ABSTRACT
Systemic acquired resistance (SAR) is a highly desirable form of resistance that protects against a broad-spectrum of pathogens. SAR involves the generation of a mobile signal at the site of primary infection, which arms distal portions of a plant against subsequent secondary infections. A number of diverse chemical signals contributing to SAR have been isolated and characterized. Among these, salicylic acid (SA) functions in parallel to azelaic acid (AzA) and glycerol-3-phosphate (G3P), and both AzA and G3P function downstream of the free radicals nitric oxide and reactive oxygen species. We now show that phloem loading of AzA and G3P occurs via the symplast, whereas that of SA occurs via the apoplast. The symplastic transport of AzA and G3P is regulated by plasmodesmata localizing protein (PDLP) 5, which together with PDLP1 also plays a signaling role in SAR. Together, these results reveal the transport routes of SAR associated chemical signals, and the regulatory role of PDLPs in SAR.
Keywords: Chemical signals plant defense, plasmodesmata, systematic acquired resistance, transport
Plants, like all living organisms, have to constantly resist pathogenic microbes and their sessile nature poses particular problems. To ensure survival plants have evolved some unique defense mechanisms including the induction of systemic defense responses such as systemic acquired resistance (SAR). During SAR, mobile signal(s) generated at the site of primary infection travel systemically to prime the systemic non-infected portions of the plant for better resistance against future infections by a broad-spectrum of pathogens.1-5 Plant cuticle, which is composed of cutin and wax layers plays an important role in SAR and mutations affecting either wax or cutin biosynthesis compromise SAR.6-8 Several mobile, SAR-inducing chemicals have been identified. Of these, the phytohormome salicylic acid (SA),9 the 9-carbon (C9) dicarboxylic acid azelaic acid (AzA,10,11), and the phosphorylated sugar G3P11-14 have been placed in a complex scheme involving 2 parallel pathways, which also include the lipid transfer protein (LTP), DIR1 (Defective in Induced Resistance,15,12) and the LTP-like AZI1 (AzA insensitive,10,11) protein.
Studies directed at dissecting the inter-relationships between various SAR components suggest that AzA is derived from C18 FAs containing double bond on carbon 9 and that reactive oxygen species (ROS) facilitate the breakage of this double bond.11,13 Nitric oxide (NO) functions in a feedback loop with ROS and mutations in either NO or ROS biosynthesis/accumulation compromises SAR.13 AzA acts upstream of G3P and stimulates G3P biosynthesis via the upregulation of genes encoding G3P dehydrogenase (G3Pdh) and glycerol kinase (GK;11). Consequently, mutations in either G3Pdh or GK render plants insensitive to exogenous AzA. Recent analysis has further shown that AzA and G3P, together with DIR1 and AZI1, orchestrate SAR via a linear pathway, with G3P serving as the vital regulator downstream of C18-FAs and AzA.11 This together with the shared dependence of AzA-, and G3P-triggered SAR on the SA pathway suggests a co-ordinated mode of induction and establishment of SAR (Fig. 1A). Relationship between NO-ROS-AzA-G3P and other SAR inducers including the diterpenoid dehdryoabietinal (DA,16), and the non-protein amino acid pipecolic acid (Pip,17), remains unknown.
Figure 1.
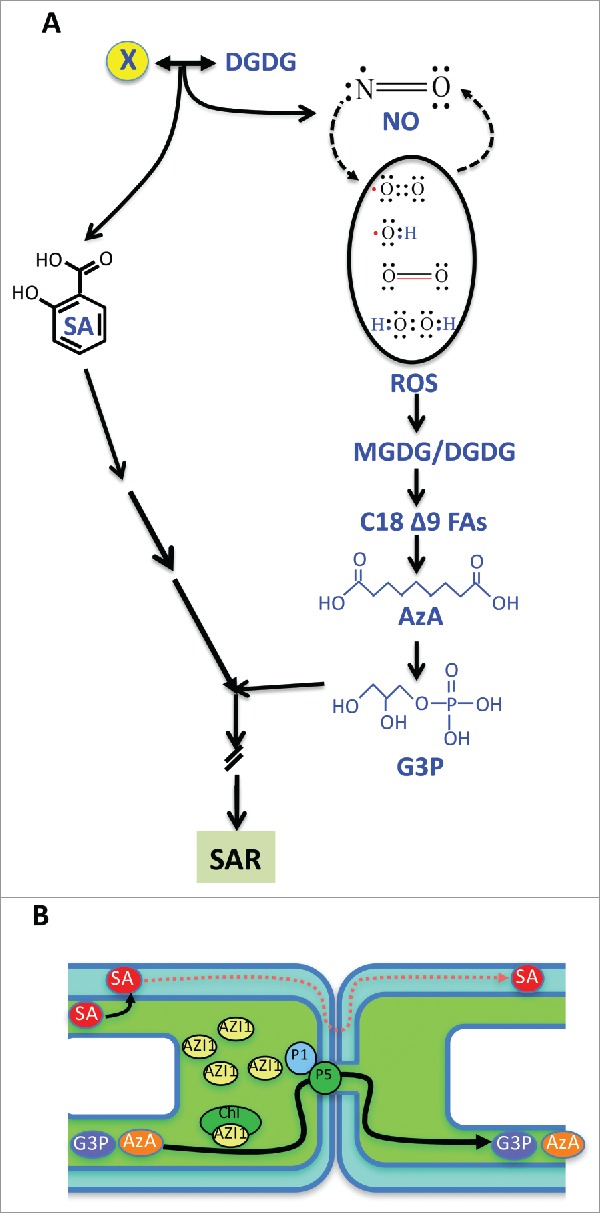
Models showing chemical signals involved in systemic signaling and their transport routes. (A) Inoculation of avirulent pathogen triggers accumulation of SA and the free radicals NO and ROS. The galactolipid DGDG functions together with an unknown signal (indicated by X) upstream of the SA and NO branch point and induces biosynthesis of SA and NO. The ROS species catalyze oxidative cleavage at carbon 9 of C18 Δ9 unsaturated fatty acids present on galactolipids MGDG and DGDG to form AzA. AzA induces biosynthesis of G3P by upregulating transcription of genes encoding G3P dehydrogenase and glycerol kinase. The NO-ROS-AzA-G3P pathway operates in parallel to the SA pathway. (B) A small percentage of SA, AzA and G3P are transported from the inoculated to the distal leaves. SA is transported to the distal leaves via the apoplast, defined as the space between the cell wall and cytoplasm. AzA and G3P are transported to the distal leaves via plasmodesmata (PD), which are cytoplasmic connections between adjacent cells. The induced levels of cytosolic SA inhibits PD permeability,27 and this in turn correlates with pathogen induced reduction in PD permeability.23 The levels of PD localizing protein 5 regulates PD permeability and thereby transport of AzA and G3P via PD but has no affect on the transport of SA. Both PDLP1 and PDLP5 proteins are required for SAR and contribute to the partitioning of the lipid transfer protein AZI1 between the plastids (chl) and cytoplasm.
Radiolabel feeding experiments suggest that a certain portion of pathogen-induced SA is transported from infected to the distal leaves.18,19 Likewise, radiolabel feeding experiments have shown that both AzA and G3P are transported to the distal tissues, and both these chemical inducers undergo derivatization into one or more, as yet unknown, chemicals.11,12 Similarly, SA is converted into various derivatives, and one of these (methyl SA) is well known to play an important role in SAR.20 The movement of SAR signal(s), which occurs within ∼6 h of primary inoculations,12,16 precedes the accumulation of SA, AzA, and G3P in the distal tissues. This suggests that SAR induction requires signal(s) that act upstream of SA, AzA, and G3P. This is consistent with the fact that a mutation in DGD1, which catalyzes the biosynthesis of digalactosyldiacylglycerol (DGDG) lipid, abolishes pathogen-mediated induction of both SA and NO-ROS-AzA-G3P branches of the SAR pathway.14 However, the dgd1 mutant plants are fully capable of generating the SAR-inducing signal as determined by the SAR-inducing ability of dgd1 petiole exudate on wild-type plants. This suggests that the induction of SA and the NO-ROS-AzA-G3P branches of SAR requires the interaction of an as yet unknown signal with the DGDG lipid (Fig. 1A). Notably, the terminal galactose in DGDG lipids is critical for the induction of SAR pathway because replenishing dgd1 plants with a lipid that contains the stereoisomer glucose, rather than the terminal galactose does not restore SAR in dgd1 plants.14
Although the phloem was the presumed site of SAR signal translocation, little was known about the intercellular transport of SA and that of other SAR-inducing chemicals, including G3P and AzA. Notably, even though the molecular size of AzA and G3P is well below the passive size exclusion limit of plasmodesmata (PD) regulated symplastic transport (800–1000 Da,21,22), only a small percentage of AzA and G3P are transported from the infected to distal leaves.11,12 Likewise, only a small percentage of SA is transported to the distal tissues.23 Together, these results suggest that the transport of SA, AzA, and G3P is unlikely to be via simple diffusion from the source (infected) to sink (distal) tissues.24 A tight regulation of the transport of AzA, G3P, and SA to the distal tissues is likely advantageous because it would be expected to better regulate the untimely induction of defenses in the distal uninfected tissues and thereby prevent metabolic perturbations that could be detrimental to overall plant health and fitness.
We recently showed that the transport of AzA and G3P from local to distal tissues occurs via the symplastic route,23 regulated by plasmodesmata (PD) channels.25,26 In contrast, SA moves via the extracytosolic apoplastic compartment (Fig. 1B). The separate primary transport route for SA is intriguing considering that these chemicals are not drastically different in their molecular sizes (SA = 138.12 Da; AzA = 188.21 Da; G3P = 172.07 Da). Notably, exogenous SA can reduce PD permeability.27 Thus, the accumulation of SA during pathogen infection would be expected to essentially counteract the movement of AzA and G3P via the PD. This is consistent with the fact that pathogen infection negatively regulates PD permeability.23 Thus, the apoplastic transport route of SA would enable its transport in spite of reduced PD permeability in pathogen-infected plants. As expected, reduced PD permeability results in decreased transport of AzA and G3P, but not SA.
We reduced PD permeability by overexpressing the PD Localizing Protein 5 (PDLP5) using constitutive and inducible promoters.28 Consistent with their impaired transport, plants overexpressing PDLP5 were defective in SAR.23 Moreover, localized application of AzA, G3P, or other SAR signals including NO, ROS and SA were unable to rescue the SAR defect of 35S-PDLP5 plants. Together, these results suggest that PD-mediated symplastic transport of AzA and G3P, and likely other molecules induced in response to pathogen infection or AzA/G3P application is critical for the normal induction of SAR. To determine if reduced PD permeability of 35S-PDLP5 conferred enhanced resistance against viral pathogens that spread symplastically, we compared the systemic spread of turnip crinkle virus (TCV) in wild-type and 35S-PDLP5 plants. Interestingly, 35S-PDLP5 plants showed wild-type-like spread of TCV (Fig. 2). Since viral pathogens are well know to alter PD aperture to facilitate their movement,29 it is possible that TCV infection restores enough PD permeability in the 35S-PDLP5 plants to allow efficient viral spread. These results further highlight a different effect produced on PD by bacterial and viral infections.
Figure 2.
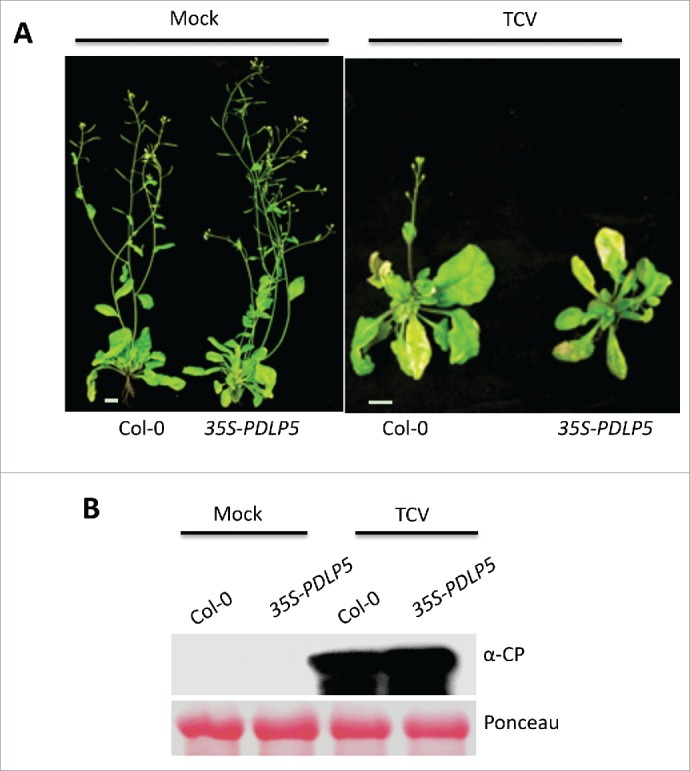
Spread and replication of TCV to uninoculated leaves. (A) Typical morphological phenotypes shown by mock- (left panel) and TCV (right panel)-inoculated plants at 17 dpi (scale bars, 0.5 cm). (B) Protein gel blot analysis showing levels of TCV in the bolt tissues of Col-0 and 35S-PDLP5 plants at 3 d post inoculation. Approximately 50 µg of total protein was fractionated on a SDS-PAGE and subjected to immunoblot analysis using anti-CP antibodies. Levels of Rubisco were used as loading control.
In addition to regulating PD permeability, PDLP5 was required for the stability of the lipid transfer-like protein AZI1, a key SAR component. Furthermore, a loss-of-function mutation in PDLP1,30 was also associated with AZI1 instability and compromised SAR phenotypes. This together with the result that a mutation in PDLP1 did not alter PD permeability suggests that PDLP proteins played a signaling role in SAR. This further correlated with our result that PDLP1 interacted with AZI1, but not DIR1. In addition, PDLP1 also showed weak interaction with PDLP5, suggesting that at least a subset of these proteins are present in a complex. Interestingly, loss of either PDLP1 or PDLP5 increased the chloroplastic localization of AZI1, suggesting that plastidal localization or partitioning of AZI1 between the plastid and cytoplasm, maybe important for SAR. The chloroplastic localization of AZI1 was also shown to increase after pathogen infection,31 suggesting that the chloroplastic localization of AZI1 is important for SAR. However, mere localization of AZI1 to the plastids is not sufficient for SAR, because the pdlp1 and pdlp5 mutants are defective in SAR despite increased chloroplastic localization of AZI1 in these plants. The infected tissues of pdlp1 or pdlp5 mutant plants accumulate normal levels of the galactolipid pool as well as SA, AzA, and G3P, suggesting that the differential partitioning of AZI1 does not impact the biosynthesis of these key SAR inducers. The biological significance of the chloroplastic localization of AZI1 remains unclear at present.
Experimental procedures
Plant growth conditions, pathogen infections and protein gel-blot analysis
Plants were grown in MTPS 144 Conviron (Winnipeg, MB, Canada) walk-in chambers at 22°C, 65% relative humidity and 14 h photoperiod. These chambers were equipped with cool white fluorescent bulbs (Sylvania, FO96/841/XP/ECO). The pdlp1, pdlp5, 35S-PDLP5, and azi1 have been described earlier.10,11,23,28
Transcripts synthesized in vitro from a cloned cDNA of TCV using T7 RNA polymerase were used for viral infections. For inoculations, the viral transcript was suspended at a concentration of 0.05 µg/ µL in inoculation buffer, and the inoculation was performed as described earlier.32 After viral inoculations, the plants were transferred to a Conviron MTR30 reach-in chamber maintained at 22°C, 65% relative humidity and 14 h photoperiod. Resistance and susceptibility was scored at 14 to 21 dpi and confirmed by northern gel blot analysis. Susceptible plants showed stunted growth, crinkling of leaves and drooping of the bolt.
Proteins were extracted in buffer containing 50 mM Tris-HCl, pH7.5, 10% glycerol, 150 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 5 mM DTT, and 1 X protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Protein concentration was measured by the Bio-RAD protein assay (Bio-Rad, CA). For small scale extractions 2–3 leaves were homogenized per sample.
For Ponceau-S staining, PVDF membranes were incubated in Ponceau-S solution (40% methanol (v/v), 15% acetic acid (v/v), 0.25% Ponceau-S). The membranes were destained using deionized water.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Science Foundation (IOS#0749731, #1457121).
References
- 1.Klessig DF, Vlot CA, Dempsey DA. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopath 2009; 47:177-206; PMID:19400653; http://dx.doi.org/24929297 10.1146/annurev.phyto.050908.135202 [DOI] [PubMed] [Google Scholar]
- 2.Wendehenne D, Gao Q-M, Kachroo A, Kachroo P. Free radical-mediated systemic immunity in plants. Curr Opin Plant Bio 2014; 20:127-34; PMID:24929297; http://dx.doi.org/ 10.1016/j.pbi.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 3.Shah J, Zeier J. Long-distance communication and signal amplification in systemic acquired resistance. Frontiers Plant Sci 2013; 4:30; PMID:23440336; http://dx.doi.org/23870750 10.3389/fpls.2013.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kachroo A, Robin GP. Systemic signaling during plant defense. Curr Opin Plant Biol 2013; 16:527-33; PMID:23870750; http://dx.doi.org/ 10.1016/j.pbi.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 5.Gao Q-M, Kachroo A, Kachroo P. Chemical inducers of systemic immunity in plants. J Exp Bot 2014; 65:1849-55; PMID:24591049; http://dx.doi.org/ 10.1093/jxb/eru010 [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Gao Q-M, Yu K, Lapchyk L, Navarre D, Hildebrand D, Kachroo A, Kachroo P. An intact cuticle in distal tissues is essential for the induction of systemic acquired resistance in plants. Cell Host & Microbe 2009; 5:151-65; PMID:19218086; http://dx.doi.org/ 10.1016/j.chom.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Xia Y, Yu K, Navarre D, Seebold K, Kachroo A, Kachroo P. The glabra1 mutation affects cuticle formation and plant responses to microbes. Plant Physiol 2010; 154:833-46; PMID:20699396; http://dx.doi.org/ 10.1104/pp.110.161646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia Y, Yu K, Gao Q-M, Wilson EV, Navarre D, Kachroo P, Kachroo A. Acyl CoA binding proteins are required for cuticle formation and plant responses to microbes. Front. Plant Sci 2012; 3:224; PMID:23060893; http://dx.doi.org/ 10.3389/fpls.2012.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaffney T, Friedrich L, Vernooij B, Negmtto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 1993; 261:754-6; PMID:17757215; http://dx.doi.org/ 10.1126/science.261.5122.754 [DOI] [PubMed] [Google Scholar]
- 10.Jung HW, Tschaplinkski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science 2009; 324:89-91; PMID:19342588; http://dx.doi.org/ 10.1126/science.1170025 [DOI] [PubMed] [Google Scholar]
- 11.Yu K, Soares JM, Mandal MK, Wang C, Chanda B, Gifford AN, Fowler JS, Navarre D, Kachroo A, Kachroo P. A feedback regulatory loop between G3P and lipid transfer proteins DIR1 and AZI1 mediates azelaic-acid-induced systemic immunity. Cell Rep 2013; 3:1266-78; PMID:23602565; http://dx.doi.org/ 10.1016/j.celrep.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 12.Chanda B, Xia Y, Mandal M, Yu K, Sekine K, Gao Q-M, et al.. (2011) Glycerol-3-phosphate, a critical mobile inducer of systemic immunity in plants. Nature Genetics 2011; 43:421-7; PMID:21441932; http://dx.doi.org/ 10.1038/ng.798 [DOI] [PubMed] [Google Scholar]
- 13.Wang C, El-Shetehy M, Shine MB, Yu K. Navarre D, Wendehenne D, Kachroo, A, Kachroo P. Free radicals mediate systemic acquired resistance. Cell Rep 2014; 7:348-55; PMID:24726369; http://dx.doi.org/ 10.1016/j.celrep.2014.03.032 [DOI] [PubMed] [Google Scholar]
- 14.Gao Q-M, Yu K, Xia Y, Shine MB, Navarre D, Kachroo A, Kachroo P. Mono- and di-galactosyldiacylglycerol function non-redundantly to regulate systemic required resistance in plants. Cell Rep 2014; 9:1681-91; PMID:25466253; http://dx.doi.org/ 10.1016/j.celrep.2014.10.069 [DOI] [PubMed] [Google Scholar]
- 15.Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK. A putative lipid transfer protein involved in systemic resistance signaling in Arabidopsis. Nature 2002; 419:399-403; PMID:12353036; http://dx.doi.org/ 10.1038/nature00962 [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi R, Venables B, Petros R, Nalam V, Li M, Wang X, Takemoto LJ, Shah J. An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J 2012; 71:161-72; PMID:22385469; http://dx.doi.org/ 10.1111/j.1365-313X.2012.04981.x [DOI] [PubMed] [Google Scholar]
- 17.Návarova H, Bernsdorff F, Doring A-C, Zeier J. Pipecolic acid, an endogenous mediator of defense amplifcation and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012; 24:5123-41; http://dx.doi.org/ 10.1105/tpc.112.103564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulaev V, Léon J, Raskin I. Is salicylic acid a translocated signal of systemic acquired resistance in tobacco? Plant Cell 1995; 7:1691-701; PMID:12242358; http://dx.doi.org/ 10.1105/tpc.7.10.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molders W, Buchala A, Metraux J-P. Transport of salicylic acid in tobacco necrosis virus-infected cucumber plants. Plant Physiol 1996; 112:787-92; PMID:12226421; http://dx.doi.org/ 10.1104/pp.112.2.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S-W, Kaimoyo E, Kumar D, Mosher S, Klessig D. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007; 318:113-6; PMID:17916738; http://dx.doi.org/ 10.1126/science.1147113 [DOI] [PubMed] [Google Scholar]
- 21.Oparka KJ. Signalling via plasmodesmata-the neglected pathway. Semin. Cell Biol 1993; 4:131-8; PMID:8318697; http://dx.doi.org/ 10.1006/scel.1993.1016 [DOI] [PubMed] [Google Scholar]
- 22.Overall RL, Blackman LM. A model of the macromolecular structure of plasmodesmata. Trends Plant Sci 1996; 1:307-11; http://dx.doi.org/ 10.1016/S1360-1385(96)88177-0 [DOI] [Google Scholar]
- 23.Lim G-H, Shine MB, Lorenzo Ld, Yu K, Cui W, Navarre D, Hunt AG, Lee J-Y, Kachroo A, Kachroo P. Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host & Microbe 2016; 19:541-9; PMID:27078071; http://dx.doi.org/ 10.1016/j.chom.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 24.Turgeon R. The role of phloem loading reconsidered. Plant Physiol 2010; 152:1817-23; PMID:20200065; http://dx.doi.org/ 10.1104/pp.110.153023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burch-Smith TM, Zambryski PC. Plasmodesmata paradigm shift: regulation from without versus within. Annu Rev Plant Biol 2012; 63:239-60; PMID:22136566; http://dx.doi.org/ 10.1146/annurev-arplant-042811-105453 [DOI] [PubMed] [Google Scholar]
- 26.Robards AW, Lucas WJ. Plasmodesmata. Annu Rev Plant Physiol Plant Mol Biol 1990; 41:369-419; http://dx.doi.org/ 10.1146/annurev.pp.41.060190.002101 [DOI] [Google Scholar]
- 27.Wang X, Sager R, Cui W, Zhang C, Lu H, Lee J-Y. Salicylic acid regulates plasmodesmata closure during innate immune responses in Arabidopsis. Plant Cell 2013; 25:2315-29; PMID:23749844; http://dx.doi.org/ 10.1105/tpc.113.110676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J-Y, Wang X, Cui W, Sager R, Modla S, Czymmek K, Zybaliov B, van Wijk K, Zhang C, Lu H, et al.. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 2011; 23:3353-73; PMID:21934146; http://dx.doi.org/ 10.1105/tpc.111.087742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoelz JE, Harries PA, Nelson RS. Intracellular transport of plant viruses: Finding the door out of the cell. Mol Plant 2011; 4:813-31; PMID:21896501; http://dx.doi.org/ 10.1093/mp/ssr070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Calvino L, Faulkner C, Walshaw J, Saalbach G, Bayer E, Benitez-Alfonso Y, Maule A. Arabidopsis plasmodesmal proteome. PLoS ONE 2011; 6:e18880; PMID:21533090; http://dx.doi.org/ 10.1371/journal.pone.0018880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cecchini NM, Steffes K, Schlappi MR, Gifford AN, Greenberg JT. Arabidopsis AZI1 family proteins mediate signal mobilization for systemic defence priming. Nat. Commun 2015; 6; 7658; PMID:26203923; http://dx.doi.org/ 10.1038/ncomms8658 [DOI] [PubMed] [Google Scholar]
- 32.Zhu S, Jeong R-D, Lim G-H, Yu K, Wang C, Chandra-Shekara AC, Navarre D, Klessig DF, Kachroo A, Kachroo P. Double-stranded RNA-binding protein 4 is required for resistance signaling against viral and bacterial pathogens. Cell Rep 2013; 4:1168-84; PMID:24055058; http://dx.doi.org/ 10.1016/j.celrep.2013.08.018 [DOI] [PubMed] [Google Scholar]


