To the Editor,
Denosumab has been approved for the prevention of skeletal-related events of bone metastases (120 mg at every 4 weeks) and for the treatment of osteoporosis (60 mg at every 6 months). Post-denosumab hypocalcemia occurred in 5.2% of the cancer patients (1), but such an event was rarely reported in osteoporosis patients (2). Recently, severe hypocalcemia because of a lower dose of denosumab for osteoporosis has been reported in chronic kidney disease (CKD) patients (3, 4). We describe the first case of rheumatoid arthritis (RA) and CKD associated with severe hypocalcemia and prolonged QT interval after the administration of a lower dose of denosumab.
A 73-year-old woman with a 10-year history of RA visited us for follow-up assessment. She denied having tetany or paresthesia but had noticed lightheadedness for a month. On conducting laboratory tests, her level of corrected calcium (cCa) was 6.7 mg/dL (Table 1). She had decreased estimated glomerular filtration rate (eGFR), consistent with moderate CKD through the equation from age and creatinine (5). Her electrocardiogram showed a corrected QT interval of 0.50 s (Figure 1). Seven months ago, the subcutaneous administration of 60-mg denosumab (Pralia, Daiichi Sankyo; Tokyo, Japan) was started for glucocorticoid-induced osteoporosis and for the multiple compression fracture of the spine (after obtaining informed consent from the patient), and the second dose was administered 4 weeks previously. She had undergone surgery for a thoracoabdominal aortic aneurysm 4 months previously. Since the surgery, her weight reduced by 6.5 kg. Her medication included prednisolone (4 mg daily), trichlormethiazide, furosemide, carvedilol, and olmesartan; subcutaneous abatacept was also administered. She had not taken any nutritional supplement during denosumab therapy. Denosumab was considered to be responsible for hypocalcemia. Following intravenous calcium gluconate administration, the level of cCa increased to 7.2 mg/dL. Two days after therapy with alfacalcidol and calcium, the level was restored to 8.9 mg/dL with the normalization of the QT interval; the level of intact PTH was elevated at 251 ng/mL (normal range, 10–65 ng/mL). Additional tests at the presentation showed a low level of 25-hydroxyvitamin D (6 ng/mL).
Table 1.
Calcium, phosphorus, and renal function according to the time course from denosumab administration
| Time from denosumab administration | 0 | 4 week | 7 week | 4 months | 6 months | 7 months |
| Corrected calcium (mg/dL) | 10.2 | 10 | 10 | 9.8 | 10.3 | 6.7 |
| Phosphorus (mg/dL) | 4 | 3.5 | 3.2 | 3.5 | 3.7 | 2.1 |
| Creatinine (mg/dL) | 0.96 | 1.18 | 1.14 | 1.07 | 1.11 | 0.97 |
| Estimated GFR for the Japanese | 44 | 35 | 36 | 39 | 37 | 43 |
| Estimated GFR from the CG | 35 | NA | 29 | NA | NA | 29 |
| Body weight (kg) | 42.1 | NA | 41.5 | NA | NA | 35.0 |
GFR: glomerular filtration rate (mL·min−1·1.73 m−2); CG: cockcroft–gault equations; NA: not available
Figure 1.
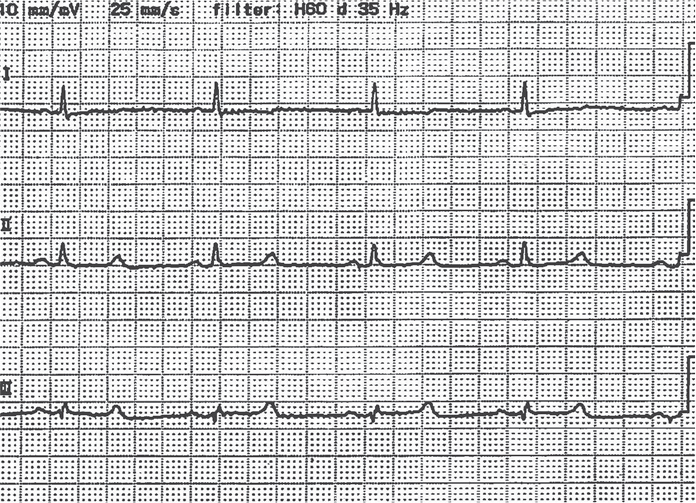
Prolongation of corrected QT interval on electrocardiogram
In a randomized-controlled trial of denosumab for post-menopausal osteoporosis, hypocalcemia (cCa <8.0 mg/dL) was not reported for 3 years among the 3886 women administered with denosumab (2). Women having a level of 25-hydroxyvitamin D of <12 ng/mL were not included in the study, and all the subjects received calcium and vitamin D. Therefore, screening and supplementation of vitamin D deficiency may have played a role in the safety profile. Recently, the effect of 60-mg denosumab on serum calcium in CKD patients was investigated (3). Hypocalcemia (cCa <7.5 mg/dL) did not occur in 13 patients with an eGFR of 30–49 mL/min−1/1.73 m−2 with or without supplemental calcium and vitamin D. However, two patients with severe CKD (eGFR <30 mL/min−1·1.73 m−2) who were initially included and did not receive supplementation showed hypocalcemia, whereas seven patients with severe CKD receiving supplementation did not. These results suggest that patients with severe CKD should receive supplemental vitamin D and calcium during denosumab therapy.
In our patient, hypocalcemia occurred with the second dose of denosumab but not with the first dose; this may be partially because of the deterioration of renal function before the second dose (Table 1). A high bone turnover mediated by secondary hyperparathyroidism and subsequent rapid reduction in bone resorption with denosumab therapy may lead to severe hypocalcemia, which is similar to the mechanism of hungry bone syndrome post-parathyroidectomy, as suggested previously (4). The assessment of vitamin D deficiency was not conducted before denosumab therapy because the measurement of 25-hydroxyvitamin D is not approved under medical insurance in Japan. Furthermore, the stage of CKD was reclassified as severe based on the Cockcroft–Gault equations including body weight (6), which may be appropriate for RA patients with low weight. Our experience suggests that the assessment of vitamin D deficiency and staging of CKD may be necessary for RA patients before starting denosumab. In addition, it is essential to monitor serum calcium level after starting denosumab therapy in RA patients with CKD.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: Written informed consent was obtained from patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.O.; Design - H.O.; Supervision - H.O.; Materials - H.O.; Data Collection and/or Processing - H.O.; Analysis and/or Interpretation - H.O.; Literature Review - H.O.; Writer - H.O.; Critical Review - S.M.
Conflict of Interest: Dr. Oiwa reports personal fees from Daiichi Sankyo, during the conduct of the study.
Financial Disclosure: The author declared that this study has received no financial support.
References
- 1.Qi WX1, Lin F, He AN, Tang LN, Shen Z, Yao Y. Incidence and risk of denosumab-related hypocalcemia in cancer patients: a systematic review and pooled analysis of randomized controlled studies. Curr Med Res Opin. 2013;29:1067–73. doi: 10.1185/03007995.2013.813840. http://dx.doi.org/10.1185/03007995.2013.813840. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. http://dx.doi.org/10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res. 2012;27:1471–9. doi: 10.1002/jbmr.1613. http://dx.doi.org/10.1002/jbmr.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dave V, Chiang CY, Booth J, Mount PF. Hypocalcemia post denosumab in patients with chronic kidney disease stage 4–5. Am J Nephrol. 2015;41:129–37. doi: 10.1159/000380960. http://dx.doi.org/10.1159/000380960. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92. doi: 10.1053/j.ajkd.2008.12.034. http://dx.doi.org/10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. http://dx.doi.org/10.1159/000180580. [DOI] [PubMed] [Google Scholar]


