ABSTRACT
Nod factors (NF) are molecules produced by rhizobia which are involved in the N2-fixing symbiosis with legume plants, enabling the formation of specific organs called nodules. Under drought conditions, nitrogen acquisition by N2-fixation is depressed, resulting in low legume productivity. In this study, we evaluated the effects of NF supply on nitrogen acquisition and on cytokinin biosynthesis of soybean plants grown under drought. NF supply to water stressed soybeans increased the CK content of all organs. The profile of CK metabolites also shifted from t-Z to cis-Z and an accumulation of nucleotide and glucoside conjugates. The changes in CK coincided with enhanced nodule formation with sustained nodule specific activity, which ultimately increased the total nitrogen fixed by the plant.
KEYWORDS: Agroecology, cytokinins, drought, Glycine max, legume, N2-fixation, nodulation factor, nodules, symbiosis
In legumes, lipo-chitooligosaccharides secreted by rhizobia, also called nodulation factors (NFs), are signals involved in the establishment of the symbiosis enabling the initiation of dinitrogen-fixing nodules.1 These molecules induce a cascade of responses within cells of the host plant such as calcium spiking, root hair curling, formation of pre-infection threads and nodule morphogenesis.2-4 Several categories of NFs can be synthesized by rhizobia, which can structurally differ in chitin chain, fatty acylation, acetylation, etc. enabling specific plant host recognition and biological activity.5-7 Previous works demonstrated that an exogenous supply in NFs could enhance seed germination and plant growth of a large range of crop species including Brassica napus, Zea mays, Gossypium hirsutum, Beta vulgaris and Glycine max.8,9 NF signaling seems to interact with programs controlled by phytohormones,10 in particular with cytokinins (CK) as NF recognition in plant epidermal cells promotes CK signaling in the cortex, most likely by increasing CK levels.11 Evidence that CK are a key differentiation signal for nodule organogenesis12 are numerous, although the underlying mechanisms still need to be further investigated.13
Symbiotic N2-fixing activity depends on 2 components, namely structural components such as total nodule biomass (specifically the number of nodules and the average biomass of one nodule) and functional components such as nodule-specific activity (g of fixed N2 per g of nodule and per day). In previous works, it has been demonstrated that a water stress occurring during the vegetative development of soybean led to a strong decrease in plant N acquisition via N2-fixation due to both a decrease of nodule biomass and a decrease of nodule specific activity.13-15 This study investigated how NF application impacted nitrogen (N) acquisition of soybean plants for which N2-fixation had been depressed by a water stress. In order to evaluate the effect of an exogenous supply of NF to soybean (Glycine max L. Merr. cv. OAC Champion) in symbiosis with N2-fixing rhizobia (Bradyrhizobium japonicum strain 532C), we followed the protocol previously described in Prudent et al.13 for plant growth and water stress imposition and treated soybean plants by adding 10–7 M nodulation factor Nod Bj (C18:1, MeFuc) isolated from Bradyrhizobium japonicum strain 532C16 to the nutrient solution, as soon as the water stress began. Plant N acquisition was based on N2 fixation only, via the use of an inert substrate and N-free nutrient solution. After 16 d of treatment, NF application under water stress increased the amount of N symbiotically fixed by plant by 25% (Fig. 1) over that of the water stressed plants. Because the lowered nodule specific activity observed for water stressed plant was not increased by NF application, their increase in total N resulted from an intensification of nodule initiation as NF application increased the number of nodules by 71% and the nodule biomass by 40%. Because roots are potential sites for nodule initiation, we observed some traits of the root system, however, NF did not impact root length or the number of root tips (Fig. 1).
Figure 1.
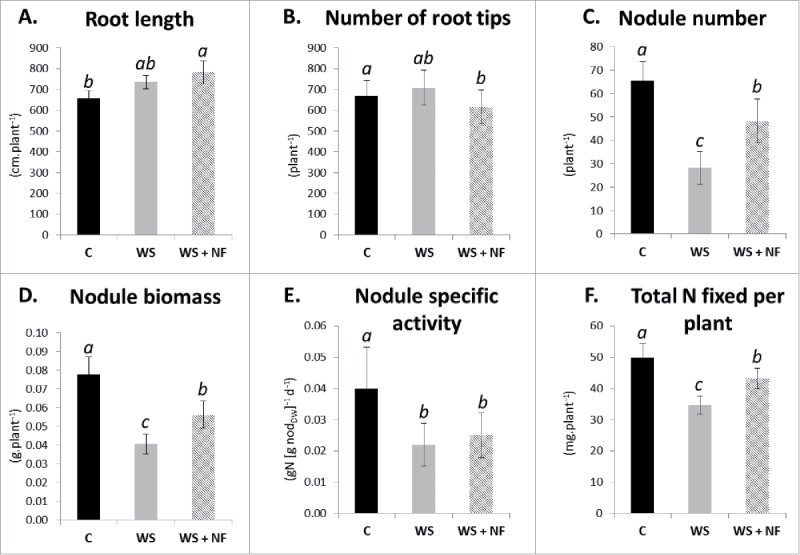
Nodulated root phenotypic traits of soybean plants grown for 15 d either under well-watered conditions (C), or subjected to a water deficit with (WS+NF) or without (WS) NF supply in the nutrient solution. Phenotypic traits comprised: root length (A), number of root tips (B), nodule number (C), nodule biomass (D), nodule specific activity (E), and the total amount of N symbiotically fixed per plant (F). Error bars represent standard deviation (n = 10), and differences among treatments significant at the 0.05 probability level, are denoted by different letters.
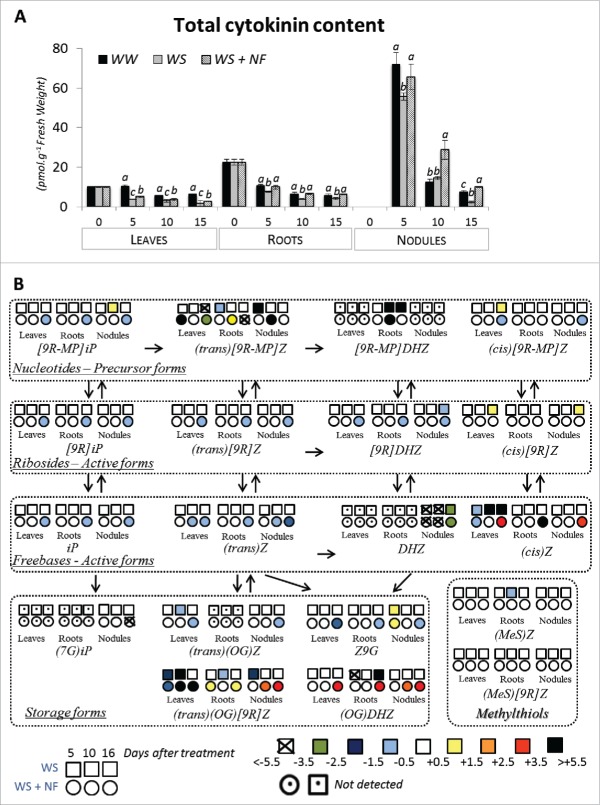
Given that CK signaling plays a key role in nodule organogenesis,12 and in lateral root formation,17,18 we decided to identify which, if any, steps of CK biosynthesis were impacted by NF, by quantifying various forms of CK at 5, 10 and 16 d after the beginning of the treatments, following the protocol described by others.19-21 Total CK content was decreased by drought in all tissues, although higher in nodules than in roots, which is in agreement with studies in soybean22 or in other species.23-25 When NF was applied under water stress conditions, CK content increased as soon as 5 d after the treatment in all plant organs (leaves, roots and nodules) till the end of the experiment (Fig. 2). In the case of leaves, the increase in total CK was significantly above the WS plants but not recovered to the level of unstressed controls. In roots the recovery to control CK levels was complete and in nodules NF treatment actually enabled the CK levels to surpass those of unstressed control plants. These results suggest that following NF application to the root system under drought conditions, a systemic signal within plant allowed a stimulation of CK biosynthesis thus increasing CK content in all plant organs, but particularly effective in roots and nodules.
Figure 2.
Effect of NF application after 5, 10, 15 d of treatment on total cytokinin content (A) of leaves, roots and nodules, and the relative abundance of each CK form (B) of plants grown for 5, 10, 15 d either under well-watered conditions (C), or subjected to a water deficit with (WS+NF) or without (WS) NF supply in the nutrient solution. Error bars represent standard deviation (n = 3 pools of 3 plants), and differences among treatments significant at the 0.05 probability level, are denoted by different letters. The colors indicate the extent of abundance modification when compared to the control plants, expressed as log (WS/C) for WS treatment and expressed as log(WS+NF/C) for WS+NF treatment. As such, a negative value means that CK form is more abundant in the control plants.
Then, the abundance of each CK derivative was measured (Table 1) and it appeared that in addition to increasing the total CK content, the NF treatment also significantly altered the individual CK metabolite profiles (Fig. 2). The most clear effect of NF treatment to WS plants was the accumulation of glucoside (i.e. (trans)(OG)[9R]Z and (OG)DHZ) and nucleotide ((trans)[9RMP]Z) conjugates and the production of greater levels of (cis)Z. Interestingly the latter coincided with a decrease in (trans) CK forms (i.e (trans)Z and (trans)[9R]Z (Fig. 2), suggesting that the profile of CK metabolites shifted from t-Z to cis-Z. The increase in cis-CK following NF application in roots and nodules is particularly interesting as the role of cis-CK has been hypothesized to maintain a minimal CK activity under growth-limiting conditions in order to enable the plant to consume its energy for more vital processes.26
Table 1.
Abundance of cytokinin derivatives measured in leaves, roots and nodules of well-watered control plants grown for 5, 10 and 16 d. Data are means and standard deviations (s.d) calculated on 3 pools of 3 plants and are expressed in pmol per g of fresh weight.
| Days after treatment | 0 |
5 |
10 |
16 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | mean | s.d | mean | s.d | mean | s.d | mean | s.d | |
| iP | 0.161 | 3.0E-02 | 0.057 | 4.5E-03 | 0.037 | 2.9E-03 | 0.030 | 3.8E-03 | |
| [9R]iP | 0.847 | 6.4E-02 | 0.739 | 1.1E-01 | 0.716 | 1.3E-01 | 0.335 | 5.7E-02 | |
| [9R-MP]iP | 1.050 | 8.7E-02 | 0.280 | 4.1E-02 | 0.134 | 2.9E-02 | 0.141 | 2.6E-02 | |
| (trans)Z | 0.369 | 2.0E-02 | 0.379 | 4.8E-02 | 0.249 | 1.5E-02 | 0.496 | 8.1E-02 | |
| (trans)[9R]Z | 0.111 | 7.0E-03 | 0.072 | 8.4E-03 | 0.032 | 6.4E-03 | 0.034 | 2.7E-03 | |
| (trans)[9R-MP]Z | nd | nd | 0.017 | 1.3E-03 | 0.033 | 2.3E-03 | |||
| (cis)Z | 0.234 | 4.7E-02 | 1.984 | 2.2E-01 | nd | nd | |||
| (cis)[9R]Z | 0.241 | 3.4E-02 | 0.097 | 7.6E-03 | 0.148 | 3.8E-02 | 0.079 | 4.7E-03 | |
| (cis)[9R-MP]Z | 0.483 | 8.4E-02 | 0.284 | 4.3E-02 | 0.402 | 4.7E-02 | 0.285 | 2.5E-03 | |
| DHZ | nd | nd | nd | nd | |||||
| [9R]DHZ | 0.037 | 4.6E-03 | 0.024 | 3.6E-03 | 0.014 | 4.6E-03 | 0.010 | 2.5E-03 | |
| [9R-MP]DHZ | nd | nd | nd | nd | |||||
| (trans)(OG)Z | 0.134 | 2.5E-02 | 0.143 | 2.5E-02 | 0.065 | 5.6E-03 | 0.069 | 2.5E-03 | |
| (OG)[9R]Z | 3.684 | 5.9E-01 | 4.366 | 3.1E-01 | nd | nd | |||
| (cis)(OG)Z | nd | nd | nd | nd | |||||
| (OG)DHZ | 0.242 | 3.5E-02 | 0.246 | 3.1E-02 | 0.135 | 3.3E-02 | 0.152 | 2.5E-02 | |
| (OG)[9R]DHZ | nd | nd | nd | nd | |||||
| [Z9G] | 0.116 | 2.6E-02 | 0.052 | 3.4E-03 | 0.053 | 9.2E-04 | 0.063 | 4.9E-03 | |
| (7G)iP | nd | nd | nd | nd | |||||
| (MeS)Z | 1.622 | 2.8E-01 | 1.353 | 2.6E-01 | 0.823 | 7.4E-02 | 0.699 | 6.5E-02 | |
| (MeS)[9R]Z | 0.663 | 4.2E-02 | 0.210 | 3.0E-02 | 0.170 | 2.9E-02 | 0.136 | 9.5E-03 | |
| (MeS)iP | nd | nd | nd | nd | |||||
| (MeS)[9R]iP | nd | nd | nd | nd | |||||
| BA | nd | nd | nd | nd | |||||
| [9R]BA | nd | nd | nd | nd | |||||
| Roots | |||||||||
| iP | 0.787 | 4.9E-02 | 0.160 | 1.5E-02 | 0.160 | 1.9E-02 | 0.132 | 2.0E-02 | |
| [9R]iP | 0.979 | 1.9E-01 | 0.661 | 4.8E-02 | 1.504 | 4.1E-01 | 0.585 | 6.2E-02 | |
| [9R-MP]iP | 2.837 | 5.2E-01 | 0.782 | 5.5E-02 | 0.574 | 5.1E-02 | 0.636 | 3.1E-02 | |
| (trans)Z | 0.572 | 2.4E-02 | 0.533 | 4.4E-02 | 0.311 | 3.8E-02 | 0.198 | 1.8E-02 | |
| (trans)[9R]Z | 0.232 | 2.3E-02 | 0.284 | 4.7E-02 | 0.111 | 3.0E-02 | 0.144 | 1.9E-02 | |
| (trans)[9R-MP]Z | 1.392 | 1.7E-01 | 1.736 | 1.0E-01 | 0.153 | 4.0E-02 | 0.107 | 1.5E-02 | |
| (cis)Z | nd | nd | nd | nd | |||||
| (cis)[9R]Z | 0.690 | 5.1E-02 | 0.470 | 4.1E-02 | 0.619 | 8.9E-02 | 0.489 | 2.4E-02 | |
| (cis)[9R-MP]Z | 4.669 | 2.3E-01 | 1.623 | 8.6E-02 | 1.145 | 2.6E-01 | 0.415 | 5.8E-02 | |
| DHZ | nd | nd | nd | nd | |||||
| [9R]DHZ | 0.080 | 3.2E-03 | 0.096 | 1.1E-02 | 0.039 | 7.7E-03 | 0.025 | 1.0E-03 | |
| [9R-MP]DHZ | nd | nd | nd | nd | |||||
| (trans)(OG)Z | nd | nd | nd | nd | |||||
| (OG)[9R]Z | 0.989 | 2.2E-02 | 0.157 | 2.5E-02 | 0.163 | 1.0E-02 | 0.122 | 2.8E-02 | |
| (cis)(OG)Z | nd | nd | nd | nd | |||||
| (OG)DHZ | 0.147 | 3.2E-02 | 0.076 | 1.1E-02 | nd | nd | |||
| (OG)[9R]DHZ | nd | nd | nd | nd | |||||
| [Z9G] | 0.038 | 2.9E-03 | 0.075 | 2.1E-03 | 0.067 | 5.2E-03 | 0.086 | 5.4E-03 | |
| (7G)iP | nd | nd | nd | nd | |||||
| (MeS)Z | 8.547 | 7.6E-01 | 3.162 | 7.2E-01 | 1.283 | 9.1E-01 | 2.698 | 6.7E-01 | |
| (MeS)[9R]Z | 0.435 | 5.9E-02 | 0.193 | 2.6E-02 | 0.181 | 1.9E-02 | 0.157 | 3.0E-02 | |
| (MeS)iP | nd | nd | nd | nd | |||||
| (MeS)[9R]iP | nd | nd | nd | nd | |||||
| BA | nd | nd | nd | nd | |||||
| [9R]BA | nd | nd | nd | nd | |||||
| Days after treatment | 5 |
10 |
16 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Nodules | 0 | mean | s.d | mean | s.d | mean | s.d | ||
| iP | 1.325 | 2.6E-01 | 0.142 | 2.2E-02 | 0.097 | 3.2E-03 | |||
| [9R]iP | 5.794 | 6.6E-01 | 1.383 | 1.2E-01 | 0.623 | 2.4E-02 | |||
| [9R-MP]iP | 10.373 | 2.6E+00 | 0.485 | 2.6E-02 | 0.165 | 1.8E-02 | |||
| (trans)Z | 1.849 | 4.1E-01 | 4.083 | 3.8E-01 | 1.122 | 1.4E-01 | |||
| (trans)[9R]Z | 0.713 | 4.7E-02 | 0.065 | 5.1E-03 | 0.110 | 1.5E-02 | |||
| (trans)[9R-MP]Z | nd | nd | nd | ||||||
| (cis)Z | nd | 2.756 | 1.7E+00 | 1.853 | 4.0E-01 | ||||
| (cis)[9R]Z | 1.752 | 2.8E-01 | 0.412 | 1.0E-02 | 0.136 | 2.5E-02 | |||
| (cis)[9R-MP]Z | 7.606 | 7.1E-01 | 0.607 | 1.9E-01 | 1.093 | 7.0E-02 | |||
| DHZ | 1.564 | 1.6E-01 | 0.094 | 7.9E-03 | 0.030 | 2.4E-03 | |||
| [9R]DHZ | 0.220 | 5.7E-02 | 0.052 | 7.7E-03 | 0.058 | 4.3E-03 | |||
| [9R-MP]DHZ | 0.553 | 1.1E-01 | nd | nd | |||||
| (trans)(OG)Z | nd | 0.380 | 1.5E-02 | 0.234 | 3.3E-02 | ||||
| (OG)[9R]Z | 22.800 | 2.7E+00 | 0.078 | 1.9E-03 | 0.158 | 1.8E-02 | |||
| (cis)(OG)Z | nd | nd | nd | ||||||
| (OG)DHZ | nd | 0.063 | 5.9E-03 | 0.089 | 4.4E-03 | ||||
| (OG)[9R]DHZ | nd | nd | nd | ||||||
| [Z9G] | 0.068 | 7.6E-02 | 0.156 | 1.3E-02 | 0.478 | 3.8E-02 | |||
| (7G)iP | nd | nd | 0.183 | 4.6E-02 | 0.058 | 3.7E-03 | |||
| (MeS)Z | 17.872 | 4.9E+00 | 1.413 | 1.8E-01 | 1.407 | 8.5E-02 | |||
| (MeS)[9R]Z | nd | 0.124 | 7.8E-03 | 0.120 | 1.2E-02 | ||||
| (MeS)iP | nd | nd | nd | ||||||
| (MeS)[9R]iP | nd | nd | nd | ||||||
| BA | nd | nd | nd | ||||||
| [9R]BA | nd | nd | nd | ||||||
Taken together, these results highlight that NF application under drought conditions increased CK production in soybeans, and enabled the plant to initiate more nodules, thus enhancing plant N acquisition. Interestingly, Muños et al.27demonstrated that under stress conditions, the N2-fixing B. japonicum USDA 138 produces NF with altered biological activities, suggesting that the addition of exogenous NF could compensate the natural decrease of efficient NF under drought. This NF supply to the plant was a way to manipulate plant hormonal status, in particular CK, whose content was increased. In the literature, increases in CK content (obtained by over expression of IPT genes encoding an enzyme involved in CK biosynthesis pathway) led to a higher tolerance to water stress28 as plants had higher photorespiration,23,29 higher stomatal conductance, higher transpiration,30 better osmotic adjustment by accumulation of amino acids and carbohydrates28,31 and enhanced root growth and root viability.32
In this study, soybean N acquisition was enhanced by NF supply under drought conditions, but further studies are still needed in order to estimate whether the use of NF as plant growth regulator under fluctuating environment could enhance the overall plant productivity in the field.
Disclosure of potential conflicts of interest
No potential conflict of interest was disclosed.
Acknowledgments
We are grateful to Alfred Souleimanov for nodulation factor isolation and to Sophie Baubil for her assistance during harvests. We would like to thank Amy Galer for her technical support during cytokinin extraction, Leonid Kurepin, Allison Hayward and Heather Francis for their help with the mass spectrometer.
References
- 1.Dénarié J, Cullimore J. Lipo-oligosaccharide nodulation factors: A new class of signaling molecules mediating recognition and morphogenesis. Cell 1993; 74:951-4; http://dx.doi.org/ 10.1016/0092-8674(93)90717-5 [DOI] [PubMed] [Google Scholar]
- 2.Ehrhardt D, Atkinson E, Long . Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science 1992; 256:998-1000; PMID:10744524; http://dx.doi.org/ 10.1126/science.10744524 [DOI] [PubMed] [Google Scholar]
- 3.Cárdenas L, Holdaway-Clarke TL, Sánchez F, Quinto C, Feijó JA, Kunkel JG, Hepler PK. Ion changes in legume root hairs responding to Nod factors. Plant Physiology 2000; 123:443-52; http://dx.doi.org/ 10.1104/pp.123.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oldroyd G E.D., Downie JA. Coordinating Nodule Morphogenesis with Rhizobial Infection in Legumes. Annual Ann Review Rev of Plant Biology 2008; 59:519-46; PMID:18444906; http://dx.doi.org/ 10.1146/annurev.arplant.59.032607.092839 [DOI] [PubMed] [Google Scholar]
- 5.Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Prome JC, Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature 1990; 344:781-4; PMID:2330031; http://dx.doi.org/ 10.1038/344781a0 [DOI] [PubMed] [Google Scholar]
- 6.Liang Y, Tóth K, Cao Y, Tanaka K, Espinoza C, Stacey G. Lipochitooligosaccharide recognition: an ancient story. New Phytologist 2014; 204:289-96; PMID:25453133; http://dx.doi.org/ 10.1111/nph.12898 [DOI] [PubMed] [Google Scholar]
- 7.Gough C, Cullimore J. Lipo-chitooligosaccharide signaling in Endosymbiotic Plant-Microbe Interactions. Molecular Mol Plant-Microbe Interactions 2011; 24:867-78; PMID:21469937; http://dx.doi.org/ 10.1094/MPMI-01-11-0019 [DOI] [PubMed] [Google Scholar]
- 8.Schwinghamer T, Souleimanov A, Dutilleul P, Smith D. Supplementation with solutions of lipo-chitooligosacharide Nod Bj V (C18:1, MeFuc) and thuricin 17 regulates leaf arrangement, biomass, and root development of canola (Brassica napus [L.]). Plant Growth Regul 2015; 78:31-41; http://dx.doi.org/ 10.1007/s10725-015-0072-8 [DOI] [Google Scholar]
- 9.Prithiviraj B, Zhou X, Souleimanov A, Kahn W, Smith D. A host-specific bacteria-to-plant signal molecule (Nod factor) enhances germination and early growth of diverse crop plants. Planta 2003; 216:437-45; PMID:12520335; http://dx.doi.org/ 10.1007/s00425-002-0928-9 [DOI] [PubMed] [Google Scholar]
- 10.Mulder L, Hogg B, Bersoult A, Cullimore JV. Integration of signalling pathways in the establishment of the legume-rhizobia symbiosis. Physiol Plant 2005; 123:207-18; http://dx.doi.org/ 10.1111/j.1399-3054.2005.00448.x [DOI] [Google Scholar]
- 11.Oldroyd GE. Nodules and hormones. Plant Sci 2007; 315:52-3; http://dx.doi.org/ 10.1126/science.1137588 [DOI] [PubMed] [Google Scholar]
- 12.Frugier F, Kosuta S, Murray JD, Crespi M, Szczyglowski K. Cytokinin: secret agent of symbiosis. Trends in Plant Science 2008; 13:115-20; PMID:18296104; http://dx.doi.org/ 10.1016/j.tplants.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 13.Prudent M, Salon C, Souleimanov A, Emery RJ, Smith DL. Soybean is less impacted by water stress using Bradyrhizobium japonicum and thuricin-17 from Bacillus thuringiensis. Agronomy for Sustainable Development 2015; 35:749-57; http://dx.doi.org/ 10.1007/s13593-014-0256-z [DOI] [Google Scholar]
- 14.Serraj R, Sinclair TR, Purcell LC. Symbiotic N2 fixation response to drought. J Exp Bot 1999; 50:143-55; http://dx.doi.org/ 10.1093/jxb/50.331.143 [DOI] [Google Scholar]
- 15.Streeter JG. Effects of drought on nitrogen fixation in soybean root nodules. Plant Cell Environment 2003; 26:1199-204; http://dx.doi.org/ 10.1046/j.1365-3040.2003.01041.x [DOI] [Google Scholar]
- 16.Souleimanov A, Prithiviraj B, Smith DL. The major Nod factor of Bradyrhizobium japonicum promotes early growth of soybean and corn. J Exp Bot 2002; 53:1929-34; PMID:12177132; http://dx.doi.org/ 10.1093/jxb/erf034 [DOI] [PubMed] [Google Scholar]
- 17.Perilli S, Moubayidin L, Sabatini S. The molecular basis of cytokinin function. Current Curr Opinion in Plant Biology 2010; 13:21-6; PMID:19850510; http://dx.doi.org/ 10.1016/j.pbi.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 18.Kiba T, Kudo T, Kojima M, Sakakibara H. Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J Exp Bot 2011; 62:1399-409; PMID:21196475; http://dx.doi.org/ 10.1093/jxb/erq410 [DOI] [PubMed] [Google Scholar]
- 19.Jones JM, Clairmont L, Macdonald ES, Weiner CA, Emery RJ, Guinel FC. E151 (sym15), a pleiotropic mutant of pea (Pisum sativum L.), displays low nodule number, enhanced mycorrhizae, delayed lateral root emergence, and high root cytokinin levels. J Exp Bot 2015; 66:4047-59; PMID:25948707; http://dx.doi.org/ 10.1093/jxb/erv201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emery RJ, Leport L, Barton JE, Turner NC, Atkins CA. cis-Isomers of cytokinins predominate in chickpea seeds throughout their development. Plant Physiology 1998; 117:1515-23; PMID:9701607; http://dx.doi.org/ 10.1104/pp.117.4.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurepin LV, Emery RJ, Chinnappa CC, Reid DM. Light irradiance differentially regulates endogenous levels of cytokinins and auxin in alpine and prairie genotypes of Stellaria longipes. Physiol Plant 2008; 134:624-35; PMID:19000197; http://dx.doi.org/ 10.1111/j.1399-3054.2008.01163.x [DOI] [PubMed] [Google Scholar]
- 22.Le DT, Nishiyama R, Watanabe Y, Mochida K, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS. Genome-Wide Expression Profiling of Soybean Two-Component System Genes in Soybean Root and Shoot Tissues under Dehydration Stress. DNA Res 2011; 18:17-29; PMID:21208938; http://dx.doi.org/ 10.1093/dnares/dsq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivero RM, Shulaev V, Blumwald E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol 2009; 150:1530-40; PMID:19411371; http://dx.doi.org/ 10.1104/pp.109.139378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies WJ, Zhang JH. Root signals and the regulation of growth and development of plants in drying soil. Annual Ann Review Rev of Plant Physiology Physiol and Plant Molecular Mol Biology 1991; 42:55-76; http://dx.doi.org/ 10.1146/annurev.pp.42.060191.000415 [DOI] [Google Scholar]
- 25.Ferguson BJ, Mathesius U. Signaling interactions during nodule development. J Plant Growth Regul 2003; 22:47-72; http://dx.doi.org/ 10.1007/s00344-003-0032-9 [DOI] [Google Scholar]
- 26.Gajdosova S, Spichal L, Kaminek M, Hoyerova K, Novak O, Dobrev PI, Galuszka P, Klíma P, Gaudinová A, Zizková E, et al.. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot 2011; 62:2827-40; PMID:21282330; http://dx.doi.org/ 10.1093/jxb/erq457 [DOI] [PubMed] [Google Scholar]
- 27.Muñoz N, Soria-Díaz ME, Manyani H, Sánchez-Matamoros RC, Serrano AG, Megías M, et al.. Structure and biological activities of lipochitooligosaccharide nodulation signals produced by Bradyrhizobium japonicum USDA 138 under saline and osmotic stress. Biology and Fertility of Soils 2014; 50:207-15; http://dx.doi.org/ 10.1007/s00374-013-0843-1 [DOI] [Google Scholar]
- 28.Merewitz EB, Gianfagna T, Huang B. Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J Exp Bot 2011; 62:5311-33; PMID:21831843; http://dx.doi.org/ 10.1093/jxb/err166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivero RM, Gimeno J, Van Deynze A, Walia H, Blumwald E. Enhanced cytokinin synthesis in tobacco plants expressing PSARK::IPT prevents the degradation of photosynthetic protein complexes during drought. Plant and Cell Physiology 2010; 51:1929-41; PMID:20871100; http://dx.doi.org/ 10.1093/pcp/pcq143 [DOI] [PubMed] [Google Scholar]
- 30.Qin H, Gu Q, Zhang J, Sun L, Kuppu S, Zhang Y, Burow M, Payton P, Blumwald E, Zhang H. Regulated expression of an Isopentenyltransferase gene (IPT) in Peanut significantly improves drought tolerance and increases yield under field conditions. Plant and Cell Physiology 2011; 52:1904-14; PMID:21920877; http://dx.doi.org/ 10.1093/pcp/pcr125 [DOI] [PubMed] [Google Scholar]
- 31.Merewitz EB, Du H, Yu W, Liu Y, Gianfagna T, Huang B. Elevated cytokinin content in ipt transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. J Exp Bot 2012; 63:1315-28; PMID:22131157; http://dx.doi.org/ 10.1093/jxb/err372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merewitz EB, Gianfagna T, Huang B. Photosynthesis, water use and root viability under water stress as affected by expression of SAG12-ipt controlling cytokinin synthesis in Agrostis stolonifera. J Exp Bot 2011; 62:383-95; PMID:20841349; http://dx.doi.org/ 10.1093/jxb/erq285 [DOI] [PMC free article] [PubMed] [Google Scholar]