ABSTRACT
In the backdrop of global warming and increase in temperatures, wheat productivity worldwide would be limited. This study was therefore undertaken to analyze the heat stress response in 12 different cultivars of Indian wheat. Three developmental stages were used i.e. germination stage, seedling stage and anthesis stage, to characterize thermotolerant and thermosusceptible cultivars on the basis of different physiological and molecular parameters. Lethal temperature stress on germinating seeds showed a clear reduction in percentage germination. At the seedling stage, higher decrease in Fv/Fm, total chlorophyll content, membrane injury and carbon isotope discrimination was observed in thermosusceptible cultivars. Results similar to seedling stage were obtained at anthesis stage. PSII efficiency of late-sown cultivars and timely-sown cultivars also indicated that thermosusceptible cultivars are more prone to terminal heat stress than thermotolerant cultivars. Heat Susceptibility Index (HSI) was calculated on the basis of physiological parameters. Based on HSI, thermotolerant and thermosusceptible cultivars were identified. HSI revealed comparatively low heat susceptibility in K7903, CBW12 and C306 and high heat susceptibility in PBW343, HD2329 and HD2428. On the basis of HSI, expression analysis of stress induced genes was performed between 2 tolerant cultivars C306 and K7903 along with 2 susceptible cultivars, HD2329 and PBW343. Higher expression of stress induced genes was observed in the 2 thermotolerant cultivars C306 and K7903 as compared to the 2 thermosusceptible cultivars HD2329 and PBW343. Thus further reconfirms that stress inducible genes can be employed for categorizing cultivars into susceptible and tolerant groups.
KEYWORDS: Delta carbon, expression profiling, heat susceptibility index, membrane injury index, photosynthetic efficiency, temperature induction response, wheat
Introduction
Global warming may unequivocally represent major challenges to plant productivity and geographic distribution.1 This is mainly due to impaired cellular metabolism, injury and even cell death. The adaptive mechanisms vary with plant age, genotype and environment. Plants have evolved mechanisms to adjust their environment and respond by bringing about short- and long-term physiological and biochemical mechanisms like leaf functioning, transpiration or changed membrane lipid composition.2 Increase in cellular temperature results in a cascade of signal pathways thereby influencing the transcriptome of cells and bringing about varying tolerance mechanism.3 This change in transcriptome invariably brings about an increase in stress proteins (HSP's), which in turn brings about necessary changes in the cell mainly through the chaperonin like function.4
Wheat, during anthesis and grain filling stages, is often exposed to terminal heat stress either singly or in combination with drought stress resulting in decreased yield, low pollen viability, decreased grain size and weight5 along with enhanced maturity, which is often an escape mechanism adapted by plants for allocating resources to developing grains.6 With a diverse gene pool available in India, variable degree of thermotolerance among different cultivars is observed, which is partly attributed to differential expression of stress responsive genes.7 Sensitivity of photosynthesis to thermal stress is reported in wheat8 and attributed to impairment of electron transport activity especially damage to OEC of PSII hence affecting PSII efficiency.9 Very often thermal stress occurs in combination with drought stress resulting in closure of stomata. The heavy isotope of carbon (δ13 C) is discriminated during the diffusion of CO2 through the stomata.10
Abiotic stress induced osmotic imbalance results in homeostatic adjustments in the form of high concentration of inorganic ions or low molecular weight solutes including proline accumulation. Proline accumulation under stressed conditions is correlated with functions of an osmolyte, ROS scavenger and molecular chaperone.11 Another aspect associated with higher temperature is the malfunctioning of the cellular membranes by increasing their permeability to ions and electrolytes. Cell membrane stability (CMS) has been used for screening against drought tolerance in wheat and wild relatives of wheat.12 However, the limitation is of screening the parental lines for thermotolerance which under natural conditions is tedious and dependent upon crop season. Commonly, field survival test is used to evaluate heat tolerance in crop plants. These tests, although efficient, are dependent upon additional factors in the field such as wind, humidity, irradiance, soil moisture content, temperature and biotic stresses which keep varying. The best alternative is thus to introduce techniques that are faster, economical and efficient in a developmental-independent pathway.
Reports related to thermotolerance in wheat are either on germinating seeds,13 seedlings14 or mature plants.8 Since a comprehensive study between germinating seeds, seedling and mature plant stage is lacking hence our study will provide an insight of the developmental stage related tolerance to thermal stress. Based on these parameters heat susceptibility index (HSI) was calculated and expression profiling of stress induced genes was undertaken. Besides being efficient these experiments can help breeders in differentiating thermotolerant cultivars from thermosusceptible cultivars. In this study we have tried to draw a correlation between physiological parameters and expression analysis of stress induced genes for a better understanding of stress tolerance mechanism operative in different cultivars of Indian bread wheat, Triticum aestivum.
Results and discussion
At present, approximately 95 million tons of wheat is produced by South Asia although the demand will increase to nearly 137 million tons by 2020.15 Hence there is a need to increase the yield gradually by breeding population in order to break the stagnant yield barrier observed in the major wheat producing areas of South Asian nations. This can be achieved with adoption of cultivars based on increased yield potential. Our aim was to find out the mechanism of thermotolerance in different wheat cultivars. For this, we have scored different physiological parameters at different stages of development in wheat plant along with expression profiling between the identified thermotolerant and susceptible cultivars following thermal stress. In the following, we summarize our main findings and discuss the level of the heat induced damage to different cultivars of wheat.
Heat stress and photosystem II efficiency
Photosystem II efficiency
Since photosynthetic machinery is the most sensitive to heat stress, therefore to check for heat stress tolerance of wheat, 12 Indian bread wheat cultivars were subjected to heat stress at 3 developmental stages including seedling stage, anthesis and late sown conditions. Although no significant difference in PSII efficiency was observed before the heat treatment, high temperatures of 37°C and 42°C magnified the discrepancy of Fv/Fm between different cultivars (Fig. 1a, b). K7903, C306, DBW17, CBW12 and BOBWHITE showed an increase in PSII efficiency at heat stress of 37°C. At 42°C, K7903, C306, DBW17, CBW12 and BOBWHITE showed higher PSII efficiency than the remaining cultivars at both seedling and mature plant stages. Under late sown conditions (Fig. S1), maximum decrease in Fv/Fm was observed in PBW343, HD2329 and HD2428, with a percentage decrease of 2.7, 2.9 and 3.1 percent, respectively.
Figure 1.
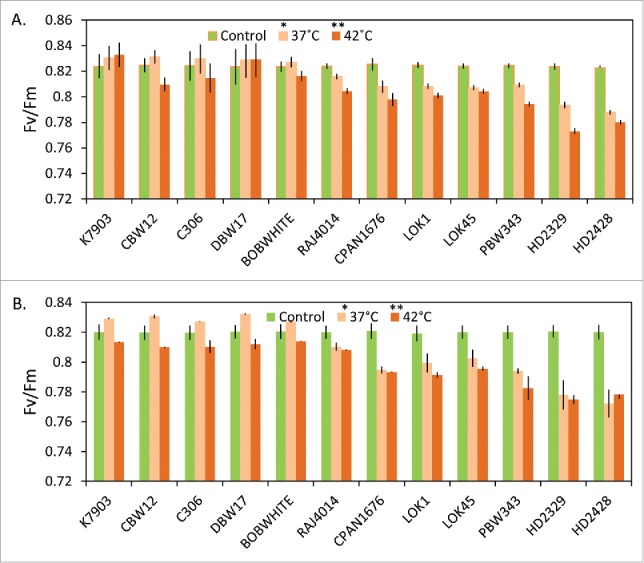
Effect of moderate and high temperature stress on Fv/Fm among wheat cultivars differing in thermotolerance. (A) Seedling at 10-day-old stage.; (B) Mature plants at anthesis stage. Significance levels: *P < 0.05; **P < 0.01.
Sensitivity of PSII toward heat stress has earlier been reported8 and attributed to impairment of electron transport activity.9 These changes in Fv/Fm were shown to be reliable diagnostic indicator of photoinhibition by earlier workers.16 At a moderately high temperature of 37°C, an increase in photorespiration can act as an electron sink resulting in faster electron transport than before the heat stress. An increase in quantum yield of PSII was observed when Arabidopsis leaves were given heat stress of 40°C for 30 min.17 However, under severe heat stress of 42°C, rubisco deactivation may occur and a decrease in PSII will be observed.18 Our result show that high temperature stress causes an increased PSII inhibition in susceptible cultivars as compared to the tolerant cultivars. Earlier studies have shown PSII efficiency to be a useful marker for identification of stress tolerance between cultivars.19
Heat susceptibility and cell membrane stability
Cell membrane stability
Under control conditions of 22°C, wheat leaves did not showed any change in membrane stability over the span of experiment, but temperature stress of 37°C and/or 42°C affected membrane stability (Figs. 2A and B). After thermal stress of 37°C and/or 42°C for 2 h, significant increase in membrane injury index (MII) was observed at seedling stage and mature plant stage; with HD2428 and HD2329 showing maximum MII followed by CPAN1676 and RAJ4014 at both seedling and mature plant stages. Cultivars which were shown to be performing better by Fv/Fm (K7903, DBW17, CBW12, LOK1, BOBWHITE) showed lesser membrane injury as compared to the remaining cultivars.
Figure 2.
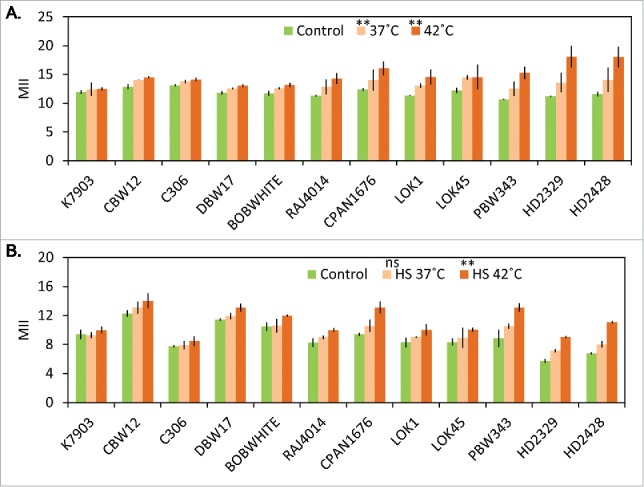
Membrane Injury Index (MII) at: seedling stage (A) and; at mature plant stage (B). Significance levels: ns (non significant) *P < 0.05; **P < 0.01.
Cell membrane stability is a heritable trait.20 It is suggested that increased membrane stability in better performing cultivars might correlate with membrane composition, as membrane fluidity is directly affected by unsaturation level of phospholipids and glycolipids. Previous results have shown changes in membrane composition under different abiotic stresses in Arabidopsis.21 A correlation can therefore be drawn between PSII efficiency and membrane composition.22 Since photosynthetic electron transport chain comprising PSI and PSII are integral thylakoid membrane protein complexes, any change in membrane fluidity is bound to have adverse effect on the efficiency of membrane associated PSI and PSII complexes and ultimately Fv/Fm.
Chlorophyll content
Decrease in total chlorophyll content was observed at seedling and mature plant stages after heat treatment at 37°C and/or 42°C for 2 h. Total chlorophyll content was higher in K7903, C306, DBW17, CBW12 and BOBWHITE after heat shock at 37°C for 2 h as against the remaining cultivars which showed a decrease in total chlorophyll content at both seedling and mature plant stages (Fig. S2A and S2B). Maximum decrease was observed at 42°C for all cultivars at seedling and anthesis stages. For particular cultivar, a comparable reduction in chlorophyll was observed at both seedling stage and anthesis stage.
The observable decrease in total chlorophyll is mainly due to decrease in the LHCII, which is a protective mechanism in plants undergoing abiotic stress.23 High temperature of 42°C could accelerate photoinhibition by inducing an imbalance between light energy absorption and utilization and could end up in ROS production, which can damage the photosynthetic apparatus.24
Temperature induction response (TIR)
All living organism maintain homeostasis in their surrounding conditions. Cells acclimatize to changing environmental conditions by changing their transcriptome and bringing about necessary changes to cell metabolism. Temperature Induction Response of seeds acclimatized at 37°C for 1.5 h showed genetic variability between cultivars. A significant decrease in percentage survival and decrease in total length was observed in all cultivars after exposure to lethal temperature stress. Up to 80% decrease in percentage survival was observed in PBW343 (Fig. 3). CBW12, K7903, BOBWHITE, DBW17 and C306 showed less than 50% reduction in survival. More than 50% reduction in total length (shoot and root) was observed in germinating seeds exposed to lethal temperature stress in HD2329, HD2428, RAJ4014 and PBW343 (Fig. 3). The data revealed a significant difference among different cultivars for percentage survival after lethal temperature stress. Cultivars that showed sensitivity to lethal temperatures showed considerably lesser growth at the end of 10-day recovery period.
Figure 3.
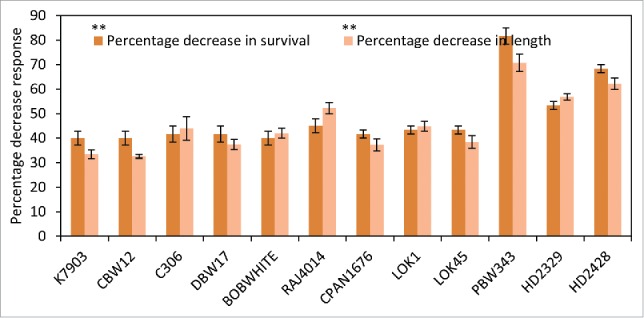
Temperature induction Response technique at germinating stage. Effect of sub-lethal temperature stress of 37°C (1.5 h) followed by a lethal stress of 51°C (3 h) on percent survival of germinating seeds (A) and reduction in recovery growth (B). The result was significant at P < 0.01.
Tolerance of cultivar toward lethal stress has been attributed to be a consequence of changing transcript levels of stress induced genes.13 The prior treatment of germinating seeds with moderately high temperature of 37°C for 1.5 h act as an inducer of the stress responsive pathways. In field grown conditions too, the plants are exposed to a gradual increase in temperature and not directly to heat shock thereby suggesting that genetic variability for thermotolerance between cultivars can be observed only after exposure to an induction temperature. Earlier reports 7 have also suggested enhanced thermotolerance of plants following exposure to a sub-lethal induction stress. Temperature Induction Response/Acquired thermotolerance had been utilized for genotyping in different crop species highlighting genotypic difference in response to heat stress.25,7
Carbon isotope discrimination (CID)
The carbon isotope composition in plants reflects the photosynthetic performance.10 Carbon isotope value was used to determine stomatal opening and closure. A low Ci reflects either a high efficiency of carboxylate ion or more commonly, a low stomatal conductance which results in diffusion limitations for both CO2 and H2O and a smaller CID. Difference in CID was observed at both 37°C and 42°C. In terms of heat, it is necessary for the stomata to remain open for heat dissipation. At 37°C an increase in CID was observed in K7903, CBW12, RAJ4014, CPAN1676, DBW17, HD2428 and PBW343 (Fig. 4). Whereas, HD2329, LOK1, BOBWHITE and LOK45 showed a decrease in carbon accumulation at 37°C. All this suggests that CID was minimally affected after heat shock at 42°C for K7903, C306, CBW12, DBW17 and RAJ4014. An increase in mean CID was observed at 42°C in K7903, suggesting toward the open state of stomata at this high temperature. At 42°C, CPAN1676, HD2329, HD2428, PBW343, LOK1, LOK45, and BOBWHITE showed a decrease in CID, with CPAN1676 and HD2329 showing a decrease of more than 5%, indicating the closed state of stomata. Under closed state of stomata, due to limited CO2, the enzyme rubisco have lesser choice to discriminate against heavy carbon and hence decreased carbon delta values.
Figure 4.
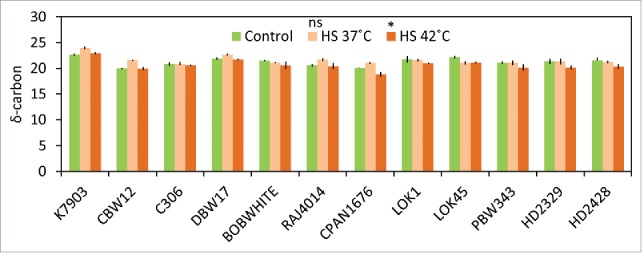
δ-Carbon isotope discrimination (CID) of wheat genotypes after thermal stress at 34°C (1 h) followed by 37°C (2 h) and/or 42°C (2 h).
Decrease in Carbon Isotope Discrimination at 42°C can be attributed to decrease in root absorption or to complete stomatal closure under water stress. Earlier reports26 have shown that higher temperature hampered the water status of leaves indicating that root hydraulic conductivity was affected. It is suggested, that at 42°C, the root hydraulic conductivity is affected, resulting in stomatal closure and hence affecting delta carbon ratio. We suggest the differential decrease in delta carbon at 42°C is due to osmotic adjustment by accumulation of osmolytes. To prove that the ability to discriminate carbon at higher temperatures is due to osmotic adjustment, we have measured the proline content, at seedling stage after giving thermal stress and between timely sown and late sown stages.
Role of proline as an osmolyte
We also measured the proline content of all cultivars after giving similar stress as that of delta carbon at seedling stage and also between timely-sown and late-sown field grown plants. Increased proline accumulation was observed after thermal stress of 37°C and 42°C at seedling stage. Cultivars that showed a relatively lower CID also showed higher proline content (Fig. 5). K7903 showed maximum proline accumulation followed by C306, BOBWHITE, CPAN1676, PBW343 whereas cultivars like HD2428, LOK45 and CBW12 showed very low proline accumulation after stress of 37°C and 42°C. Under late sown conditions, cultivars like HD2329 and HD2428 showed comparatively low proline accumulation as compared to the remaining cultivars (Fig. S3).
Figure 5.
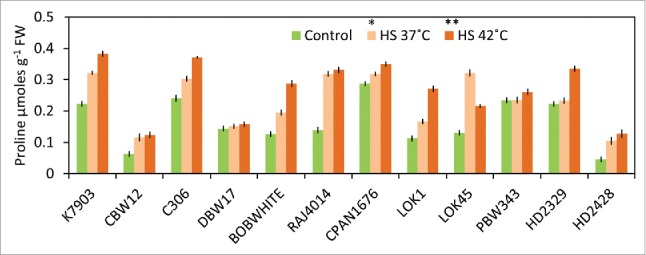
Differential accumulation of proline under thermal stress at seedling stage. Significance levels: *P < 0.05; **P < 0.01.
Under thermal stress conditions, plants try to bring down the temperature through transpiration via stomata. Under higher temperatures, to maintain the open state of stomata, plants increase osmolytes including proline accumulation that usually results in a hypertonic plant internal condition and increased suction pressure. As a highly significant interaction (P < 0.01) was observed between proline accumulation and CID, we suggests an increased accumulation of proline is acting as an osmolyte in wheat plant for maintaining suction pressure.
Heat susceptibility index
To determine the heat tolerance of different cultivars, Heat Susceptible Index (HSI) was estimated (Fig. 6). This was based on a comprehensive analysis of different physiological parameters at 2 developmental stages, i.e., seedling stage and mature plants at anthesis stage, Fv/Fm, Membrane Injury Index, Temperature Induction Response technique, Carbon Isotope Discrimination and total proline content. HSI was calculated based on earlier calculations.27 Higher Heat Susceptibility Index generally depicts high susceptibility toward heat stress. In this study we found K7903, CBW12, C306 and DBW17 showed low HSI as compared to PBW343, HD2329 and HD2428, which showed comparatively high HSI in all parameters under consideration, pointing toward the higher susceptibility of PBW343, HD2329 and HD2428 under heat stress.
Figure 6.
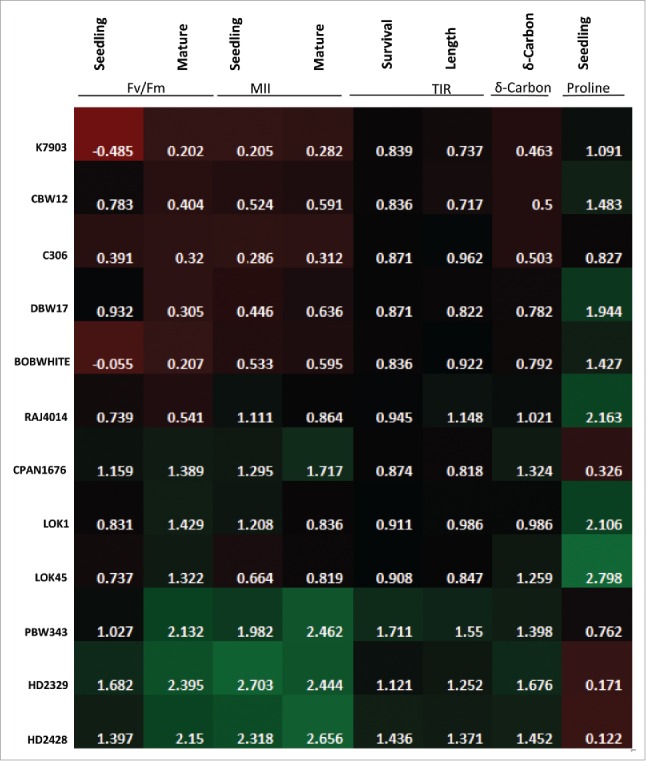
Heat map based on the Heat Susceptibility index of different biochemical and physiological parameters in different wheat cultivars.
Expression analysis of stress inducible genes
Based on HSI, thermotolerant and thermosusceptible wheat cultivars were identified (Fig. 6). Expression analysis was carried out between 2 thermotolerant cultivars C306 and K7903 and 2 thermosusceptible cultivars, HD2329 and PBW343. Ten-day-old seedlings were subjected to thermal stress of 37°C and/or 42°C for 2 h. Expression analysis was carried out via northern blotting (Fig. 7) and qRT-PCR (Fig. 8). The genes taken in this study were earlier reported to be differentially expressed in response to high temperature in our lab.28 The selection of these genes were done from different categories including HSP's, stress related genes, transcription factors, photosynthesis and ROS scavengers. Further, since wheat shows susceptibility toward terminal heat stress, especially fertilization, hence expression analysis was carried out via semi-quantitative RT-PCR between spike, anther and ovary in field grown plants under control conditions and after giving thermal stress at 37°C and/or 42°C for 2 h for spike and 37°C/2 h for anther and ovary (Fig. 9).
Figure 7.
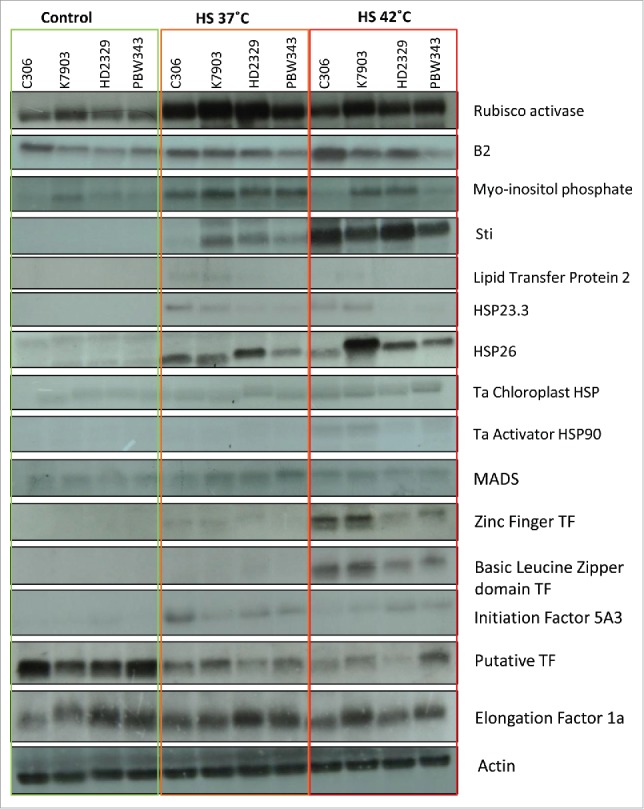
Northern analysis of heat stress induced genes in different wheat cultivars. Seedlings were given heat stress at 37°C/2 h and 42°C/2h. (HSP-Heat Shock Protein, TF-Transcription Factor).
Figure 8.
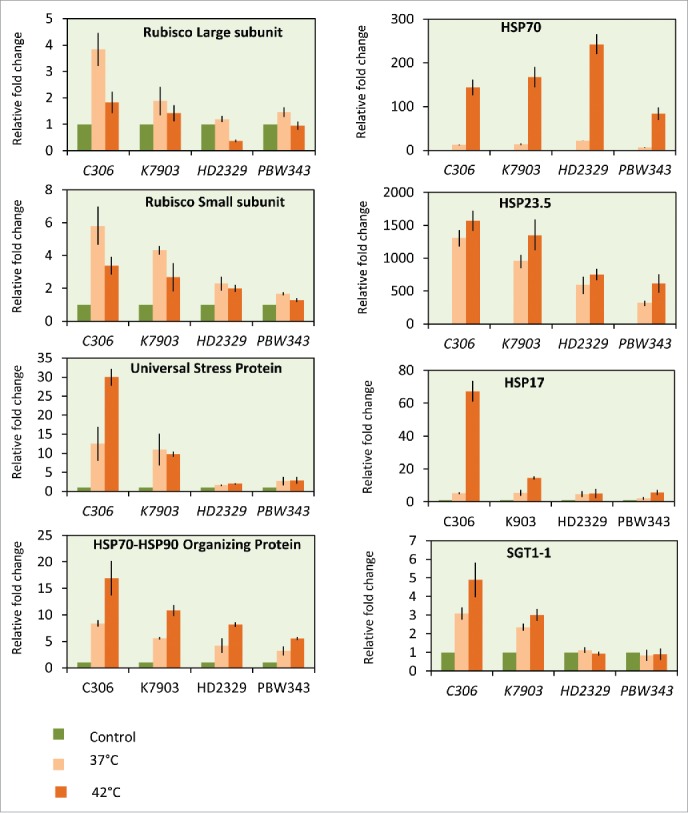
Expression analysis of stress associated genes via qRT-PCR. in selected wheat cultivars.
Figure 9.
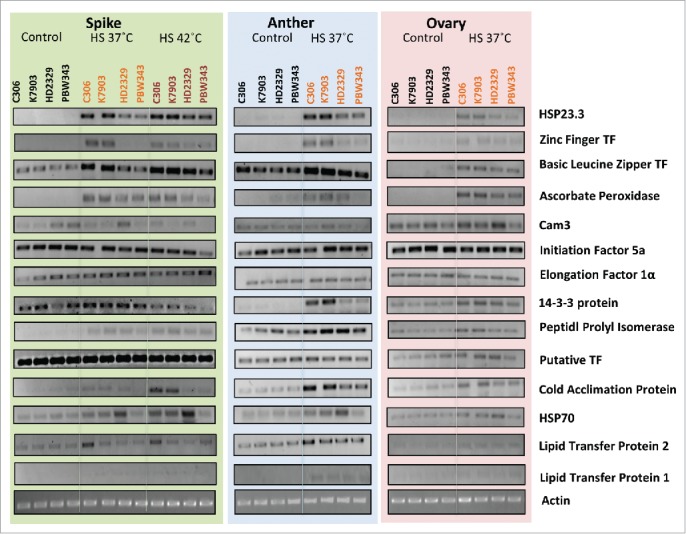
Semi-Quantitative RT-PCR analysis of heat stress induced genes in different cultivars of wheat (spike, anther and ovary). HSP-Heat Shock Protein, TF-Transcription Factor.
A comparative expression analysis of heat stress induced genes showed higher expression of stress induced genes in at least one of the tolerant cultivar as compared to the 2 thermosusceptible cultivars taken in this study.
Photosynthesis and ROS scavenging
Rubisco activase B (RcaB) showed high expression at 37°C in all cultivars but its transcript level showed relative reduction at 42°C in tolerant cultivar C306 as compared to other cultivars. A decrease in Rubisco activase in tolerant cultivar C306 at 42°C is advantageous in coping with high temperature. Deactivation of Rubisco and its activator will prevent photorespiration.29 Further a decrease rate of dark reaction of photosynthesis will result in the formation of strong proton motive force and this could contribute to tolerance toward heat stress. Under high temperature, higher ROS production due to decreased efficiency of PSII can be scavenged by APX (Ascorbate Peroxidase) as part of the ascorbate glutathione or Asada-Halliwell-Foyer pathway.30
Heat shock proteins
Since Heat Shock Proteins (HSPs) are molecular chaperones, their higher expression in tolerant cultivars can be correlated with tolerance of these cultivars toward thermal stress. HSP expression in response to heat stress seems to be a universal response with respect to temperature stress, and is observed in all organisms ranging from bacteria to humans.31 In our study, we have observed low expression of members of HSP gene family in PBW343. The low expression of HSPs might be acting as a significant player in imparting thermosusceptibility to PBW343. Studies show that acquisition of thermotolerance is directly related to the synthesis and accumulation of HSPs.31 An observable increase in HSP26 transcript was seen at 42°C in tolerant cultivars K7903 as compared to other cultivars. Overexpression lines of TaHSP26 in Arabidopsis have earlier been shown to be imparting thermotolerance.32
Lipid transfer proteins
Similar to HSP's, higher expression of Lipid Transfer Protein 2 (LTP2) was observed in floral tissue at 37°C and/or 42°C in thermotolerant cultivars C306. nsLTP gene family has earlier being associated with abiotic stress tolerance.33 Role of LTP OsC6 in post meiotic anther development have earlier been reported.34
Transcription factors
A comparatively high level of bZIP and Zinc Finger transcripts were also observed in both the thermotolerant cultivars as compared to the thermosusceptible cultivars. In plants, bZIP transcription factors form a diverse group of transcription factors which are involved in imparting stress tolerance.35 Wheat Zinc Finger proteins have also been identified in-silico and are found to be responsive to both salt and drought stress.36
Stress associated genes
Higher expression of stress induced genes was observed in flower, anther and ovary of thermotolerant cultivars as compared to thermosusceptible cultivars, except HSP70 and Cam3, which showed higher expression in thermosusceptible cultivar HD2329 as compared to the 2 tolerant cultivars C306 and K7903. Higher expression of Myo-inositol phosphate (MIPS) gene transcripts were observed in all cultivars. Myo-inositol is reported in signaling, cell wall biosynthesis and stress signaling.37 Role of Stress Inducible Protein (Sti) in stressful conditions has earlier been reported.38 Majority of the TPR containing proteins like Sti1 or its mammalian homolog (HSP70-HSP90 Organizing Protein (HOP) act as co-chaperones.39 Higher expression of Sti in all the cultivars at 42°C especially the 2 tolerant cultivars suggest its role in imparting thermal stress tolerance in wheat.
Materials and methods
Seeds of wheat (Triticum aestivum) K7903, CBW12, C306, DBW17, BOBWHITE, RAJ4014, CPAN1676, LOK1, LOK45, PBW343, HD2329 and HD2428 were obtained from Directorate of Wheat Research (Karnal, Haryana, India).
Experimental conditions
For 10-day-old seedlings, the seeds were germinated in trays (40 cm × 20 cm) with cotton bed. Mature plant studies between timely sown (on 15th November) and late sown (on 25th December) were carried out for 2 consecutive years i.e 2011 and 2012. Plants were grown in the departmental garden and experimental studies were done 90 d after sowing. Potted plants for all cultivars were also grown in green house at 22°C by mixing loam soil with farm yard manure (FYM) in 3:1 ratio. Heat stress at 37°C and 42°C were given to all plants in growth chamber (Conviron, Canada).
Temperature induction response (TIR)
For Temperature Induction Response (TIR), 20 seeds of uniform size were imbibed for 16 h in petri plates with approximately 15 ml distilled water. Seeds showing germination were subjected to lethal temperature stress of 51°C for 3 h with prior induction at sublethal temperature of 37°C for 1.5 h. Immediately after the treatment, seedlings were allowed to recover at 22 ± 1°C. On the 10th day, from the date of imbibition of seeds, the percentage survival of seeds were scored along with the total decrease in plant length.40
Chlorophyll estimation
Total chlorophyll were estimated by taking 100 mg leaf tissue in 20 ml of DMSO at 65°C for 4 h in dark for 2 growth stages namely, anthesis stage and 10-day-old seedlings. Absorbance was recorded at 645 nm and 663 nm in a UV-Vis spectrophotometer (Hitachi U-2810, Tokyo, Japan) and chlorophyll content were calculated.41
Proline estimation
Frozen samples at −80°C were grounded in liquid nitrogen and free proline content was measured by colorimetric assay accordingly.42
Membrane injury index (MII)
Membrane injury index was measured with the central portion of the primary leaf of 10-day-old seedling or leaf disc of mature plant leaf. 50 mg leaf tissue were taken per tube for both seedling and mature plant stage after giving thermal stress of 37°C and/or 42°C for 2 h. The leaves were washed with distilled water twice before dipping in 10 mL double distilled water at 25°C/100 rpm for 1 h. Electrical conductivity (EC) was measured with an EC-meter (Eutech, Singapore). Test tubes were autoclaved for 10 min at 0.10 MPa pressure to release all the electrolytes. Final EC was measured after bringing down the temperature to 25°C. Percentage Membrane Injury Index(MII%), an indicator of cell membrane thermostability (CMT) was than calculated accordingly.43
PSII efficiency
For measuring Fm, leaves were dark adapted for 30 min followed by exposure to 0.8 s saturating pulse at 8000 µmol m−2 s−1 after 30 min of dark adaptation. Fv/Fm between timely sown (15th November) and late sown (25th December) field grown plants for 2 consecutive years, namely 2011 and 2012 were measured with Junior PAM-250 (Walz, Germany) after 30 min dark adaptation.
Carbon isotope discrimination (CID)
For determination of carbon isotope discrimination, primary leaf of 10-day-old seedlings of different cultivars with treatment of 34°C/1 h followed by 37°C and/or 42°C for 2 h were detached, oven dried at 80°C till constant weight was achieved and crushed to fine powder in a mortar and pestle. The powdered samples were employed for δ-carbon discrimination at the Isotope Ratio Mass Spectrometer (IRMS) at the National Facility for Stable Isotope studies in biological science at the Department of Crop Physiology, UAS, GKVK, Bangalore, India.
RNA isolation
Total RNA was isolated from different tissues by Trizol Reagent (Sigma, USA) as per the manufacturer's protocol and were treated by on column DNase treatment to remove genomic DNA using RNA cleanup kit (Qiagen, Germany).
Northern blotting
RNA isolated from control and treated samples were resolved in 1.2% agarose gel containing formaldehyde. The gel was further washed in R.O water for 20 min and transferred to hybond N membrane (Amersham Pharmacia Biotech, UK). After PCR amplification and gel purification, probes were labeled with α32 P dATP using megaprime DNA labeling system (Amersham Pharmacia Biotech, UK) and purified through Probquant G-25 column (Amersham Pharmacia Biotech, UK). Hybridization was further performed overnight at 60°C and differential band intensity was visually observed.
Semi-quantitative RT-PCR
A two-step RT-PCR was used to study the differential expression of heat stress induced genes among the thermotolerant and thermosusceptible wheat cultivars. Total 2 µg of RNA from each sample was used to synthesize the first strand using the Superscript III first strand cDNA synthesis kit (Invitrogen) in a 20 µl reaction volume. After reaction completion, RNase H treatment was given, subsequently 80 µl MQ was added to each tube. One µl each of this diluted cDNA template was used for subsequent PCR reactions. Wheat gene specific primers used were similar to those used earlier (See also Table 1).28
Table 1.
List of semi-quantitative PCR primers used in the experiment.
| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| Ascorbate Peroxidase | F | GTAGCACGCATTCTCATCAC |
| R | TGGCGTGGGGGTGTAGAGAC | |
| HSP23.5 | F | CCAAGAAAACCGGATCCATTGTTTG |
| R | GCTCAGGGTGGTCGTGCCCAAGG | |
| Peptidyl Prolyl Isomerase | F | CGCCTGGCAACCTCAAAAC |
| R | AAGGAAGGATAGGAGTCCCGGAGTG | |
| Initiation Factor 5A3 | F | CTGGATCAAACTTGTTTCACCCAAC |
| R | GATGACCTGAAGCTTCCCACTGATG | |
| Putative Transcription Factor | F | GGAATCGAAATAGAGTATTTAAACGAC |
| R | CTGTTGGTGTCATCAAGGGCG | |
| Zinc Finger C3HC4 | F | AAGGAGTCAAAACTACAAAACACAGG |
| R | CAGGCAGATGTCTACCTGAAGGC | |
| Elongation Factor 1 α | F | CATCAACAGGGCGCATATCA |
| R | CGGCATAGTGAAGATGATTCCCAC | |
| bZIP | F | CAACGCGAACAGAGAGAGCAG |
| R | GGCTAGTGAGCATCGTGTGCC | |
| HSP70C | F | CGATACAACGCCGGCTACA |
| R | CGGCCCATCTACAAATGTGTGAAC | |
| TaCAM3 | F | ACAAGAGGTCTGGAAACACAC |
| R | CCTCGGCGAGAAGCTCACCGACGAG | |
| Cold Acclimation Protein | F | CCCGTGACCGGACCTCGATTG |
| R | CAACATCCAGGACCTGACTCTG | |
| 14-3-3 | F | CTCTCGGCCTTTGCGCCATTGG |
| R | CTCGCTTGTTCAGTGTAGATTGTAATG | |
| LTP1 | F | CACCATGGCTCGCACTGCAGC |
| R | TCAGTGGATCTTAGAGCAGTCCA | |
| LTP2 | F | CACCATGGCTCGCACTGCAGC |
| R | TCAGTGGATCTTAGAGCAGTCCA | |
| Actin | F | GATACACGCTTCCTCATGCTATCC |
| R | AGAGCCACCGATCCAGACACTG |
Real-time expression analysis
1.5 µg of the total RNA was used as template for cDNA synthesis employing the High Capacity cDNA Archive kit (Applied Biosystems, USA) and mixed with 200 nM of forward and reverse primers and SYBR Green PCR Master Mix (Agilent). The primer pairs were designed by using Primer Express 2.0 software (PE Applied Biosystems) and checked for specificity by the BLAST program with wheat sequences available in the NCBI database (Table 2). The gene specificity was verified by melting curve analysis. Normalization was done by using internal control, actin. Three independent RNA isolations (biological replicates) were used for cDNA synthesis.
Table 2.
List of qRT-PCR primers used in the experiment.
| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| HOP | F | GCATTACACAAAGGCCTTGGA |
| R | CGCTGCTCGGTTAGTGAGGTA | |
| HSP 17 | F | CAAGATGATGCGCAAGTTCGT |
| R | CACACGGCGGAGATCTTCTC | |
| USP | F | GGTCTCCGTGGTTT AAAAGTC |
| R | GGATGGCTTGGCACAGCTT | |
| Rubisco Large subunit | F | AACGAAGGGCGCGATCTT |
| R | CATTTGCAAGCTGCTCGGATA | |
| Rubisco Small subunit | F | CCTTCTCCTTGTGTTAGCATCGA |
| R | TTGCACGGATGACCATTAGG | |
| SGT1-1 | F | CAGGATGGCGCAAATATGG |
| R | GGGCTTGCTTGGCACTTCTA | |
| HSP23.3 | F | CGCGAGCGCGACATC |
| R | TGAACGGATCACGGAACACA | |
| HSP70 | F | AAGAACTGGAAGGCATTT |
| R | CCCCGGCTCCCCTGGTA | |
| Actin | F | CCTTGTTTGCGACAATGGAA |
| R | AGCCCTTGGTGCATCATCTC |
Heat susceptible index (HSI)
Heat Susceptible Index was calculated accordingly27 using the following equation HSI = (1 – Xh/X)/(1 – Yh/Y), where Xh and X are the mean of physiological trait for each cultivar under heat stressed and control conditions, respectively. Whereas, Yh and Y are the physiological mean of all genotypes under heat stress and control conditions. The values for HSI was used for generation of a heat map.
Statistical analysis
The data collected was the mean of values from at least 3 replications. The significant differences among the treatment averages were analyzed using one way ANNOVA at P < 0.05 (Supplimentary Table 1)
Conclusions
In summary, the bioassay's along with the gene expression profiling described in this paper can be useful in identification of thermotolerant and susceptible cultivars. The cultivars C306 and K7903 were identified as heat tolerant based on their expression profiling along with other physiological parameters. Further work is required for a fool proof phenotyping and in adapting the core method for screening breeding populations.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by Department of Biotechnology, Government of India, and partially by Indo-Swiss Collaboration in Biotechnology (ISCB). SH thanks Council for Scientific and Industrial Research for Junior and Senior Research Fellowships.
References
- 1.Semenov MA, Shewry PR. Modelling predicts that heat stress, not drought, will increase vulnerability of wheat in Europe. Sci Rep 2011; 1:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: An overview. Environ Exp Bot 2007; 61:199-223; http://dx.doi.org/ 10.1016/j.envexpbot.2007.05.011 [DOI] [Google Scholar]
- 3.Mangelsen E, Kilian J, Harter K, Jansson C, Wanke D, Sundberg E. Transcriptome analysis of high-temperature stress in developing barley caryopses: early stress responses and effects on storage compound biosynthesis. Mol Plant 2011; 4:97-115; PMID:20924027; http://dx.doi.org/ 10.1093/mp/ssq058 [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Zhan C, Huang B. Heat Shock Proteins in Association with heat tolerance in Grasses. Int J Proteomics 2011; 1-11; PMID:22084689; http://dx.doi.org/ 10.1155/2011/529648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asseng S, Foster I, Turner NC. The impact of temperature variability on wheat yields. Glob Change Biol 2011; 17:997-1012; http://dx.doi.org/ 10.1111/j.1365-2486.2010.02262.x [DOI] [Google Scholar]
- 6.Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. Plant Signal Behav 2006; 15:227-8; http://dx.doi.org/ 10.4161/psb.1.5.3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikanthbabu V, Ganeshkumar , Krishnaprasad BT, Gopalakrishna R, Savitha M, Udayakumar M. Identification of pea genotypes with enhanced thermotolerance using temperature induction response technique (TIR). J Plant Physiol 2002; 159:535-45; http://dx.doi.org/ 10.1078/0176-1617-00650 [DOI] [Google Scholar]
- 8.Hairat S, Khurana P. Improving photosynthetic responses during recovery from heat treatments with brassinosteroid and calcium chloride in Indian bread wheat cultivars. Am J Plant Sci 2015a; 6:1827; http://dx.doi.org/ 10.4236/ajps.2015.611184 [DOI] [Google Scholar]
- 9.Hairat S, Khurana P. Evaluation of Aegilops tauschii and Aegilops speltoides for acquired thermotolerance: Implications in wheat breeding programmes. Plant Physiol Biochem. 2015b; 95:65-74; PMID:26188500; http://dx.doi.org/ 10.1016/j.plaphy.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 10.Farquhar GD, Ehleringer IJR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol Biol 1989; 40:503-37. [Google Scholar]
- 11.Szabados l, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci 2009; 15:89-97; PMID:20036181 [DOI] [PubMed] [Google Scholar]
- 12.Maheshwari M, Joshi DK, Saha R, Nagarajan S, Gambhir PN. Transverse Relaxation Time of Leaf Water Protons and Membrane injury in Wheat (Triticum aestivum L.) in Response to high temperature. Ann Bot 1999; 84:741-745; http://dx.doi.org/ 10.1006/anbo.1999.0974 [DOI] [Google Scholar]
- 13.Natu PS, Savitha M, Babu S, Udayakumar M. Heat shock response of wheat cultivars differing in thermotolerance. Indian JPlant Physiol 2007; 12:327-36. [Google Scholar]
- 14.Mathur S, Jajoo A, Mehta P, Bharti S. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol 2009; 13:1-6; PMID:21143718; http://dx.doi.org/ 10.1111/j.1438-8677.2009.00319.x [DOI] [PubMed] [Google Scholar]
- 15.Chatrath R, Mishra B, Ferrara GO, Singh SK, Joshi AK. Challenges to wheat production in South Asia. Euphytica 2007; 157:447-56; http://dx.doi.org/ 10.1007/s10681-007-9515-2 [DOI] [Google Scholar]
- 16.Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. J Exp Bot 2000; 51:659-68; PMID:10938857; http://dx.doi.org/ 10.1093/jexbot/51.345.659 [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Sharkey TD. Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth Res 2009; 100:29-43; PMID:19343531; http://dx.doi.org/ 10.1007/s11120-009-9420-8 [DOI] [PubMed] [Google Scholar]
- 18.Feller U, Crafts-Brandner SJ, Salvucci E. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase activase-mediated activation of Rubisco. Plant Physiol 1998; 116:539-46; PMID:9490757; http://dx.doi.org/ 10.1104/pp.116.2.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagaram M, Burns JK. Leaf chlorophyll fluorescence parameters and huanglongbing. J Am Soc Hortic Sci 2009; 134:194-201. [Google Scholar]
- 20.Fokar M, Blum A, Nguyen HT. Heat tolerance in spring wheat. II. Grain filling. Euphytica 1998; 104:9-15; http://dx.doi.org/ 10.1023/A:1018322502271 [DOI] [Google Scholar]
- 21.Routaboul JM, Skidmore C, Wallis JG, Browse J. Arabidopsis mutants reveal that short- and long-term thermotolerance have different requirements for trienoic fatty acids. J Exp Bot 2011; 63:1435-1443; PMID:22140238; http://dx.doi.org/ 10.1093/jxb/err381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol 2004; 4:17; PMID:15377388; http://dx.doi.org/ 10.1186/1471-2229-4-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishida A, Toma T, Marjenah M. Leaf gas exchange and canopy structure under wet and drought years in Macaranga conifera, a tropical pioneer tree. Ecol Stud 2000; 140:129-42; http://dx.doi.org/ 10.1007/978-4-431-67911-0_12 [DOI] [Google Scholar]
- 24.Muller P, Li XP, Niyogi KK. Non-photochemical quenching: A response to excess light energy. Plant Physiol 2001; 125:1558-66; PMID:11299337; http://dx.doi.org/ 10.1104/pp.125.4.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvaraj MG, Burow G, Burke JJ, Belamkar V, Puppala N, Burow MD. Heat stress screening of peanut (Arachis hypogaea L.) seedlings for acquired thermotolerance. Plant Growth Regul 2011; 65:83-91; http://dx.doi.org/ 10.1007/s10725-011-9577-y [DOI] [Google Scholar]
- 26.Wahid A, Close TJ. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biologia Plantarum 2007; 51:104-9; http://dx.doi.org/ 10.1007/s10535-007-0021-0 [DOI] [Google Scholar]
- 27.Fisher RA, Maurer R. Drought resistance in wheat cultivars I Grain yield response. J Agr Res 1978; 29:898-907. [Google Scholar]
- 28.Chauhan H, Khurana N, Tyagi AK, Khurana JP, Khurana P. Identification and characterization of high temperature stress responsive genes in bread wheat (Triticum aestivum L.) and their regulation at various stages of development. Plant Mol Biol 2010; 75:35-51; PMID:20972607; http://dx.doi.org/ 10.1007/s11103-010-9702-8 [DOI] [PubMed] [Google Scholar]
- 29.Sharkey TD. Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 2005; 28:269-77; http://dx.doi.org/ 10.1111/j.1365-3040.2005.01324.x [DOI] [Google Scholar]
- 30.Mittler R, Poulos TL. Ascorbate peroxidase In Antioxidants and Reactive Oxygen Species in Plants (ed. Smirnoff N.) pp. 87-100. Blackwell Publishing, Oxford, 2005; UK. [Google Scholar]
- 31.Vierling E. The role of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol Biol 1991; 42:579-620; http://dx.doi.org/ 10.1146/annurev.pp.42.060191.003051 [DOI] [Google Scholar]
- 32.Chauhan H, Khurana N, Nijhavan A, Khurana JP, Khurana P. The wheat chloroplastic small heat shock protein (sHSP26) is involved in seed maturation and germination and imparts tolerance to heat stress. Plant Cell Environ 2012; 35:1912-31; http://dx.doi.org/ 10.1111/j.1365-3040.2012.02525.x [DOI] [PubMed] [Google Scholar]
- 33.Cameron KD, Teece MA, Smart LB. Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol 2006; 140:176-83; PMID:16361524; http://dx.doi.org/ 10.1104/pp.105.069724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D, Liang W, Yin C, Zong J, Gu F, Zhang D. OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice. Plant Physiol 2010; 154:149-62.; PMID:20610705; http://dx.doi.org/ 10.1104/pp.110.158865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K. ARED1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances stress tolerance in Arabidopsis. Plant Cell 2005; 17:3470-88; PMID:16284313; http://dx.doi.org/ 10.1105/tpc.105.035659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kam J, Gresshoff PM, Shorter R, Xue GP. The Q-type C2H2 zinc finger subfamily of transcription factors in Triticum aestivum is predominantly expressed in roots and enriched with members containing an EAR repressor motif and responsive to drought stress. Plant Mol Biol 2008; 67:305-22; PMID:18347915; http://dx.doi.org/ 10.1007/s11103-008-9319-3 [DOI] [PubMed] [Google Scholar]
- 37.Downes CP, Gray A, Lucocq JM. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol 2005; 15:259-68; PMID:15866030; http://dx.doi.org/ 10.1016/j.tcb.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 38.Nicolet CM, Craig EA. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol 1989; 9:3638-46; PMID:2674681; http://dx.doi.org/ 10.1128/MCB.9.9.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearl LH, Prodromou C. Structure and Mechanism of the Hsp90 molecular chaperone machinery. Ann Rev Biochem 2006; 75:271-94; PMID:16756493; http://dx.doi.org/ 10.1146/annurev.biochem.75.103004.142738 [DOI] [PubMed] [Google Scholar]
- 40.Senthil-Kumar M, Srikanthbabu V, Mohan Raju B, Ganeshkumar , Shivaprakash N, Udayakumar M. Screening of inbred lines to develop a thermotolerant sunflower hybrid using the temperature induction response (TIR) technique: a novel approach by exploiting residual variability. J Exp Bot 2003; 54:2569-78; PMID:14565951; http://dx.doi.org/ 10.1093/jxb/erg278 [DOI] [PubMed] [Google Scholar]
- 41.Hiscox JD, Israelstam GF. A method for extraction of chlorophyll from leaf tissue without maceration. Can J Bot 1979; 57:1332-4; http://dx.doi.org/ 10.1139/b79-163 [DOI] [Google Scholar]
- 42.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil 1973; 39:205-7; http://dx.doi.org/ 10.1007/BF00018060 [DOI] [Google Scholar]
- 43.Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. Protective role of antioxidant enzymes under high temperature stress. Plant Sci 2006; 171:382-8; PMID:22980208; http://dx.doi.org/ 10.1016/j.plantsci.2006.04.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


