Abstract
Objectives
Based on data from clinical practice, we evaluated the effectiveness and safety of switching to abacavir/lamivudine plus rilpivirine (ABC/3TC+RPV) treatment in virologically suppressed HIV-1-infected patients.
Methods
We performed a multicenter, non-controlled, retrospective study of HIV-1-infected patients who switched treatment to ABC/3TC+RPV. Patients had an HIV-RNA <50 copies/mL for at least 24 weeks prior to changing treatments. The primary objective was HIV-1 RNA <50 copies/mL at week 48. Effectiveness was analyzed by intention-to-treat (ITT), missing = failure and on-treatment (OT) analyses. The secondary objectives analyzed were adverse effects changes in renal, hepatic or lipid profiles, changes in CD4+ cell count and treatment discontinuations.
Results
Of the 205 patients included, 75.6% were men and the median age was 49. At baseline, before switching to ABC/3TC+RPV, median time since HIV diagnosis was 13.1 years, median time with undetectable HIV-1 RNA was 6.2 years and median time of previous antiretroviral regimen was 3.1 years (48.3% patients were taking efavirenz and ABC/3TC was the most frequent backbone coformulation in 69.7% of patients). The main reasons for switching were drug toxicity/poor tolerability (60.5%) and simplification (20%). At week 48, the primary objective was achieved by 187 out of 205 (91.2%) patients by ITT analysis, and 187 out of 192 (97.4%) patients by OT analysis. The CD4+ lymphocyte count and CD4+ percentage increased significantly from baseline to week 48 by a median of 48 cells/μL (−50 to 189) and 1.2% (−1.3% to 4.1%), respectively, P<0.001. Thirty-eight adverse events (AE) were detected in 32 patients. Of these, 25 had no clear association with treatment. Three patients interrupted therapy due to AE. We observed a decrease in all lipid parameters, P<0.001, and a slight improvement in the glomerular filtration rate, P<0.01. Therapy was considered to have failed in 18 patients owing to virological failure (5 [2.4%]), toxicity/poor tolerability (4 [2%]), clinical decision (3 [1.5%]), loss to follow-up (3 [1.5%]), death (1 [0.5%]), and no clinical data (2 [1%]).
Conclusions
The results of this study confirms that ABC/3TC+RPV is an effective, safe, and cost-effective option for the treatment of patients with virologically stable HIV-1 infection.
Introduction
First-line combined antiretroviral therapies (cART) are currently highly effective [1–2]. However, toxicity in the short and long term still arises as a main concern for clinicians managing HIV infection.
Therapy regimens are sometimes switched to improve or maintain the patient’s quality of life and, in a few cases, to reduce treatment costs [3]. Strategies used when switching cART regimens are aimed at improving a patient’s quality of life without compromising treatment effectiveness. This is accomplished through the discontinuation of toxic drugs, the identification of poorly tolerated drugs and the management of drug interactions [4–6].
The combination of abacavir/lamivudine (ABC/3TC) is widely used in both clinical trials and real-world practice, especially when clinicians wish to avoid the toxicity of older nucleoside/nucleotide analogues, such as zidovudine, didanosine, stavudine and tenofovir (TDF) [7–9].
Based on the ECHO and THRIVE clinical trials [10–14] the second-generation non-nucleoside reverse-transcriptase inhibitor (NNRTI) rilpivirine (RPV) has been approved for treatment-naïve HIV-1-infected patients. Information about its combination with ABC/3TC in clinical trials is scarce, although favorable efficacy and safety results were observed in a small group of 35 treatment-naïve patients in the THRIVE clinical trial [12]. Clinical studies with RPV as a switching strategy have only been performed in combination with tenofovir/emtricitabine (TDF/FTC), demonstrating high efficacy, good tolerability profiles and high adherence [15–18].
The combination ABC/3TC+RPV might simultaneously address the issues of effectiveness, tolerability and ease of administration as an alternate therapy in routine clinical practice.
Given the lack of evidence on switching to ABC/3TC+RPV, it is important to test the feasibility of this strategy. We carried out a study to explore the effectiveness and safety of switching to a regimen comprised of a fixed-dose ABC/3TC and RPV in virologically suppressed HIV-1-infected patients.
Methods
Study design and patients
This was a multicenter, non-controlled and retrospective study of HIV-1-infected patients switching to ABC/3TC+RPV conducted in 13 hospitals in Spain. All included patients had to fulfill the following criteria: i) age ≥18 years, ii) documented HIV-1 infection, iii) switch from another therapy regimen to coformulated ABC/3TC (600/300 mg fixed-dose combination) and RPV (25 mg) once daily for any reason from March 2013 to March 2014 and iv) serum HIV RNA <50 copies/mL for at least 24 weeks before switching to ABC/3TC+RPV. Analysis was carried out in 2015, to ensure that the final patient included in 2014 had at least 48 weeks of follow up. Patients tested negative for HLA*B5701 before initiating ABC/3TC+RPV.
Eligible individuals were identified by a systematic search of the databases of each center. Data for the study was collected retrospectively from patient´s medical records, anonymized and entered into an electronic database. The information collected included: baseline demographic and HIV-related data, comorbidities, adverse events and laboratory results (blood count, biochemical parameters including lipid profile and liver and kidney function, CD4+ lymphocyte count, and HIV RNA), at baseline and thereafter according to the routine clinical protocols in each hospital (every 12 to 24 weeks), reasons for treatment discontinuation in patients who stopped or changed therapy, and results of genotypic resistance testing after virological failure, when available.
The study protocol was approved by the Hospital Universitario Gregorio Marañón Ethics Committee with code SEI-RIL-2014-02, in accordance with the principles of the Declaration of Helsinki 2008. The study required local approval of several other Clinical Research Ethics Committees: Hospital Universitario Vall d´Hebrón Ethics Committee, Hospital de Mataró-Consorcio Sanitario del Maresme Ethics Committee, Hospital Universitario de Bellvitge Ethics Committee, Hospital Clínico Universitario de Valencia Ethics Committee and Ethics Committee of the province of Huelva.
Analysis with a view to scientific publication was based on anonymized routine clinical data, thus obviating the need for written informed consent.
Outcomes
The primary objective was the percentage of patients who maintained virological suppression (HIV-1 RNA <50 copies/mL) after 48 weeks of treatment. This outcome was analyzed using intention to treat (ITT), missing = failure and on treatment (OT) analyses. Treatment was considered to have failed when any of the following events occurred: virological failure (VF), defined as two consecutive HIV RNA measurements >50 copies/mL or a single measurement of >50 copies/mL if treatment was changed afterwards; treatment interruption by patient; change of treatment regimen due to non-VF reasons and incomplete data. Viral load was evaluated 3 or 4 times during the 48-week period of the study to ascertain virologic control, according to the routine clinical protocols in each hospital. The secondary objectives were adverse events, changes in CD4 count, lipid, hepatic or renal profile changes, treatment discontinuations and reasons for discontinuations.
Statistical analysis
All analyses were carried out using IBM SPSS Statistics for Windows®, Version 21.0 (SPSS, Chicago, IL, USA).
We analyzed the variables available at baseline and at 48 weeks. Variables that did not follow a normal distribution were described using the median and interquartile range (IQR). Nominal variables were described as numbers and percentages. Paired samples were compared using the t test (normally distributed variables) and the Wilcoxon test (non-normally distributed variables). Normality of the variables was tested with the Kolmogorov-Smirnov-Lilliefords test. The association between qualitative variables was assessed using the χ2 test when the sample was sufficiently large or Fisher's exact test when it was not. The 95% confidence intervals were calculated based on the Newcombe-Wilson hybrid score.
For the primary outcome (ITT analysis), we included all registered patients (incomplete or missing patients were defined as failures). We also performed an analysis excluding patients whose treatment was discontinued for non-virological reasons (OT analysis).
Results
The study population comprised 205 patients. Baseline patient characteristics are summarized in Table 1. Men accounted for 76% of the sample, median age was 49 years, a quarter of patients had prior AIDS-defining conditions, and a fifth were co-infected with hepatitis C virus (HCV). At baseline, median CD4+ lymphocyte count was 667 cells/μL, and the median CD4+ lymphocyte nadir was 198 cells/μL.
Table 1. Baseline characteristics of the study patients.
| (n = 205) | |
|---|---|
| Age (years); median (IQR) | 49 (41–54) |
| Gender; n (%) | |
| Male | 155 (75.6) |
| Female | 50 (24.4) |
| HIV risk factors; n (%) | |
| Sexual relations between MSM | 62 (30.2) |
| Heterosexual relations | 65 (31.7) |
| IDU | 61 (29.7) |
| Other/Unknown | 17 (8.3) |
| Baseline CD4; median (IQR) | |
| CD4 count (cells/μ) | 667 (471–870) |
| CD4% | 31 (23–38) |
| Nadir CD4 (cells/μ); median (IQR) | 198 (87–288) |
| AIDS diagnosis; n (%) | 54 (26.3) |
| Time since HIV diagnosis (years); median (IQR) | 13.1 (6.1–18.7) |
| Months since undetectable viral load (<50 copies/mL); median (IQR) | 74.8 (35.8–117.0) |
| Comorbidities; n (%) | |
| HBV (HbsAg +) | 4 (2) |
| HCV (PCR +) | 41 (20) |
| Hypertension | 44 (22.5) |
| Diabetes mellitus | 19 (9.3) |
| Dyslipidemia | 59 (28.8) |
| Ischemic heart disease | 12 (5.9) |
| Kidney disease | 10 (4.9) |
| Reasons for switching; n (%) | |
| Drug toxicity/tolerability | 124 (60.5%) |
| Regimen simplification | 41 (20%) |
| Physician’s criteria* | 23 (11.2%) |
| Cost savings | 3 (1.5%) |
| Unknown reasons | 14 (6.8%) |
| Previous cART; n (%) | |
| Nucleos(t)ide | |
| Abacavir | 144 (70.2) |
| Tenofovir | 53 (25.8) |
| Zidovudine | 11 (5.4) |
| Protease inhibitors | |
| Atazanavir | 24 (11.7) |
| Darunavir (monotherapy: 2) | 22 (10.7) |
| Lopinavir (monotherapy: 2) | 9 (4.4) |
| Other | 8 (3.9) |
| Non-nucleoside | |
| Efavirenz | 99 (48.3) |
| Etravirine | 13 (6.3) |
| Nevirapine | 6 (2.9) |
| Rilpivirine | 4 (1.9) |
| Integrase inhibitor | |
| Raltegravir | 3 (1.5) |
MSM: men who have sex with men; IDU: injection drug user; AIDS: acquired immunodeficiency syndrome; HBV: hepatitis B virus; HCV: hepatitis C virus; cART: combined antiretroviral treatment.
* Potential drug interactions, better profile, a personal decision.
At baseline, before switching to ABC/3TC+RPV, median time from HIV diagnosis was 13.1 years, median time from first antiretroviral treatment was 9.6 years, median time of previous antiretroviral regimen was 3.1 years and median time with undetectable HIV-1 RNA was 6.2 years.
Prior to switching, 63 patients (30.7%) were taking a ritonavir-boosted protease inhibitor (bPI) and 122 patients (59.4%) a NNRTI (efavirenz, EFV, 48.3%). TDF was part of the previous backbone in 53 patients (25.8%) and ABC in 144 (70.2%). Of note, ABC/3TC was the most frequent nucleoside reverse transcriptase inhibitor (NRTI) backbone coformulation before switching (69.7%). The main reasons for switching were drug toxicity/tolerability in 124 cases (60.5%) and regimen simplification in 41 (20%). In the toxicity/tolerability group of patients, 82 (65.3%) were on EFV-based regimens and 25 (20.2%) on bPI-based regimens. Regimen simplification (doses per day or number of pills) resulted primarily from bPI and etravirine-based regimens (51.2% and 22%, respectively).
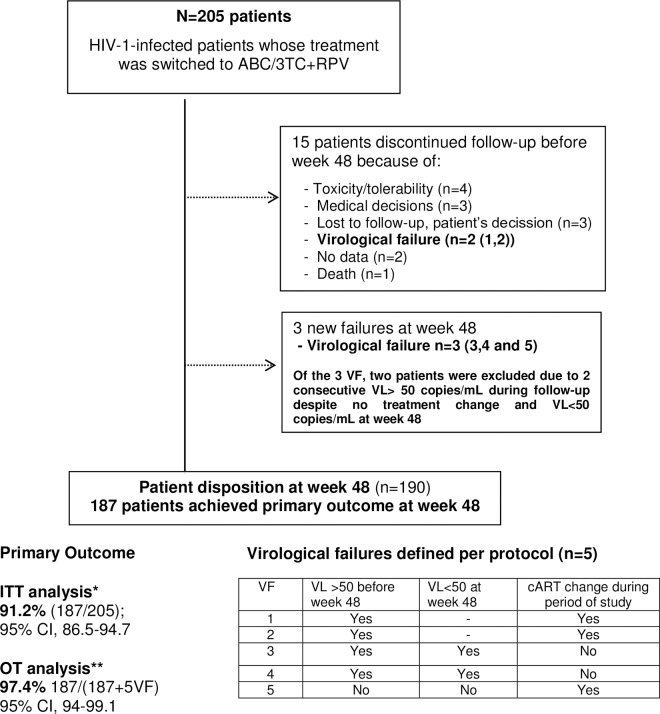
The primary objective was observed in 187 out of 205 patients (91.2% [95% CI, 86.5–94.7]) by ITT analysis. When patients who had discontinued therapy for non-virological reasons were excluded, 187 out of 192 patients (97.4% [95% CI, 94–99.1]) reached the primary outcome per protocol (OT analysis) (Fig 1). Therapy failed in 18 patients (8.8%), including 15 who discontinued ABC/3TC+RPV. The reasons for discontinuation were toxicity/poor tolerability (4 [2%]), virological failure (2 [1%]), physician’s decision (3 [1.5%]), loss of clinical patient monitoring or patient’s decision (3 [1.5%]), death (1 [0.5%]), and no clinical data at the evaluation time point (2 [1%]). At week 48, five patients (2.4%) experienced protocol-defined virological failure. Of these, two continued with ABC/3TC+RPV after two consecutive low-level detectable HIV-1 RNA determinations (at weeks 6 and 24), and both had undetectable HIV-1 RNA at week 48. The other three patients had detectable HIV-1 RNA during the study period (two patients between weeks 4 and 8, and one patient at week 48). All three patients underwent resistance testing at the time of virological failure. Genotyping could not be performed in one patient due to an HIV-1 RNA level below 200 copies/mL, and in the other two patients, the resistance mutations found were as follows: NRTI mutations (M41L, M184V, T215F, K219Q) and NNRTI mutations (A98G, K103N, K238T) in one patient; and NRTI mutations (A62V, M184V), a NNRTI mutation (E138K), and a bPI mutation (L33V) in the other patient. Before switching to ABC/3TC+RPV, the available genotypic resistance tests did not show resistance mutations that would have compromised this treatment strategy. Therefore, no patients were excluded from the study for previous resistance mutations.
Fig 1. Study Flowchart.
* ITT analysis: including all registered patients (incomplete or missing patients were included with patients whose treatment had failed). **OT analysis: excluding patients who discontinued treatment for non virological reasons. VF: virological failure, VL: viral load, cART: combined antiretroviral treatments.
The median CD4+ lymphocyte count and median CD4+ percentage increased significantly from baseline to week 48 by a median of 48 cells/μL (−50 to 189) and 1.2% (-1.3% to 4.1%), respectively, P<0.001. In patients with a previous AIDS diagnosis (CDC stage C3), the CD4+ lymphocyte count and CD4+ percentage also increased significantly by a median of 50 cells/μL (−19 to 182.5) and 1.7% (−0.5% to 4.3%), respectively, P<0.001.
In this study cohort, 38 adverse events (AEs) were registered among 32 patients (15.6%) (Table 2). Clinical intervention was unnecessary for 33 of the events, and 25 resolved. Medication was discontinued in three (1.5%) of the five patients who required clinical intervention. The reasons for discontinuation were insomnia/depression, dizziness, and epigastralgia. Severe AEs were not related to medication, but moderate AEs could have been associated to the treatment in 3 patients. In 25 events, clinicians did not find a clear correlation between the event and antiretroviral therapy.
Table 2. Overview of adverse events (AEs).
| Summary of AE | |
| Patients with ≥ 1 AE | 32 |
| Total number of AE | 38 |
| Patients with ≥1 Grade 3–4 AE | 4 |
| Total number of Grade 3 or 4 AE | 5 |
| Patients with serious AE | 1 |
| Total number of serious AE | 1 |
| Discontinuation due to AE | 3* |
| Deaths | 1 |
| Types of AE | |
| Digestive | 11 |
| Neuropsychiatric | 7 |
| Infectious | 7 |
| Systemic | 7 |
| Dermatological | 3 |
| Cardiovascular | 3 |
| Severity of adverse events | |
| Severe (fatal or life-threatening) | 1 |
| Moderate (requires medical treatment or hospitalization) | 5 |
| Mild (symptoms do not require major medical intervention) | 32 |
| Association with combination ABC/3TC+RPV | |
| Related | 2 |
| Likely | 5 |
| Possible | 3 |
| Unlikely | 3 |
| Not related | 25 |
* Insomnia/depression, dizziness, epigastralgia.
Median changes in lipid, hepatic, and renal profiles were analyzed at week 48 (Table 3). In this cohort of patients a small but statistically significant decrease in lipid levels were observed: total cholesterol (T-chol), with a percent change of −9.1% (median change, IQR, −18 [−47 to 3] mg/dL); low-density lipoprotein cholesterol (LDL-chol), −8.9% (−10.8 mg/dL [−43 to 9 mg/dL]); high-density lipoprotein cholesterol (HDL-chol), −7% (−4 mg/dL [−9 to 1 mg/dL]) and triglycerides (TG), −15% (−19 mg/dL [−51 to 12 mg/dL]); P<0.001 for all differences. There was no significant difference in lipid profiles in patients previously on a bPI-based regimen, but patients previously on an EFV-based regimen presented changes in T-chol, −15.4% (−29.6 mg/dL [−55.5 to −9 mg/dL], P<0.01), LDL-chol -14.6% (−20.5 mg/dL [−47 to 1.6 mg/dL], P<0.01), HDL-chol, −9.8% (−5 mg/dL [−9 to 0 mg/dL], P<0.01) and TG −20.3% (−28 mg/dL [−55 to 1.2 mg/dL], P<0.01).
Table 3. Laboratory values at baseline and at week 48.*.
| LIPID PROFILE | N | Median | P. 25 | P. 75 | p | |
|---|---|---|---|---|---|---|
| Total cholesterol | Baseline (mg/dL) | 192 | 202.9 | 178.0 | 233.1 | |
| At week 48 (mg/dL) | 179 | 186.0 | 158.0 | 209.0 | ||
| Change | 167 | −18.0 | −47.0 | 3.0 | <0.001$ | |
| Percentage change | 167 | −9.1 | −22.1 | 1.6 | ||
| High-density lipoprotein cholesterol | Baseline (mg/dL) | 151 | 49.0 | 40.2 | 62.0 | |
| At week 48 (mg/dL) | 152 | 46.0 | 39.0 | 58.0 | ||
| Change | 123 | −4.0 | −9.0 | 1.0 | <0.001¥ | |
| Percentage change | 123 | −7.0 | −17.1 | 2.0 | ||
| Low-density lipoprotein cholesterol | Baseline (mg/dL) | 144 | 123.7 | 93.5 | 153.5 | |
| At week 48 (mg/dL) | 146 | 113.8 | 91.0 | 135.0 | ||
| Change | 118 | −10.8 | −43.0 | 9.0 | <0.001$ | |
| Percentage change | 118 | −8.9 | −27.1 | 10.7 | ||
| Triglycerides | Baseline (mg/dL) | 190 | 134.5 | 84 | 204 | |
| At week 48 (mg/dL) | 180 | 108.0 | 75.5 | 163.5 | ||
| Change | 167 | −19.0 | −51.0 | 12.0 | <0.001¥ | |
| Percentage change | 167 | −15.0 | −33.5 | 12.3 | ||
| LIVER PROFILE | ||||||
| Aspartate aminotransferase | Baseline (IU/L) | 164 | 25.0 | 20.0 | 34.0 | |
| At week 48 (IU/L) | 155 | 24.0 | 20.0 | 31.0 | ||
| Change | 140 | 0.5 | −4.0 | 4.0 | 0.953¥ | |
| Alanine aminotransferase | Baseline (IU/L) | 189 | 25.8 | 17.0 | 40.0 | |
| At week 48 (IU/L) | 181 | 25.0 | 18.0 | 40.0 | ||
| Change | 167 | 1.0 | −9.0 | 8.0 | 0.931¥ | |
| Gamma-glutamyl transpeptidase | Baseline (IU/L) | 167 | 39.0 | 23.0 | 71.4 | |
| At week 48 (IU/L) | 165 | 29.0 | 21.0 | 53.0 | ||
| Change | 143 | −7.0 | −26.0 | 2.0 | <0.001¥ | |
| Alkaline phosphatase | Baseline (IU/L) | 177 | 87.0 | 68.0 | 108.0 | |
| At week 48 (IU/L) | 151 | 75.0 | 62.0 | 90.0 | ||
| Change | 145 | −12.0 | −29.0 | -1.0 | <0.001¥ | |
| Bilirubin | Baseline (mg/dL) | 154 | 0.5 | 0.4 | 0.70 | |
| At week 48 (mg/dL) | 139 | 0.5 | 0.4 | 0.6 | ||
| Change | 122 | 0.0 | −0.1 | 0.1 | 0.812¥ | |
| RENAL PROFILE | ||||||
| Estimated glomerular filtration rate | Baseline | 127 | 82.0 | 71.0 | 97.0 | |
| At week 48 | 147 | 89.4 | 74.6 | 99.6 | ||
| Change | 107 | 1.1 | −4.8 | 10.0 | <0.01¥ | |
| CD4+ LYMPHOCYTE COUNT | ||||||
| Baseline (cells/μL) | 197 | 667 | 471 | 870 | ||
| Absolute CD4 | At week 48 (cells/μL) | 174 | 741 | 505 | 942 | |
| Change | 167 | 48 | −50 | 189 | <0.001¥ | |
| Baseline | 199 | 31.0 | 23.7 | 38.0 | ||
| % CD4 | At week 48 | 175 | 34.0 | 27.0 | 38.8 | |
| Change | 170 | 1.2 | −1.3 | 4.1 | <0.001¥ | |
* Taking into account the retrospective nature of the study and according to the different routine clinical protocols in each hospital not all the patients had a baseline or 48-week lipid, hepatic or renal profiles.
$ p value Paired Samples T-test (parametric variables).
¥ p value Wilcoxon Signed-Rank test (nonparametric variables).
No changes were observed in liver profiles (aspartate aminotransferase, alanine aminotransferase, and bilirubin) at week 48, except for a slight decrease in gamma-glutamyl transpeptidase and alkaline phosphatase levels. This decrease was especially significant in the group of patients previously on an EFV-based regimen, median gamma-glutamyl transpeptidase of −18.5 IU/L (−41.7 to −7 IU/L) and a median alkaline phosphatase of −16.4 IU/L (−35 to −5 IU/L), P<0.01.
The estimated glomerular filtration rate (eGFR; Modification of Diet in Renal Disease equation) increased by a median (IQR) of 1.1 mL/min/1.73 m2 (−4.8 to 10) by week 48, P<0.01. Most of the patients who attained an increase in eGFR were taking TDF before switching (n = 55) and these patients had a median increase in eGFR of 7 mL/min/1.73 m2 (−5.8 to 13.1) at the end of follow-up, P<0.04. There were no significant changes in eGFR in patients previously on EFV or bIP-based regimens.
Discussion
To our knowledge, there is little data showing that switching to ABC/3TC+RPV is effective, safe, and well tolerated in virologically suppressed HIV-1-infected patients. Previous studies have demonstrated that when switched from EFV+FTC/TDF, RPV+FTC/TDF is not inferior in terms of efficacy, has a better tolerability profile (resolving neuropsychiatric toxicity), and patients have a high adherence to this regimen [17, 18]. In terms of effectiveness, our findings also revealed a high virological suppression rate (above 90% at 48 weeks of follow-up), which in fact is similar to other reported studies on treatment switching [15–20].
Of the 205 patients included, 18 experienced treatment failure, although only five experienced protocol-defined virological failure during the 48 weeks of the study period. Interestingly, two of those patients presenting as virological failures had two consecutive HIV-1 RNA values between 50 and 200 copies/mL and later achieved and maintained HIV-1 RNA <50 copies/mL without having to modify their treatment. Resistance testing performed on samples from the other three patients who experienced virological failure revealed mutations that may have compromised the efficacy of the regimen in two cases. A high proportion of patients maintained an undetectable viral load at week 48 after the switch, probably because of the excellent tolerability profile of ABC/3TC+RPV and the good adherence motivated by the low pill burden and once-daily regimen.
We documented a median increase of 48 cells/μL (7.2%) in the CD4+ lymphocyte count but only a 1.2% increase in the percentage of CD4+ cells. Immune function was largely unchanged given the small and clinically insignificant change in CD4%. The absolute CD4 change may have been driven by increases in white blood cells or total lymphocyte count. Further follow-up would be needed in order to observe potential clinically significant changes.
The frequency of adverse events was low (15.6%). The attending physicians considered most events to be unrelated to the study medication, and most AES resolved. Only three patients discontinued the regimen owing to adverse events. The low number of discontinuations due to poor tolerability indicates that the regimen was well tolerated.
A small irrelevant decrease was observed in the serum concentrations of all fasting lipids. This observation was more evident in the group of patients with a previous EFV-based regimen, comprising nearly half of the patients who switched to RPV+ABC/3TC. This finding is similar to findings described in other studies with EFV, in naïve or treated patients [21, 22]. No significant changes were observed in the subgroup of previous bPI-based regimens despite findings in other studies [23].
Additionally, serum biochemistry analysis revealed a small but statistically significant decrease in alkaline phosphatase and gamma-glutamyl transpeptidase levels, especially among the group of patients with a previous EFV-based regimen. EFV is known to produce enzyme induction in the liver through CYP3A4 and has been described in the EFV data sheet in 7% of the patients who receive 600 mg of this drug [24].
Despite a significant median increase in eGFR of 1.1 mL/min/1.73 m2, no clinical relevance could be established. This increase is much more striking in the subgroup of patients who switched to ABC/3TC+RPV from a regimen containing TDF with a median increase in the eGFR of 7 mL/min/1.73 m2. This finding may reflect either a decrease in the inhibition of the tubular secretion of creatinine or an improvement in renal function after removing TDF from the regimen [25, 26]. Future availability of tenofovir-alafenamide (TAF) resulting in a better renal profile could avoid the potential renal damage observed in some patients using TDF [27].
Our study has several limitations. First, it was a retrospective study. Second, there were differences in clinical protocols and visit timetables between the study centers. It also lacked a comparative arm.
Nevertheless, our findings are relevant, because ABC/3TC+RPV proved to be highly effective in virologically suppressed HIV-1-infected patients. Therefore, this combination could potentially form the basis for a switching strategy, especially when TDF cannot be taken because of toxicity issues, resistance, or clinical reasons. Safety is yet another key advantage of ABC/3TC+RPV. For example, adverse events are infrequent and a regimen free of TDF has potential long-term benefits for the kidneys and bones.
In addition, this switching strategy could be cheaper than the most common antiretroviral regimens that include protease and integrase inhibitors. These savings can have a significant economic impact on the hospital drug expenses.
In summary, the results of the SIMRIKI study support the use of ABC/3TC+RPV as an effective, safe, well-tolerated and cost-effective switching strategy in patients with virologically stable HIV-1 infection.
Acknowledgments
We thank the SIMRIKI study patients and all investigators and Institutions of GESIDA-8314 study group**. We also thank Cristina Jiménez and Xolimar Pereiro (ViiV Healthcare Medical Scientific Liaison) for their continued support. Data presented at the 15th European AIDS Conference, Barcelona (21–24 October 2015).
Contributors
Members of the GESIDA-8314 Study Group are: J. Troya***, P. Ryan, G. Cuevas, J. Solís. Hospital Universitario Infanta Leonor, Madrid, Spain); E. Ribera. Hospital Universitario Vall d’Hebrón, Barcelona, Spain; D. Podzamczer, A. Imaz, L. Acerete. Hospital Universitario de Bellvitge, Barcelona, Spain; J. González-García, V. Hontañón. Hospital Universitario La Paz, Madrid, Spain; J.A. Terrón. Hospital Jerez de la Frontera, Jerez de la Frontera, Spain; V. Boix, J. Portilla, E. Merino, S. Reus, D. Torrús, L. Giner, I. Portilla, M. Pampliega, M. Díez. Hospital General Universitario de Alicante, Alicante, Spain; S. Moreno, MJ. Pérez, A. Díaz, J.L. Casado, A. Moreno, F. Dronda, C. Gómez, S. Serrano Hospital Universitario Ramón y Cajal, Madrid, Spain; P. Barrufet, L Force. Hospital de Mataró, Mataró, Spain; M. Castaño, M.I. Mayorga, F. Orihuela, F. Jiménez. Hospital Carlos Haya, Málaga, Spain; J. Berenguer, A. Carrero. Hospital Universitario Gregorio Marañón, Madrid, Spain; J. Galindo. Hospital Clínico de Valencia, Valencia, Spain; I. Suárez-Lozano, M. Raffo, M.D. Merino, F. Rodriguez, J.M. Fajardo, F.J. Martínez, A. Hidalgo-Castejón; S. de la Rosa. Hospital Universitario Infanta Elena, Huelva, Spain; H. Knobel. Hospital del Mar, Barcelona, Spain; H. Esteban, M. Yllescas. Fundación SEIMC-GESIDA, Madrid, Spain.
*** Lead author for this group: Jesús Troya García (email: jestrogar@hotmail.com)
Data Availability
Anonymized data from the SIMRIKI database will be available upon request from the corresponding author due to the potentially identifying nature of this data.
Funding Statement
The study was supported by ViiV Healthcare and SEIMC-GESIDA Foundation (grant number Gesida-8314). ViiV Healthcare provided SEIMC-GESIDA funds for the coordination and operational aspects of this study (administrative costs, payment for including different hospitals, collecting patient data and data analysis) but did not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. SEIMC-GESIDA Foundation was the sponsor of the study and managed all the operational aspects, including design, data collection and data analysis. All authors are responsible for the veracity and completeness of the data reported. The corresponding author had full access to all the study data and had final responsibility for the decision to submit for publication.
References
- 1.European Guidelines for treatment of HIV-infected adults in Europe. Guidelines version 8.0. European AIDS Clinical Society (EACS), October, 2015. Available: http://www.eacsociety.org/files/2015_eacsguidelines_8.0-english_revised-20151104.pdf. Accessed 2015 Nov 9.
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services USA, January 28, 2016. Available: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 2016 Feb 11.
- 3.Waters L, Nelson M. Long-term complications of antiretroviral therapy: lipoatrophy. Int J Clin Pract. 2007;61 (Suppl 6): S999–S1014. 10.1111/j.1742-1241.2007.01385.x [DOI] [PubMed] [Google Scholar]
- 4.Nachega JB, Mugavero MJ, Zeier M, Vitoria M, Gallant JE. Treatment simplification in HIV-infected adults as a strategy to prevent toxicity, improve adherence, quality of life and decrease healthcare costs. Patient Prefer Adherence. 2011;5: 357–367. 10.2147/PPA.S22771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford N, Flexner C, Vella S, Ripin D, Vitoria M. Optimization and simplification of antiretroviral therapy for adults and children. CurrOpin HIV AIDS. 2013;8 (Suppl 6): S591–S599. 10.1097/COH.0000000000000010 [DOI] [PubMed] [Google Scholar]
- 6.Casado JL, Moreno S. Potential role of rilpivirine in simplification regimens. Enferm Infecc Microbiol Clin. 2013;31 (Suppl 2): S30–S35. 10.1016/S0213-005X(13)70140-X [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen TA, Jensen D, Tolstrup M, Nielsen US, Erlandsen EJ, Birn H, et al. Comparison of bone and renal effects in HIV-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. Plos One. 2012;7 (Suppl 3): e32445 10.1371/journal.pone.0032445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51: 963–972. 10.1086/656417 [DOI] [PubMed] [Google Scholar]
- 9.Moyle GJ, Stellbrink HJ, Orkin C, Arribas JR, Domingo P, Granier C, et al. 96-week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz in antiretroviral-naive, HIV-1 infected adults: ASSERT study. Antivir Ther. 2013;18 (Suppl 7): S905–S913. 10.3851/IMP2667 [DOI] [PubMed] [Google Scholar]
- 10.Edurant®. Data Sheet. European Medicines Agency. Available: http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information /human/002264/WC500118874.pdf. Accessed 2016 Jul 12.
- 11.Molina JM, Cahn P, Grinsztejn B, Lazzarin A, Mills A, Saag M, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naïve adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378: 238–246. 10.1016/S0140-6736(11)60936-7 [DOI] [PubMed] [Google Scholar]
- 12.Cohen CJ, Andrade-Villanueva J, Clotet B, Fourie J, Johnson MA, Ruxrungtham K, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011;378: 229–237. 10.1016/S0140-6736(11)60983-5 [DOI] [PubMed] [Google Scholar]
- 13.Nelson MR, Elion RA, Cohen CJ, Mills A, Hodder SL, Segal-Maurer S, et al. Rilpivirine versus efavirenz in HIV-1-infected subjects receiving emtricitabine/tenofovir DF: pooled 96-week data from ECHO and THRIVE Studies. HIV Clin Trials. 2013;14 (Suppl 3): S81–S91. 10.1310/hct1403-81 [DOI] [PubMed] [Google Scholar]
- 14.Putcharoen O, Kerr SJ, Ruxrungtham K. An update on clinical utility of rilpivirine in the management of HIV infection in treatment-naïve patients. HIV AIDS. 2013;16 (Suppl 5): S231–S241. 10.2147/HIV.S25712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward D, Grant R. Rilpivirine/tenofovir/emtricitabine fixed-dose combination is an efficacious and well-tolerated “switch” regimen for patients on therapy. J Int AIDS Soc. 2012;15 (Suppl 4): S18351 10.7448/ias.15.6.18351 [DOI] [Google Scholar]
- 16.Cevik M, Sonecha S, Nelson M. Safety and efficacy of fixed-dose combination rilpivirine-tenofovir-emtricitabine (RPV/TDF/FTC) in treatment-experienced patients infected with HIV-1. J Int AIDS Soc. 2012;15 (Suppl 4): S18291 10.7448/ias.15.6.18291 [DOI] [Google Scholar]
- 17.Mills AM, Cohen C, Dejesus E, Brinson C, Williams S, Yale KL, et al. Efficacy and safety 48 weeks after switching from efavirenz to rilpivirine using emtricitabine/tenofovir disoproxil fumarate-based single-tablet regimens. HIV Clin Trials. 2013. Sep-Oct;14(5):216–223 10.1310/hct1405-216 [DOI] [PubMed] [Google Scholar]
- 18.Cazanave C, Reigadas S, Mazubert C, Bellecave P, Hessamfar M, Le Marec F, et al. Switch to Rilpivirine/Emtricitabine/Tenofovir Single-Tablet Regimen of Human Immunodeficiency Virus-1 RNA-Suppressed Patients, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales CO3 Aquitaine Cohort, 2012–2014. Open Forum Infect Dis. 2015; 2(1): ofv041. 10.1093/ofid/ofv018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy RL, Smith WJ. Switch studies: a review. HIV Medicine. 2002;3: 146–155. 10.1046/j.1468-1293.2002.00102.x [DOI] [PubMed] [Google Scholar]
- 20.Pozniak A, Markowitz M, Mills A. Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis. 2014;14 (Suppl 7): S590–S599. 10.1016/S1473-3099(14)70796-0 [DOI] [PubMed] [Google Scholar]
- 21.Tebas P, Sension M, Arribas J, Duiculescu D, Florence E, Hung CC, et al. Lipid levels and changes in body fat distribution in treatment-naive, HIV-1-Infected adults treated with rilpivirine or Efavirenz for 96 weeks in the ECHO and THRIVE trials. Clin Infect Dis. 2014,1;59(3):425–34 10.1093/cid/ciu234 [DOI] [PubMed] [Google Scholar]
- 22.Ward DJ, Curtin JM. Switch from Efavirenz to Nevirapine Associated with Resolution of Efavirenz-Related Neuropsychiatric Adverse Events and Improvement in Lipid Profiles AIDS Patient Care STDS. 2006;20(8):542–8. 10.1089/apc.2006.20.542 [DOI] [PubMed] [Google Scholar]
- 23.Haubrich RH, Riddler SA, DiRienzo AG, Komarow L, Powderly WG, Klingman K, et al. Metabolic outcomes in a randomised trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23 (Suppl 9): S1109–S1118. 10.1097/QAD.0b013e32832b4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efavirenz®. Data Sheet. European Medicines Agency. Available: http://ec.europa.eu/health/documents/communityregister/2012/20120109113509/anx_113509_es.pdf. Accessed 2016 Jul 12.
- 25.Mocroft A, Kirk O, Reiss P, De Wit S, Sedlacek D, Beniowski M, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24: 1667–1678. 10.1097/QAD.0b013e328339fe53 [DOI] [PubMed] [Google Scholar]
- 26.Nishijima T, Kawasaki Y, Tanaka N, Mizushima D, Aoki T, Watanabe K, et al. Long-term exposure to tenofovir continuously decrease renal function in HIV-1 infected patients with low body weight: results from 10 years of follow-up. AIDS. 2014;28 (Suppl 13): S1903–S1910. 10.1097/QAD.0000000000000347 [DOI] [PubMed] [Google Scholar]
- 27.Pozniak A, Arribas JR, Gathe J, Gupta SK, Post FA, Bloch M, et al. Switching to Tenofovir Alafenamide, Coformulated With Elvitegravir, Cobicistat, and Emtricitabine, in HIV-Infected Patients With Renal Impairment: 48-Week Results From a Single-Arm, Multicenter, Open-Label Phase 3 Study. Acquir Immune Defic Syndr. 2016; 15; 71(5): 530–537 10.1097/QAI.0000000000000908 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from the SIMRIKI database will be available upon request from the corresponding author due to the potentially identifying nature of this data.