48–53]. Recent work indicates that some of the symptom sub-groups and immune abnormalities can be correlated with genetic variation [54,55]. Our study indicates that a two-day cardiopulmonary exercise test, in conjunction with molecular analyses of blood and microbiomes, could provide valuable information about the illness, particularly if applied to a larger cohort of monozygotic twins.
Acknowledgments
We thank Dr. Julia Goodrich (Cornell University) for advice about 16S rRNA analysis.
Footnotes
Declaration of conflicting interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References:
- 1.(IOM) IoM . Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. Washington, D.C: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 2.VanNess JM, Stevens SR, Bateman L, et al. Postexertional malaise in women with chronic fatigue syndrome. J Womens Health (Larchmt) 2010;19:239–44. doi: 10.1089/jwh.2009.1507. [DOI] [PubMed] [Google Scholar]
- 3.Hansen JE, Sun XG, Yasunobu Y, et al. Reproducibility of cardiopulmonary exercise measurements in patients with pulmonary arterial hypertension. Chest. 2004;126:816–24. doi: 10.1378/chest.126.3.816. [DOI] [PubMed] [Google Scholar]
- 4.Snell CR, Stevens SR, Davenport TE, Van Ness JM. Discriminative validity of metabolic and workload measurements for identifying people with chronic fatigue syndrome. Phys Ther. 2013;93:1484–92. doi: 10.2522/ptj.20110368. [DOI] [PubMed] [Google Scholar]
- 5.White AT, Light AR, Hughen RW, et al. Differences in metabolite-detecting, adrenergic, and immune gene expression after moderate exercise in patients with chronic fatigue syndrome, patients with multiple sclerosis, and healthy controls. Psychosom Med. 2012;74:46–54. doi: 10.1097/PSY.0b013e31824152ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimas NG, Broderick G, Fletcher MA. Biomarkers for chronic fatigue. Brain Behav Immun. 2012;26:1202–10. doi: 10.1016/j.bbi.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornig M, Gottschalk G, Peterson DL, et al. Cytokine network analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue syndrome. Mol Psychiatry. 2016;21:261–69. doi: 10.1038/mp.2015.29. [DOI] [PubMed] [Google Scholar]
- 8.Hornig M, Montoya JG, Klimas NG, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. 2015;1(1):e1400121. doi: 10.1126/sciadv.1400121. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maes M, Kubera M, Uytterhoeven M, et al. Increased plasma peroxides as a marker of oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Med Sci Monit. 2011;17(4):SC11–15. doi: 10.12659/MSM.881699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patarca-Montero R, Antoni M, Fletcher MA, Klimas NG. Cytokine and other immunologic markers in chronic fatigue syndrome and their relation to neuropsychological factors. Appl Neuropsychol. 2001;8:51–64. doi: 10.1207/S15324826AN0801_7. [DOI] [PubMed] [Google Scholar]
- 11.Barnden LR, Kwiatek R. Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome. Neuroimage Clin. 2016;11:530–37. doi: 10.1016/j.nicl.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boissoneault J, Letzen J, Lai S, et al. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: An arterial spin-labeling fMRI study. Magn Reson Imaging. 2016;34:603–8. doi: 10.1016/j.mri.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy FH, McAnulty GB, McCreary MC, et al. EEG spectral coherence data distinguish chronic fatigue syndrome patients from healthy controls and depressed patients – a case control study. BMC Neurol. 2011;11:82. doi: 10.1186/1471-2377-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan ZY, Kwiatek R, Burnet R, et al. Progressive brain changes in patients with chronic fatigue syndrome: A longitudinal MRI study. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25283. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shungu DC, Weiduschat N, Murrough JW, et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25:1073–87. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–59. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bell DS. Reading, Mass. Addison-Wesley; 1995. The Doctor’s Guide to Chronic Fatigue Syndrome. [Google Scholar]
- 18.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–39. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 19.Giloteaux L, Goodrich JK, Walters WA, et al. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4(1):30. doi: 10.1186/s40168-016-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Environmental and Occupational Medicine . 4th ed. Philadelphia, PA: Wolters Kluwer/Lippincott, Williams & Wilkins; 2007. [Google Scholar]
- 21.E K MA. Ergonomics guide to assessment of physical work capacity. Am Indust Hygiene Assoc J. 1976;37:1–9. [Google Scholar]
- 22.Ehrman JK, Gordon PM, Visich PS, Keteyian SJ. Clinical exercise physiology. 3rd ed. Champaign, Il: Human Kinetics; Champaign, IL: Human Kinetics, Inc.; 2013. [Google Scholar]
- 23.Davis JA, Vodak P, Wilmore JH, et al. Anaerobic threshold and maximal aerobic power for 3 modes of exercise. J Appl Physiol. 1976;41:544–50. doi: 10.1152/jappl.1976.41.4.544. [DOI] [PubMed] [Google Scholar]
- 24.Baschetti R. Chronic fatigue syndrome, decreased exercise capacity, and adrenal insufficiency. Arch Intern Med. 2001;161:1558–59. doi: 10.1001/archinte.161.12.1558-a. [DOI] [PubMed] [Google Scholar]
- 25.Buono MJ, Yeager JE, Sucec AA. Effect of aerobic training on the plasma ACTH response to exercise. J Appl Physiol. 1987;63:2499–501. doi: 10.1152/jappl.1987.63.6.2499. [DOI] [PubMed] [Google Scholar]
- 26.Viru A. Plasma hormones and physical exercise. Int J Sports Med. 1992;13:201–9. doi: 10.1055/s-2007-1021254. [DOI] [PubMed] [Google Scholar]
- 27.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 28.Buchwald D, Wener MH, Pearlman T, Kith P. Markers of inflammation and immune activation in chronic fatigue and chronic fatigue syndrome. J Rheumatol. 1997;24:372–76. [PubMed] [Google Scholar]
- 29.Raison CL, Lin JMS, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav Immun. 2009;23:327–37. doi: 10.1016/j.bbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Spence VA, Kennedy G, Belch JJF, et al. Low-grade inflammation and arterial wave reflection in patients with chronic fatigue syndrome. Clin Sci. 2008;114:561–66. doi: 10.1042/CS20070274. [DOI] [PubMed] [Google Scholar]
- 31.Edrisi M, Keshavarz A, Hessari FT. Inflammatory effect of single bout exercise on resistin non-trained individuals with type II diabetes. Indian Journal of Fundamental and Applied Life Sciences. 2014;4:701–5. [Google Scholar]
- 32.Bo S, Gambino R, Pagani A, et al. Relationships between human serum resistin, inflammatory markers and insulin resistance. Int J Obes (Lond) 2005;29:1315–20. doi: 10.1038/sj.ijo.0803037. [DOI] [PubMed] [Google Scholar]
- 33.Grewal IS, Flavell RA. The CD40 ligand. At the center of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 34.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–94. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 35.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 36.Jiang S, Moriarty-Craige SE, Li C, et al. Associations of plasma-soluble fas ligand with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:1345–49. doi: 10.1167/iovs.07-0308. [DOI] [PubMed] [Google Scholar]
- 37.Adamopoulos S, Parissis J, Karatzas D, et al. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653–63. doi: 10.1016/s0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- 38.Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–54. e1841. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 39.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–36. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–83. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 41.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–85. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saemann MD, Bohmig GA, Osterreicher CH, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14:2380–82. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 43.Segain JP, Raingeard de la Bletiere D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi: 10.1186/1475-2891-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norman JM, Handley SA, Baldridge MT, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–60. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner J, Maksimovic J, Farries G, et al. Bacteriophages in gut samples from pediatric Crohn’s disease patients: Metagenomic analysis using 454 pyrosequencing. Inflamm Bowel Dis. 2013;19:1598–608. doi: 10.1097/MIB.0b013e318292477c. [DOI] [PubMed] [Google Scholar]
- 47.Brussow H, Canchaya C, Hardt WD. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ball N, Buchwald DS, Schmidt D, et al. Monozygotic twins discordant for chronic fatigue syndrome: objective measures of sleep. J Psychosom Res. 2004;56:207–12. doi: 10.1016/S0022-3999(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 49.Ciregia F, Giusti L, Da Valle Y, et al. A multidisciplinary approach to study a couple of monozygotic twins discordant for the chronic fatigue syndrome: A focus on potential salivary biomarkers. J Transl Med. 2013;11:243. doi: 10.1186/1479-5876-11-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claypoole K, Mahurin R, Fischer ME, et al. Cognitive compromise following exercise in monozygotic twins discordant for chronic fatigue syndrome: Fact or artifact? Appl Neuropsychol. 2001;8:31–40. doi: 10.1207/S15324826AN0801_5. [DOI] [PubMed] [Google Scholar]
- 51.Mahurin RK, Claypoole KH, Goldberg JH, et al. Cognitive processing in monozygotic twins discordant for chronic fatigue syndrome. Neuropsychology. 2004;18:232–39. doi: 10.1037/0894-4105.18.2.232. [DOI] [PubMed] [Google Scholar]
- 52.Sabath DE, Barcy S, Koelle DM, et al. Cellular immunity in monozygotic twins discordant for chronic fatigue syndrome. J Infect Dis. 2002;185:828–32. doi: 10.1086/339194. [DOI] [PubMed] [Google Scholar]
- 53.Watson NF, Kapur V, Arguelles LM, et al. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2003;26:324–28. doi: 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- 54.Billing-Ross P, Germain A, Ye K, et al. Mitochondrial DNA variants correlate with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. 2016;14:19. doi: 10.1186/s12967-016-0771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall-Gradisnik S, Huth T, Chacko A, et al. Natural killer cells and single nucleotide polymorphisms of specific ion channels and receptor genes in myalgic encephalomyelitis/chronic fatigue syndrome. Appl Clin Genet. 2016;9:39–47. doi: 10.2147/TACG.S99405. [DOI] [PMC free article] [PubMed] [Google Scholar]