Abstract
Objective
Systemic lupus erythematosus (SLE) can present limitations to exercise capacity and quality of life (QoL) because of various clinical conditions, such as pulmonary disease or heart disease. Tissue Doppler echocardiography (TDE) offers the promise of an objective measurement to quantify regional and global ventricular function through the assessment of myocardial velocity data. This study aimed to assess the intensity of left ventricular (LV) and right ventricular (RV) systolic and diastolic dysfunction in SLE patients by means of TDE and cardiopulmonary exercise (CPX) testing to determine their impact on QoL.
Material and Methods
Overall, 56 SLE patients within two tertiary healthcare centers as well as 50 healthy controls were examined with TDE after the exclusion of cardiovascular risk factors. TDE was performed for maximal systolic (S), early diastolic (E′), and late diastolic (A′) velocities of the mitral and tricuspid annulus. Pulsed wave (PW) Doppler of mitral and tricuspid valve inflow was performed in addition to the estimation of the left ventricle ejection fraction and assessment of right ventricle systolic function by tricuspid annular plane systolic excursion (TAPSE). Disease activity was assessed by the Systemic Lupus Activity Measure (SLAM), and the damage index was assessed by the Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI). CPX tests according to the modified Bruce protocol were performed.
Results
SLE patients in both subgroups had more or less similar laboratory data and statistically higher values of ESR, CRP, and anticardiolipin (aCL) antibodies compared to the control group. LV function showed statistically insignificant EF compared to the control group, being lower in the patient group. Tissue Doppler image revealed that E′ and A′ of the mitral annulus were lower in the patient group than in the control group. Concerning RV, TAPSE in the patient group was statistically lower than in the control group, and there was a statistical difference between SLE groups Ia and Ib; also, S wave was lower in group Ib than in group Ia. RV diastolic dysfunction in the form of lower E′ and A′ values was observed for the SLE group compared to the control group, especially in the medial annulus of the tricuspid valve. A higher A wave velocity with PWD of mitral and tricuspid inflows was observed in the patient group compared to the control group.
Conclusion
SLE patients have an increased prevalence of subclinical systolic and diastolic LV and RV dysfunction. This result advocates for regular follow-up and early screening of SLE patients. Accordingly, treatment focused on improving diastolic heart function may have a role in enhancing QoL and improving the prognosis of SLE patients.
Keywords: Systemic lupus erythematosus, cardiopulmonary exercise test, tissue Doppler echocardiography, LV and RV diastolic dysfunction
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune and multisystem disease that affects women of childbearing age (1–3). The disease course is characterized by remission and exacerbation and is difficult to be controlled. Cardiovascular disease (CVD) is the most common cause of death (4). In view of the hypothesis that atherosclerosis is not only a degenerative process but also may be an autoimmune-inflammatory disease, SLE is an interesting example of inflammatory disease in which atherosclerosis risk is increased because of its autoimmune nature (5, 6).
SLE can manifest with limitations in exercise capacity, which reduces the quality of life secondary to disease complications such as pulmonary disease, heart disease, and association with other rheumatic diseases such as fibromyalgia (7). SLE is associated with high cardiovascular morbidity and mortality. Cardiovascular affection is often underestimated in SLE patients by routine imaging techniques (8).
Routine echocardiographic evaluation of left ventricular (LV) wall motion is usually subjective because it depends on the visual determination of endocardial excursion as well as wall thickening. Tissue Doppler echocardiography (TDE) provides the promise of an objective evaluation to quantify regional and global ventricular function by the assessment of myocardial velocity data such as regional contraction phenomena, especially delayed and reduced systolic shortening as well as late systolic contraction. The combination of tissue Doppler imaging of the mitral annulus and conventional pulsed wave Doppler (PWD) imaging of mitral inflow velocities allows better estimates of LV filling pressures than other methods, such as pulmonary vein and preload reduction (9).
Cardiopulmonary exercise (CPX) testing assesses global exercise responses involving multiple systems, including the pulmonary, cardiovascular, neuropsychological, hematopoietic, and skeletal muscle systems, which are not properly reflected through individual organ system function measurements. The functional capacity and overall health status usually correlates with exercise tolerance rather than resting measurements (10).
The modified Bruce protocol, a CPX test, is a treadmill exercise with an incremental protocol which determines cardiovascular endurance (11).
Tissue Doppler echocardiography gives a quantitative objective assessment of LV and RV systolic and diastolic function that is more accurate than mitral and tricuspid inflow examination because there is no preload dependence. CPX classifies the levels of heart and respiratory failure in an objective manner.
The aim of this study was to assess the intensity of LV and RV systolic and diastolic dysfunction in SLE patients by means of echocardiography with TDE and CPX. This may have a role in enhancing the quality of life and improving the prognosis of SLE patients.
Material and Methods
Patients
Fifty-six SLE patients who fulfilled the American College of Rheumatology (ACR) classification criteria of SLE (11) and who previously attended the Rheumatology and Rehabilitation Department at Al Hada Armed Forces Hospital and King Abdulaziz Specialist Hospital (KSA) between January 2013 and January 2015 were studied.
Exclusion criteria
Smoking, DM, dyslipidemias, thyroid dysfunction, HTN, hemoglobin <10, neurologic disease or psychosis, presence of respiratory diseases such as pulmonary hypertension or pulmonary fibrosis, heart failure (NYHA class ≥II), history of myocardial infarction or ischemic heart disease, active nephritis with creatinine levels >3.0 mg/dL, Systemic Lupus Activity Measure (SLAM) scores >28, Systemic Lupus Damage Index (SDI) >1, hip and/or knee joint prosthesis or aseptic bone necrosis, severe arthritis in weight-bearing joints, pregnancy, and practicing regular physical exercise ≥3 times a week for patients and controls; and SLE with concomitant rheumatic disease, such as rheumatoid arthritis, polymyositis, dermatomyositis, or systemic sclerosis.
Control group
A group of 50 age- and sex-matched healthy individuals without known systemic, immunological, or atherosclerotic vascular disease served as a control group.
The patients gave informed consent, and the Commission Hospital Ethics and Research Committee approved the study.
Methods
At the time of the study, the following studies were performed: detailed disease and medication history (steroid intake duration, dosage, and frequency), body mass index (BMI), laboratory assessment of complete blood count (CBC) using a Coulter Counter (T660), erythrocyte sedimentation rate (ESR) by the Westergren method, C-reactive protein (CRP) using the enzyme-linked immunosorbent assay (ELISA), antinuclear antibodies (ANA) via indirect immune-florescence by Kallestad kit, anti-double stranded DNA by the indirect immune-fluorescence technique, anti-cardiolipin (aCL) antibodies (IgG and IgM) by ELISA (13), and lipid profiles using a CX-5 system (Beckman US).
The disease activity was evaluated by SLAM, which consists of clinical and laboratory manifestations of SLE within nine organ systems (14). The damage index was assessed using the Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index (SDI) (15).
Echocardiography - Doppler protocol
A Philips iE 33 ultrasound machine system (The Netherlands) using an X-matrix (X5-1) transducer was used for the assessment of echocardiographic parameters. Patients were studied in long axis parasternal, short axis parasternal, apical 4-chamber, apical 2-chamber, and apical 5-chamber views, using M-mode and 2D-mode, in addition to PWD and TDE (16).
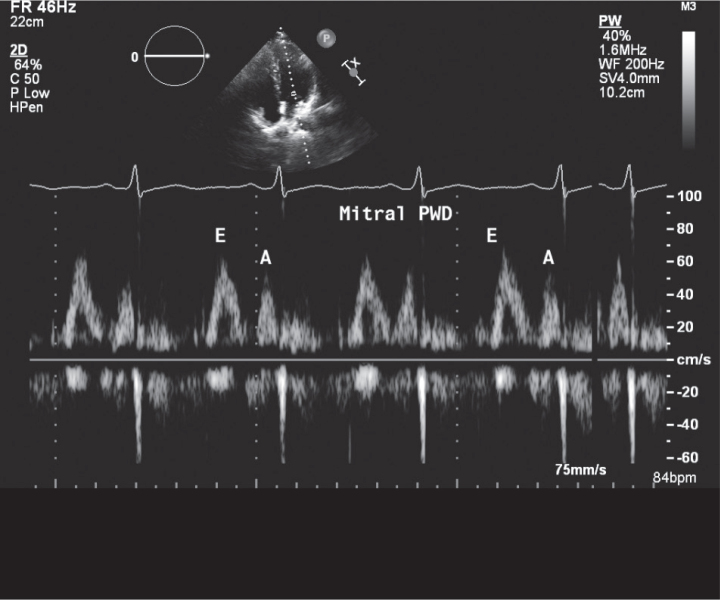
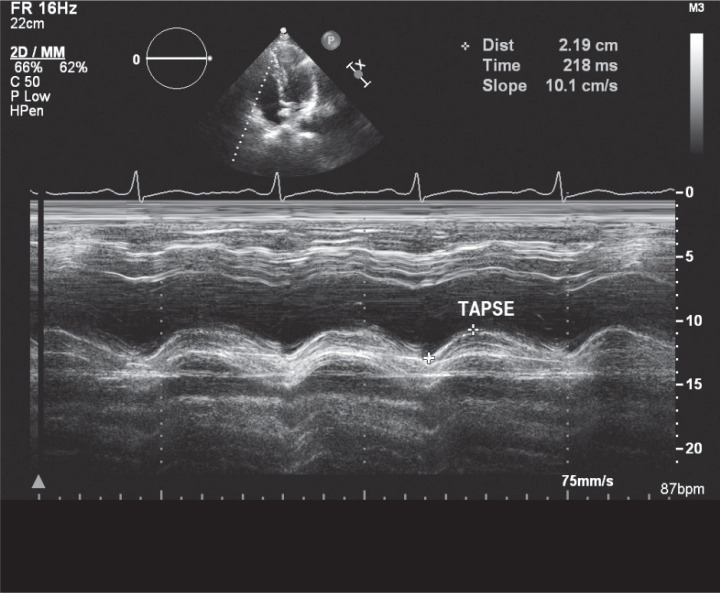
Left ventricle ejection fraction and tricuspid annular plane systolic excursion (TAPSE) of the lateral tricuspid annulus were obtained from M-mode, and cardiac valves were assessed by other echocardiographic modalities to confirm the absence of significant valvular disease. PW Doppler of mitral and tricuspid valves was performed where early diastolic velocity (E), late diastolic velocity (A), and E/A ratio for both valves were obtained (Figure 1, 2).
Figure 1.
Mitral pulsed wave Doppler (PWD)
Figure 2.
Tricuspid annular plane systolic excursion (TAPSE) by M-mode
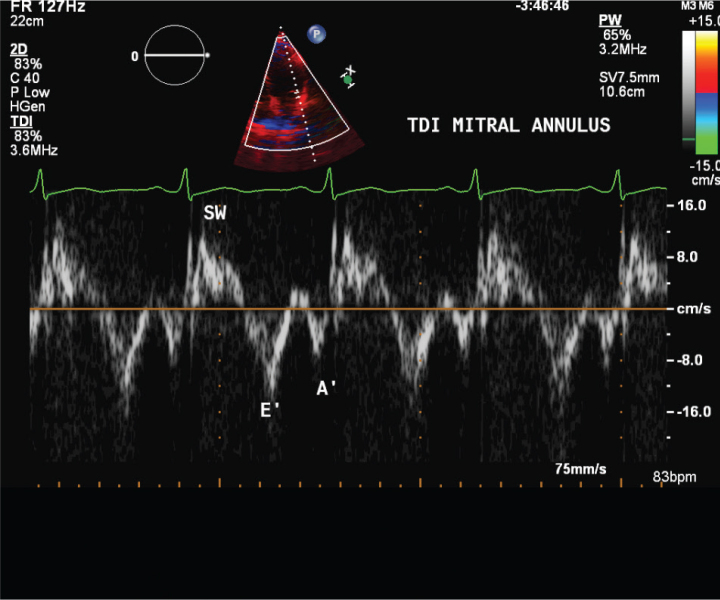
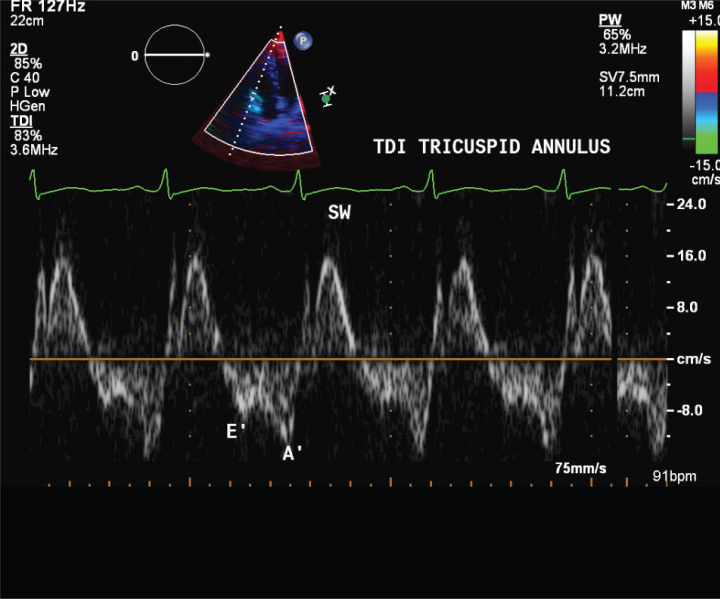
Tissue Doppler echocardiography parameters, including maximal systolic velocity (S wave) of lateral tricuspid annulus, early diastolic velocity (E′), late diastolic velocity (A′), and E′/A′ of the medial and lateral aspects of the mitral and tricuspid annuli, were calculated (Figure 3, 4).
Figure 3.
Mitral annulus tissue Doppler imaging (TDI)
Figure 4.
Tricuspid annulus tissue Doppler imaging (TDI)
CPX testing by modified Bruce protocol was performed using an Enraf-Nonius (The Netherlands) treadmill exercise test (11, 17). Exercise times and metabolic equivalents (METs) were estimated in the CPX test.
Statistical analysis
Data were collected, tabulated, and analyzed using IBM SPSS Statistics 20 (IBM Corp; NY, USA). The mean, standard deviation, and statistical significance were calculated by Student’s t test for paired data. The Mann–Whitney test using the standard error of the mean to calculate z was used for the comparison of the tissue Doppler parameters. The Chi-square test was used to compare the probability of variables. A value of p<0.05 was considered statistically significant. The correlation coefficient r for the relationship of different variables was calculated using Pearson’s coefficient for quantitative data and Spearman’s correlation for qualitative (non-parametric) data.
Results
This study included two main groups: The first group included 56 SLE patients, whereas the second included control healthy individuals (50). They all matched in age, sex, BMI, and systemic blood pressure (Table 1). In addition, the lipid profiles were similar in both groups. However, aCL antibodies were significantly higher in the SLE group.
Table 1.
Mean values and SD of age, BMI, BP, CRP, and laboratory findings of the studied subjects
| SLE Patients | Control | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Group Ia | p1 | Group Ib | p2 | p3 | ||
| Age | 36.9±11.9 | >0.05 | 35.3±9.8 | >0.05 | 34.9±10.1 | >0.05 |
| BMI | 26.8±4.3 | >0.05 | 25.7±6.8 | >0.05 | 25.7±3.9 | >0.05 |
| BP | ||||||
| SBP | 126±15.2 | >0.05 | 124.3±16.2 | >0.05 | 119±18 | >0.05 |
| DBP | 75±9.2 | >0.05 | 82.1±15.8 | >0.05 | 80±6.6 | >0.05 |
| CRP | 7.6±2.4 | >0.05 | 6.2±5.3 | <0.05 | 1.9±0.8 | <0.05 |
| ESR | 36.8±8.4 | >0.05 | 37.3±5.8 | <0.01 | 8.4±1.2 | <0.01 |
| Total cholesterol | 5.1±1.14 | >0.05 | 4.99±0.95 | >0.05 | 5.06±0.93 | >0.05 |
| LDL cholesterol | 2.82±0.91 | >0.05 | 2.74±0.81 | >0.05 | 2.82±0.7 | >0.05 |
| HDL cholesterol | 1.78±0.45 | >0.05 | 1.51±0.53 | >0.05 | 1.78±0.75 | >0.05 |
| Triglycerides | 103.2±25.6 | >0.05 | 113±31.8 | >0.05 | 93.2±21.6 | >0.05 |
| IgGaCL (GPL units/mL) | 26.1±13.4 | >0.05 | 23.2±11.5 | <0.05 | 10.84±5.8 | <0.05 |
| IgMaCL (MPL units/mL) | 15.2±11.1 | >0.05 | 12.9±10.3 | <0.001 | 4.14±2.9 | <0.001 |
p1: Ia subgroup versus Ib subgroup; p2: Ib subgroup versus controls; p3: Ia subgroup versus controls; SBP: systolic blood pressure; DBP: diastolic blood pressure, aCL: anticardiolipin; SLE: systemic lupus erythematosus; BMI: body mass index; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; LDL: low density lipoprotein; HDL: high density lipoprotein; IgGaCL: immunoglobulin G anticardiolipin; GPL: G phospholipids; IgMaCL: immunoglobulin M anticardiolipin; MPL: M phospholipids
All our patients (100%) in the SLE group were on corticosteroids with a mean dose of 20± 9.34 mg/day, ranging between 5 and 30 mg. In addition, 32% of the patients were on a combination therapy of steroids, hydroxychloroquine, and azathioprine; however, 68% of the patients were on a combination therapy of steroids and hydroxychloroquine only.
However, Table 1 reveals statistically higher CRP and ESR in SLE patients than in control cases.
The SLE group was divided into two subgroups according to disease duration: group Ia, with disease duration less than 10 years (35 patients) and group Ib, with disease duration more than 10 years (21 patients). The SLAM score was statistically insignificant in the two subgroups (9.8±2.7 vs 11.6±4.4).
Regarding the exercise data, Table 1 and 3 revealed insignificant data in time and METs between the SLE and control groups and between subgroups Ia and Ib. Left ventricle systolic function showed a statistically insignificant difference between the SLE cases and the control group regarding EF (Table 2).
Table 3.
Mean values and SD of exercise and echocardiographic data of the studied SLE subgroups
| SLE Patients | Control | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| <10 years | p1 | >10 years | p2 | p3 | ||
| Exercise data: | ||||||
| Time: | 12.4±1.1 | >0.05 | 12.4±2.3 | >0.05 | 12.6±3.5 | >0.05 |
| METs: | 8.5±0.9 | >0.05 | 8.4±1.5 | >0.05 | 8.6±1.7 | >0.05 |
|
| ||||||
| Systolic unction: | ||||||
| EF% (LV) | 62±2.8 | >0.05 | 63.5±2.3 | >0.05 | 64.2±14.6 | >0.05 |
|
| ||||||
| Diastolic function: | ||||||
| Mitral PWD: | ||||||
| E | 75.8±11.9 | >0.05 | 80.9±17.9 | >0.05 | 80.4±14.1 | >0.05 |
| A | 71.5±12.1 | >0.05 | 72.2±15.8 | <0.05 | 50.3±11.4 | <0.05 |
| E/A | 1.04±0.3 | >0.05 | 1.08±0.4 | <0.05 | 1.59±0.6 | <0.05 |
|
| ||||||
| Mitral lateral TDI: | ||||||
| E′ | 11.3±3.4 | >0.05 | 9.95±4.4 | <0.01 | 19.4±3.1 | <0.01 |
| A′ | 6.9±2.5 | >0.05 | 7.2±3.8 | <0.01 | 10.01±2.3 | <0.01 |
| E′/A′ | 1.45±0.5 | >0.05 | 0.98±0.6 | <0.01 | 1.94±0.4 | <0.01 |
|
| ||||||
| Mitral medial TDI: | ||||||
| E′ | 9.07±3.04 | >0.05 | 7.2±3.7 | <0.001 | 12.8±1.9 | <0.05 |
| A′ | 8.14±1.4 | <0.05 | 9.5±3 | >0.05 | 9.39±1.8 | <0.05 |
| E′/A′ | 1.5±0.2 | >0.05 | 1.3±0.3 | >0.05 | 1.36±0.3 | <0.05 |
|
| ||||||
| Right ventricle: | ||||||
| R V systolic: | ||||||
| TAPSE: | 20.75±2.9 | <0.05 | 17.7±1.2 | <0.05 | 20.6±0.7 | >0.05 |
| S wave | 11±0.4 | <0.001 | 10.25±0.3 | <0.001 | 12±0.3 | <0.01 |
|
| ||||||
| Tricuspid PWD: | ||||||
| E | 60.7±3 | <0.05 | 64.1±3.8 | <0.01 | 59.3±7.9 | <0.05 |
| A | 35.7±3 | <0.05 | 36.1±7.8 | <0.01 | 31±8 | <0.01 |
| E/A | 1.8±0.1 | <0.0001 | 1.9±0.3 | >0.05 | 1.91±0.6 | >0.05 |
|
| ||||||
| Tricuspid TDI: | ||||||
| Medial annulus | ||||||
| E′ | 13.5±1.7 | >0.05 | 11.7±2.2 | <0.05 | 14.7±2.9 | <0.05 |
| A′ | 11.1±1.3 | >0.05 | 10.5±3.7 | <0.001 | 12.4±3.5 | <0.05 |
| E′/A′ | 1.2±0.4 | >0.05 | 1.1±0.3 | <0.01 | 1.18±0.34 | <0.001 |
|
| ||||||
| Lateral annulus | ||||||
| E′ | 12.7±1.4 | >0.05 | 12.4±2 | <0.05 | 14.5±3.2 | <0.05 |
| A′ | 10.7±1.9 | >0.05 | 9.1±1.4 | <0.05 | 12.5±3.6 | <0.05 |
| E′/A′ | 1.26±0.4 | <0.05 | 1.7±0.3 | <0.05 | 1.16±0.3 | <0.05 |
p1: Ia subgroup versus Ib subgroup; p2: Ib subgroup versus controls; p3: Ia subgroup versus controls; E wave: maximal early diastolic; SW: maximal systolic wave; E/A ratio: early (E) to late (A) ventricular filling velocities; EF: ejection fraction; TAPSE: tricuspid annular plane systolic excursion; PWD: pulsed wave Doppler; TDI: tissue Doppler image
Table 2.
Mean values of exercise and echocardiographic findings of the studied subjects
| SLE (n=56) | Control (n=50) | p | |
|---|---|---|---|
| Age: | |||
| Mean±SD | 35.4±11.9 | 34.9±10.1 | >0.05 |
| 95% CI | 31.3–42.5 | 31.2–43.6 | |
|
| |||
| Sex: male/female | 10/46 | 3/25 | >0.05 |
|
| |||
| Disease duration | 8.6±3.7 (6.9–10.3) | ||
|
| |||
| Exercise: | |||
| Duration (m.) Mean±SD | 12.38±2.1 | 12.6±3.5 | >0.05 |
| 95% CI | 11.4–13.4 | 11.2–14 | |
| METs Mean±SD | 8.5±1.6 | 8.6±1.7 | >0.05 |
| 95% CI | 7.7–9.2 | 7.9–9.3 | |
|
| |||
| Cardiac function: | |||
| LV Systolic: | |||
| EF% Mean±SD | 62.6±3.8 | 64.2±14.6 | >0.05 |
| 95% CI | 60.8–64.4 | 58.2–70.2 | |
|
| |||
| RV Systolic: | |||
| TAPSE Mean±SD: | 18.8±3.9 | 20.6±0.7 | <0.05 |
| 95% CI systolic | 16.2–21.4 | 20.3–20.9 | |
|
| |||
| PWD: | |||
| Mitral: | |||
| E Mean±SD | 77.9±19.5 | 80.4±14.1 | >0.05 |
| 95% CI | 68.7–86.9 | 74.6–86.2 | |
| A Mean±SD | 71.8±18.5 | 50.3±11.4 | <0.0001 |
| 95% CI | 63.7–80.5 | 45.6–55 | |
| E/A Mean±SD | 1.16±0.43 | 1.59±0.6 | <0.0001 |
| 95% CI | 0.96–1.36 | 1.34–1.84 | |
|
| |||
| Tricuspid: | |||
| E Mean±SD | 62.7±8 | 59.3±7.9 | 0.08 |
| 95% CI | 58.9–66.5 | 56.04–62.6 | |
| A Mean±SD | 35.9±10.9 | 31±8 | <0.05 |
| 95% CI | 30.8–40.9 | 27.7–34.3 | |
| E/A Mean±SD | 1.86±0.46 | 1.91±0.6 | >0.05 |
| 95% CI | 1.64–2.1 | 1.66–2.16 | |
|
| |||
| Mitral TDI: | |||
| Lateral annulus: | |||
| E′ Mean±SD | 12.9±3.6 | 19.4±3.1 | <0.0001 |
| 95% CI | 11.2–14.6 | 18.1–20.7 | |
| A′ Mean±SD | 8.8±3.7 | 10.01±2.3 | >0.05 |
| 95% CI | 7.1–10.6 | 9.1–10.9 | |
| E′/A′ Mean±SD | 1.68±0.69 | 1.94±0.4 | 0.08 |
| 95% CI | 1.36–2 | 1.77–2.1 | |
|
| |||
| Medial annulus: | |||
| E′ Mean±SD | 11.6±2.4 | 12.8±1.9 | <0.05 |
| 95% CI | 10.4–12.7 | 12.01–13.6 | |
| A′ Mean±SD | 8.7±2.9 | 10.39±1.8 | <0.001 |
| 95% CI | 7.3–10 | 9.6–11.1 | |
| E′/A′ Mean±SD | 1.45±0.48 | 1.23±0.3 | <0.05 |
| 95% CI | 1.2–1.67 | 1.1–1.35 | |
|
| |||
| Tricuspid TDI: | |||
| Medial annulus: | |||
| E′ Mean±SD | 12.4±3.4 | 14.7±2.9 | <0.001 |
| 95% CI | 10.1–14.7 | 13.5–15.9 | |
| A′ Mean±SD | 10.8±2.9 | 12.4±3.5 | <0.05 |
| 95% CI | 3.48–3.8 | 10.9–13.8 | |
| E′/A′ Mean±SD | 1.37±0.55 | 1.18±0.34 | >0.05 |
| 95% CI | 0.98–1.77 | 1.04–1.3 | |
|
| |||
| Lateral annulus: | |||
| E′ Mean±SD | 12.6±3.5 | 14.5±3.2 | <0.05 |
| 95% CI | 10.9–14.2 | 13.8–15.9 | |
| A′ Mean±SD | 9.7±3.5 | 12.5±3.6 | <0.001 |
| 95% CI | 8.1–11.4 | 11–13.3 | |
| E′/A′ Mean±SD | 1.5±0.83 | 1.15±0.3 | <0.01 |
| 95% CI | 1.12–1.88 | 1.25–1.2 | |
E: early diastolic filling velocity (cm/s); A: late diastolic filling velocity (cm/s); E/A ratio: early (E) to late (A) ventricular filling velocities; LV: left ventricle; RV: right ventricle; EF: ejection fraction; TAPSE: tricuspid annular plane systolic excursion (mm); PWD: pulsed wave Doppler; TDI: tissue Doppler image
Regarding diastolic function of the left ventricle, the A wave of mitral PWD was statistically significantly lower in the control group than in the SLE patients; the E′ and A′ waves of the medial and lateral mitral annulus by tissue Doppler image (TDI) were lower in the patient group than in the control group, being statistically significant for E′ of the lateral annulus and A′ of the medial mitral annulus (Table 2).
Right ventricle systolic function (Table 2) showed statistically lower TAPSE values in the SLE cases (though still not below normal) than in the control group. However, further analysis (Table 3) revealed statistically lower values in subgroup Ib than in the control group and subgroup Ia. Subgroup Ia showed statistically higher values of S wave velocity of the lateral tricuspid annulus than subgroup Ib (Table 3).
Regarding diastolic function of the right ventricle with PWD, Table 2 shows that there were statistically higher A wave values and statistically insignificant E wave values in SLE cases than in the control group. On the other hand, Table 3 shows statistically higher E wave values in subgroup Ib than in subgroup Ia and the control group.
Medial annulus TDI showed statistically lower E′ and A′ waves in SLE cases than in the control group (Table 2). Similarly, Table 3 revealed statistically insignificant difference in which the values were lower subgroup Ib than in subgroup Ia.
Lateral annulus TDI (Table 2, 3) revealed statistically lower E′ and A′ wave values in the SLE group (either the entire group or the subgroups) than in the control group.
Discussion
The current study revealed that SLE patients had high possibilities of diastolic dysfunction. The late ventricular filling component (A wave) was found to be the first affected in the diastolic function with PWD (increasing A wave indicates impaired ventricular relaxation). Similar findings as reported by several authors (18, 19).
Cardiac involvement is prevalent in more than 50% of SLE cases. This includes coronary arterial disease, myocarditis, pericarditis, valvular heart disease, and conduction abnormalities (20). Exercise stress tests have been used to screen patients to identify early cardiac affection, e.g., early stage of pulmonary hypertension (21). This is the reason for the inclusion of exercise tests in the current study.
Systemic lupus erythematosus is a chronic inflammation with a prominent feature of atherosclerotic lesions (4). Puntmann et al. (22) added that the underlying pathology includes immune complex- and complement-mediated injury with diffuse inflammation and myocardial fibrosis. This confirms the current study findings of higher ESR and CRP in the SLE group of the studied patients.
In 1993, Giunta et al. (23) demonstrated that cardiac involvement is quite frequent in SLE patients: the disease duration affects both endocardial and myocardial involvement; the aCL antibodies appear to be related to endocardial but not to myocardial damage. The current study showed that the two subgroups of the SLE group according to disease duration had similar aCL antibodies but different echocardiographic findings, with lower systolic parameters of LV (EF) and RV (TAPSE and S wave) in group Ib than those in group Ia. The same was found for the diastolic parameters, especially for the tricuspid annulus, by TDI. Myocarditis is shown to be higher in cardiac magnetic resonance imaging by up to 40%–70% (22). TDE is a sensitive echocardiographic technique for the quantitative assessment of subclinical myocardial dysfunction (9).
Paran et al. (24) reported abnormal echocardiographic findings in asymptomatic patients with SLE or antiphospholipid syndrome (APS). The SLE patients were younger and LV systolic function was more impaired in patients with SLE than in PAPS; meanwhile, LV and RV diastolic function, as reflected by the E/A ratios, were significantly more impaired in patients with APS. The current study showed an insignificant difference concerning LV (EF) between the SLE and control groups. It was also noted that there was a statistically significant difference between the two subgroups of SLE patients concerning the S wave of the lateral tricuspid annulus, which was lower in group Ib than in group Ia. This supported that cardiac affection is correlated with disease duration.
Gin et al. (25) reported that in SLE patients, the peak systolic velocity of the tricuspid annulus was significantly lower than that in the control group; this is in concordance with our study, where TAPSE (reflecting the systolic function of the right ventricle) is lower in the patient group than in the control group.
In a large cohort study, Tektonidou et al. (19) showed an abnormal pattern of diastolic function, especially of the right ventricle, in SLE patients with positive aCL antibodies. This adds further support to the hypothesis that RV diastolic dysfunction is related to aCL antibodies and the APS disease process. The current study confirmed these data for positive aCL antibodies in the SLE group, where the A wave of PWD of the tricuspid valve is higher and E′ and A′ of the tricuspid annulus by TDI are lower in SLE patients than in the control group. However, APS cases were not included in the current study. Meanwhile, Duman et al. (26) revealed an association between pulmonary artery stiffness and right ventricular dysfunction (mainly TAPSE) in SLE patients without cardiovascular symptoms.
The current study revealed that right ventricular function was more impaired than left ventricular function, similar to findings by Tektonidou et al. (19) who declared that all parameters of RV diastolic function showed more pronounced impairment than the respective LV parameters. This dissociation may reflect a preferential involvement or a higher susceptibility of the right ventricle, which can emphasize the role of microvascular disease in the RV more than in the LV, due to its smaller mass than the LV (27); pulmonary hypertension may also have a role (20, 27). In that context, pulmonary hypertension and primary APS were the strongest independent predictors of a prolonged duration time.
However, Gin et al. (25) stated that systolic tricuspid annular velocity reflects exercise tolerance in SLE patients with pulmonary hypertension (PH), who had a significantly shorter 6-min walk distance than patients with SLE without PH; this is not the case in the current study, as all patients had more than 6 min of exercise, which might exclude the existence of PH.
We can conclude that SLE patients have an increased prevalence of subclinical LV and RV dysfunction. Although the parameters of 2D echocardiography were within normal ranges in the SLE patients, tissue Doppler showed systolic and diastolic affection of the LV and RV measurements, which were significantly impaired in SLE patients compared to controls. Disease duration can also affect the systolic and diastolic parameters of the left and right ventricles, which are more affected with longer duration. Right ventricle dysfunction is relatively more frequent than left ventricle dysfunction in SLE. Accordingly, treatment focused on improving diastolic heart function may have a role in enhancing the quality of life and improving the prognosis of SLE patients.
Acknowledgements
The authors would like to thank Al Hada Armed Forces Hospital and King Abdulaziz Specialist Hospital for the valuable support.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Al Hada Armed Forces Hospital and King Abdulaziz Specialist Hospital, Saudi Arabia.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - B.M.; Design - B.M., M.D., M.E.; Supervision - A.S., E.A.; Resources - B.M., A.S.; Materials - B.M., M.G.; Data Collection and/or Processing - B.M.; Analysis and/or Interpretation - A.S., E.A.; Literature Search - A.S., B.M.; Writing Manuscript - A.S.; Critical Review - A.S., E.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Sinicato NA, Cardoso PA, Appenzeller S. Risk Factors in Cardiovascular Disease in Systemic Lupus Erythematosus. Curr Cardiol Rev. 2013;9:15–9. doi: 10.2174/157340313805076304. http://dx.doi.org/10.2174/157340313805076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster MH. T cells and B cells in lupus nephritis. Semin Nephrol. 2007;27:47–58. doi: 10.1016/j.semnephrol.2006.09.007. http://dx.doi.org/10.1016/j.semnephrol.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–18. doi: 10.1191/0961203306lu2305xx. http://dx.doi.org/10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 4.Svenungsson E, Jensen-Urstad K, Heimburger M, Silveira A, Hamsten A, de Faire U, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104:1887–93. doi: 10.1161/hc4101.097518. http://dx.doi.org/10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]
- 5.Othman KMS, Assaf NY. Early detection of premature subclinical coronary atherosclerosis in systemic lupus erythematosus patients. The Egyptian Heart Journal. 2013;65:281–8. http://dx.doi.org/10.1016/j.ehj.2012.12.003. [Google Scholar]
- 6.Doria A, Shoenfeld Y, Wu R, Gambari PF, Puato M, Ghirardello A, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62:1071–7. doi: 10.1136/ard.62.11.1071. http://dx.doi.org/10.1136/ard.62.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho MR, Sato EI, Tebexreni AS, Heidecher RT, Schenkman S, Neto TL. Effects of supervised cardiovascular training program on exercise tolerance, aerobic capacity, and quality of life in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;53:838–44. doi: 10.1002/art.21605. http://dx.doi.org/10.1002/art.21605. [DOI] [PubMed] [Google Scholar]
- 8.Forte S, Carlone S, Vaccaro F, Onorati P, Manfredi F, Serra P, et al. Pulmonary gas exchange and exercise capacity in patients with systemic lupus erythematosus. J Rheumatol. 1999;26:2591–4. [PubMed] [Google Scholar]
- 9.Gorcsan J, Manning WJ, Yeon SB. Up to date. Dec, 2015. Tissue Doppler echocardiography. [Google Scholar]
- 10.American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. http://dx.doi.org/10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 11.Wilmore JH, Costill DL. Physiology of Sport and Exercise. Vol. 3. Champaign: USA; IL: Human Kinetics; 2005. [Google Scholar]
- 12.Inês L, Silva C, Galindo M, López-Longo FJ, Terroso G, Romão VC, et al. Rheumatic Diseases Registry of the Portuguese Society of Rheumatology; Registry of Systemic Lupus Erythematosus Patients of the Spanish Society of Rheumatology. Classification of Systemic Lupus Erythematosus: Systemic Lupus International Collaborating Clinics versus American College of Rheumatology Criteria. A Comparative Study of 2,055 Patients from a Real-Life, International Systemic Lupus Erythematosus Cohort. Arthritis Care Res (Hoboken) 2015;67:1180–5. doi: 10.1002/acr.22539. http://dx.doi.org/10.1002/acr.22539. [DOI] [PubMed] [Google Scholar]
- 13.Harris EN, Gharavi AE, Patel SP, Hughes GR. Evaluation of anticardiolipin antibody test: report of an international workshop held for April. Clin Exp Immunol. 1987;68:215. [PMC free article] [PubMed] [Google Scholar]
- 14.Liang HL, Socher SA, Larson MA, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–18. doi: 10.1002/anr.1780320909. http://dx.doi.org/10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 15.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. http://dx.doi.org/10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 16.Feigenbaum H. Echocardiography. 5th ed. Baltimore: Williams & Wilkins; 1994. [Google Scholar]
- 17.Bruce RA, Pearson R, Lovejoy FW, Jr, Yu PNG, Brothers GB. Variability of respiratory and circulatory performance during standardized exercise. J Clin Invest. 28:1431–8. doi: 10.1172/JCI102208. http://dx.doi.org/10.1172/JCI102208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giunta A, Picillo U, Maione S, Migliaresi S, Valentini G, Arnese M, et al. Spectrum of cardiac involvement in systemic lupus erythematosus: echocardiographic, echo-Doppler observations and immunological investigation. Acta Cardiol. 1993;48:183–97. [PubMed] [Google Scholar]
- 19.Tektonidou MG, Ioannidis JP, Moyssakis I, Boki KA, Vassiliou V, Vlachoyiannopoulos PG, et al. Right ventricular diastolic dysfunction in patients with anticardiolipin antibodies and antiphospholipid syndrome. Ann Rheum Dis. 2001;60:43–8. doi: 10.1136/ard.60.1.43. http://dx.doi.org/10.1136/ard.60.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tincani A, Rebaioli CB, Taglietti M, Shoenfeld Y. Heart involvement in systemic lupus erythematosus, anti-phospholipid syndrome and neonatal lupus. Rheumatology (Oxford) 2006;45(Suppl 4):iv8–13. doi: 10.1093/rheumatology/kel308. http://dx.doi.org/10.1093/rheumatology/kel308. [DOI] [PubMed] [Google Scholar]
- 21.Kusunose K, Yamada H, Hotchi J, Bando M, Nishio S, Hirata Y, et al. Prediction of Future Overt Pulmonary Hypertension by 6-Min Walk Stress Echocardiography in Patients with Connective Tissue Disease. J Am Coll Cardiol. 2015;66:376–84. doi: 10.1016/j.jacc.2015.05.032. http://dx.doi.org/10.1016/j.jacc.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Puntmann VO, D’Cruz D, Smith Z, Pastor A, Choong P, Voigt T, et al. Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ Cardiovasc Imaging. 2013;6:295–301. doi: 10.1161/CIRCIMAGING.112.000151. http://dx.doi.org/10.1161/CIRCIMAGING.112.000151. [DOI] [PubMed] [Google Scholar]
- 23.Giunta A, Picillo U, Maione S, Migliaresi S, Valentini G, Arnese M, et al. Spectrum of cardiac involvement in systemic lupus erythematosus: echocardiographic, echo-Doppler observations and immunological investigation. Acta Cardiol. 1993;48:183–97. [PubMed] [Google Scholar]
- 24.Paran D, Caspi D, Levartovsky D, Elkayam O, Kaufman I, Litinsky I, et al. Cardiac dysfunction in patients with systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis. 2007;66:506–10. doi: 10.1136/ard.2005.044073. http://dx.doi.org/10.1136/ard.2005.044073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gin PL, Wang WC, Yang SH, Hsiao SH, Tseng JC. Right heart function in systemic lupus erythematosus: insights from myocardial Doppler tissue imaging. J Am Soc Echocardiogr. 2006;19:441–9. doi: 10.1016/j.echo.2005.10.018. http://dx.doi.org/10.1016/j.echo.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Duman D, Masatlioğlu S, Demirtunç R, Karadağ B. Increased pulmonary artery stiffness and its relation to right ventricular function in patients with systemic lupus erythematosus. Turk Kardiyol Dern Ars. 2008;36:82–9. [PubMed] [Google Scholar]
- 27.Leal GN, Silva KF, França CM, Lianza AC, Andrade JL, Campos LM, et al. Subclinical right ventricle systolic dysfunction in childhood-onset systemic lupus erythematosus: insights from two-dimensional speckle-tracking echocardiography. Lupus. 2015;24:613–20. doi: 10.1177/0961203314563135. http://dx.doi.org/10.1177/0961203314563135. [DOI] [PubMed] [Google Scholar]