Abstract
Background
Dengue is a leading cause of morbidity throughout the tropics; however, accurate population-based estimates of mortality rates are not available.
Methods/Principal Findings
We established the Enhanced Fatal Acute Febrile Illness Surveillance System (EFASS) to estimate dengue mortality rates in Puerto Rico. Healthcare professionals submitted serum and tissue specimens from patients who died from a dengue-like acute febrile illness, and death certificates were reviewed to identify additional cases. Specimens were tested for markers of dengue virus (DENV) infection by molecular, immunologic, and immunohistochemical methods, and were also tested for West Nile virus, Leptospira spp., and other pathogens based on histopathologic findings. Medical records were reviewed and clinical data abstracted. A total of 311 deaths were identified, of which 58 (19%) were DENV laboratory-positive. Dengue mortality rates were 1.05 per 100,000 population in 2010, 0.16 in 2011 and 0.36 in 2012. Dengue mortality was highest among adults 19–64 years and seniors ≥65 years (1.17 and 1.66 deaths per 100,000, respectively). Other pathogens identified included 34 Leptospira spp. cases and one case of Burkholderia pseudomallei and Neisseria meningitidis.
Conclusions/Significance
EFASS showed that dengue mortality rates among adults were higher than reported for influenza, and identified a leptospirosis outbreak and index cases of melioidosis and meningitis.
Author Summary
Dengue is a major public health problem in the tropics. Despite its global importance, population-based mortality rates attributable to dengue are largely unknown. Dengue vaccines are now in late stage clinical trials and one vaccine has been licensed in several countries. Evidence-based decisions regarding the future use of dengue vaccines will depend on robust estimates of disease burden which should include mortality. To estimate mortality due to dengue in Puerto Rico, where dengue is endemic, we developed an enhanced surveillance system to detect fatalities due to a preceeding dengue-like acute febrile illness using more sensitive case identification and laboratory methods than the previous passive method. This surveillance system found the dengue mortality rate was 1.05 per 100,000 Puerto Rico residents in 2010, the highest rate ever detected. Among adults aged 19–64 years, mortality from dengue (1.17 deaths per 100,000) was higher than from other infectious diseases, including influenza. The utility of this enhanced surveillance system was further proven through the identification of an outbreak of leptospirosis as well as detection of other diseases of public health importance, including melioidosis and meningitis.
Introduction
Dengue is a major public health problem worldwide. While most dengue virus (DENV) infections are asymptomatic or result in a mild acute febrile illness (AFI), some are life-threatening due to plasma leakage [1, 2]. With no antivirals to treat dengue or prevent its severe manifestations [3], early recognition of severe dengue and timely supportive care is used to reduce mortality [4–6]. Several dengue vaccines are in late stage clinical trials and one was recently licensed in several countries [7]. Decisions regarding their use will depend on vaccine performance and safety, and reduction of disease burden, including deaths.
Globally, an estimated 3.9 billion people are at risk of DENV infection, and 96 million dengue cases are estimated to have occurred in 2010 alone [8]. Despite its global presense, robust estimates of population-based dengue mortality rates are lacking. Most estimates have been derived from passive surveillance data [9–14] or hospital-based, retrospective case reviews [15, 16]. During epidemic periods, these methods have produced annual mortality rates that ranged from 0.30–0.59 deaths per 100,000 population. However, these approaches have not been validated as to under recognition due to misdiagnosis or under reporting [14].
Dengue has been endemic in Puerto Rico since the late 1960s [17, 18], and after the first deaths were reported in 1986, surveillance for deaths due to dengue was established in 1987 [19]. Mortality data have been collected through the island-wide Passive Dengue Surveillance System (PDSS), a hospital-based Infection Control Nurse Dengue Surveillance System (ICNDSS) that operated until 2007, and review of death certificates. Evaluations of these systems identified misdiagnosis and underreporting of cases, and failure to include dengue on death certificates of known laboratory-positive dengue cases [14, 20]. Few suspected fatal cases had tissue specimens or appropriately timed pre-mortem serum specimens for diagnostic testing, which resulted in a high proportion of indeterminate diagnostic results [14, 19–21].
In 2009, the Centers for Disease Control and Prevention Dengue Branch (CDC-DB), Puerto Rico Department of Health (PRDH), Instituto de Ciencias Forenses de Puerto Rico (in English, Puerto Rico Institute of Forensic Sciences [PRIFS]), Demographic Registry of Puerto Rico, and CDC Infectious Diseases Pathology Branch (CDC-IDPB) established the Enhanced Fatal Acute Febrile Illness Surveillance System (EFASS) to define mortality due to dengue-like AFI, and determine the etiology of these cases. We describe the findings from the first three years of EFASS.
Methods
Ethics Statement
This project underwent CDC institutional review and formal institutional review board review was not required since the case-patients were deceased. Because cases were reported in the context of public health surveillance, the informed consent of patients’ families was not sought. Patient identifiers were removed from the dataset prior to analysis.
Data Sources and Case Detection
EFASS used enhanced surveillance to detect dengue-like AFI deaths, improve reporting, and standardize collection of specimens at autopsy. While PDSS provided retrospective diagnostic data on fatal suspected dengue cases reported early in their illness, the primary source of EFASS cases was reporting by participating epidemiologists, pathologists, and registry statisticians. Specifically, they were asked to report and submit samples from all fatal cases whose death occurred during or immediately following a dengue-like AFI defined by the presence of fever (body temperature ≥38.0C axillary) or history of fever for ≤7 days. This included deaths with pre-defined diagnostic codes on the medical record, autopsy report or death certificate (S1 Appendix); the list of ICD codes was developed in 2009 after a review of the 1994–2007 fatal laboratory-positive dengue cases was conducted. Surveillance was enhanced by collaboration with pathologists and epidemiologists at hospitals most likely to encounter severe dengue cases, and included training and provision of standardized protocols. Dengue-like AFI fatalities that occurred at home or within 24 hours of hospital admission and referred to PRIFS were included. Collaborators were contacted weekly, and death certificates, PDSS, and National Notifiable Diseases Surveillance System (NNDSS) were routinely queried to identify suspected cases.
Specimen and Data Collection
Once a suspected case was identified, serum, whole blood, and tissue specimens were obtained, and PRIFS pathologists completed a Surgical Pathology and Autopsy Report (SPAR) (S2 Appendix). Cases with history of respiratory failure had a nasopharyngeal swab submitted for testing.
Data was abstracted from medical records of all health care visits during the illness for laboratory-positive dengue cases using a standard instrument that captured demographic characteristics, past medical history, clinical course, and management.
Diagnostic Testing
Serum specimens were tested by a DENV-serotype specific real time, reverse transcriptase-polymerase chain reaction (rRT-PCR) assay [22], an anti-DENV IgM enzyme-linked immunosorbent assay (MAC ELISA) [23], and an anti-DENV IgG ELISA [24]. Serum specimens were also tested by anti-West Nile virus (WNV) MAC-ELISA and, if positive, WNV-specific rRT-PCR and 90% plaque reduction neutralization tests (PRNT90) were performed [25]. Serum specimens with sufficient volume were sent to CDC Bacterial Special Pathogens Branch and tested for Leptospira IgM using the ELISA ImmunoDOT kit (GenBio, Inc., San Diego, CA). Acute specimens were tested for nucleic acid by rRT-PCR and 20 Leptospira reference antigens representing 17 serogroups by microscopic agglutination test (MAT) [26]. RNA was extracted from nasopharyngeal specimens and tested for Influenza A and B viral genome by rRT-PCR [27].
Tissue specimens were tested at the CDC-IDPB for DENV antigen or nucleic acid by immunohistochemistry (IHC) and RT-PCR, respectively [28]. If clinical presentation or histopathology were suggestive of another etiology pathogen-specific diagnostic testing was performed [26, 29].
Definitions
A fatal suspected dengue-like AFI case had a dengue-like AFI that immediately preceded death in a Puerto Rico resident. A fatal laboratory-positive dengue case was a suspected case with DENV nucleic acid in serum or tissue; DENV antigen in tissue; IgM seroconversion in paired specimens; or IgM in a single specimen. A fatal laboratory-negative dengue case was a suspected case with no molecular, immunodiagnostic or IHC markers of DENV infection. A fatal laboratory-indeterminate dengue case was a suspected case with no DENV nucleic acid or anti-DENV IgM in the acute serum specimen (collected ≤5 days post-illness onset [DPO]) and no available convalescent serum specimen (≥6 DPO). A fatal dengue co-infection was a fatal suspected dengue-like AFI case with DENV nucleic acid in serum or tissue and another pathogen detected by PCR or IHC. A primary DENV infection was a fatal laboratory-positive dengue case without anti-DENV IgG in the acute serum specimen [30] and a secondary DENV infection had anti-DENV IgG in the acute specimen.
A fatal laboratory-confirmed leptospirosis case was a suspected dengue-like AFI case with ≥4-fold increase in MAT titers in paired specimens, MAT titer ≥800 in a single specimen, or detection of Leptospira spp. nucleic acid in serum by PCR or antigen in tissue by IHC. A probable fatal leptospirosis case had a MAT titer >200 but <800 in a serum specimen.
Dengue fever (DF), dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) were defined according to the 1997 World Health Organization (WHO) guidelines [31] (Table 1). Dengue, dengue with warning signs, and severe dengue were defined according to 2009 WHO guidelines [2]. Definitions for severe dengue, other clinical features and medical complications are shown in Table 1.
Table 1. Clinical findings used to define fatal laboratory-positive dengue cases.
| Clinical Syndrome or Condition | Laboratory or Clinical Definitions Used | Ref. |
|---|---|---|
| Dengue fever, dengue hemorrhagic fever, dengue shock syndrome | Case had signs or symptoms as defined in the 1997 WHO Guidelines. Our definitions include: | [31] |
| Leucopenia | White cell count less than 5.0 × 109 cells/L. | |
| Plasma Leakage | Case met at least one of following criteria: | [32–36] |
| ||
| Dengue, dengue with warning signs, severe dengue | Case had signs or symptoms as outlined in the 2009 WHO Guidelines. Our more specific definition of severe dengue criteria included: | [2] |
| Severe plasma leakage | Case had plasma leakage (defined above) leading to shock or effusions resulting in acute respiratory distress, respiratory failure or ARDS. | |
| Severe bleeding | Case had intracranial bleed, or bleeding that resulted in hemodynamic instability requiring fluid replacement and/or blood transfusion. | |
| Severe organ impairment | Case had acute liver failure, myocarditis, or neurologic impairment necessitating intubation or resulting in death. | |
| Jaundice | Case had clinically apparent jaundice, or plasma bilirubin greater than 3 mg/dL. | |
| Acute liver failure (ALF) | Case with no chronic liver disease had acute hepatitis plus hepatic encephalopathy of any grade, jaundice, and new onset coagulopathy defined by international normalized ratio ≥1.5. | [37] |
| Myocarditis | Case had dyspnea, chest pain, dizziness, or weakness; echocardiographic evidence of global dysfunction; left ventricular ejection fraction <30%, and pericardial effusion or elevated serum troponin T or I. | |
| Coma | Case had a Glasgow Coma Score of less than 9 and/or was unconscious and unresponsive to painful or verbal stimuli for more than 6 hours. | |
| Other Clinical Outcomes | ||
| Acute hepatitis | Case had serum alanine aminotransferase 10 times the upper limit of normal (ULN) or >400 U/L and no underlying chronic liver disease (e.g., hepatitis C or B, cirrhosis of other or unknown etiology). | |
| Acute acalculous cholecystitis (AAC) | Case had severe abdominal pain plus two major, or one major and two minor sonographic or CT scan criteria. Gallbladder wall thickening not used as ACC criteria when ascites or hypoalbuminemia present. | [38] |
| Acute renal failure | Case had at least one of the following criteria: | [39] |
| ||
| Medical Complications of Dengue | ||
| Prolonged shock | Case had hypotension for age for ≥8 hours. | |
| Metabolic acidosis | Case had an arterial pH <7.35 and bicarbonate <24 mmol/L with a serum bicarbonate within 2 mmol/L, and a normal or low arterial carbon dioxide. | [40] |
| Fluid overload | Case had periorbital edema, dyspnea, weight gain, or abdominal compartment syndrome. | |
| Abdominal compartment syndrome | Case had intraabdominal pressure >20 mm Hg with attributable organ failure. | [41] |
| Acute hypoxemic respiratory failure | Case had an arterial oxygen <60 mm Hg and normal or low arterial carbon dioxide level while on oxygen. | |
| Acute respiratory distress syndrome (ARDS) | Case met criteria outlined in American-European Consensus Conference definition. | [42] |
| Healthcare-associated infections (HAI) | Case had infection that became clinically evident >48 hours after hospitalization. | [43] |
| Disseminated intravascular coagulation (DIC) | Case had a DIC score of ≥5 that accounted for platelet count, D-dimer, PT, and fibrinogen. | [44] |
Data Analysis
Frequencies were calculated for demographic, clinical and laboratory features of fatal laboratory-positive dengue cases. Rates of fatal laboratory-positive dengue cases per 100,000 Puerto Rico population were calculated by age group, sex, and municipality using US Census data [45]. Incidence rate ratios (IRR) were calculated to compare females to males. Case fatality rates (CFR) were calculated by dividing the number of fatal laboratory-positive dengue cases by PDSS laboratory-positive dengue cases. Statistical differences in proportions were tested by Chi-square or Fisher's exact tests. Differences between municipality-specific fatal laboratory-positive dengue cases and PDSS laboratory-positive dengue cases were examined by calculation of Pearson correlation coefficients. A geographically weighted regression model was used to determine if the number of fatal cases differed from the expected based on PDSS laboratory-positive dengue cases in the municipality and neighboring municipalities. Data analyses were conducted using STATA (Stata Corporation, College Station, TX) and ArcGIS (Environmental Systems Research Institute, Redlands, CA); maps were created using ArcMap.
Results
Identification of Fatal Cases and Diagnostic Testing
During 2010–2012, a total of 311 fatalities following a dengue-like AFI were detected by EFASS and 40,881 suspected dengue cases were reported to PDSS, of which 17,929 (44%) were dengue laboratory-positive. Of all fatalities detected, 146 (47%) were identified and reported by PRIFS pathologists, 93 (30%) by death certificate review, 50 (16%) by hospital epidemiologists, 15 (5%) by PDSS, four (1%) by NNDSS, and three (1%) by chart review as part of another study. Serum and tissue specimens were available for 148 (48%) cases, serum alone for 138 (44%) cases, tissue alone for 16 (5%) cases, and 9 (3%) cases had no diagnostic specimens. Of the 164 cases (53%) with tissue, one case was not tested because of sample quality. Of evaluable cases, 159 (98%) had liver, 156 (96%) lung, 155 (95%) kidney, 142 (87%) spleen, 98 (60%) lymph nodes, and 66 (41%) intestine. A nasopharyngeal swab was submitted for 27 (9%) cases.
A pathogen was identified in 120 (40%) of 302 cases with a diagnostic specimen. A pathogen was more likely to be identified in cases with tissue specimens than in those without (69% versus 45% respectively, P <0.0001). Overall, 58 (19%) fatal cases were dengue laboratory-positive, 167 (54%) were dengue laboratory-negative, and 77 (25%) were dengue laboratory-indeterminate. Other pathogens identified included: Leptospira spp. in 34 (11%) cases (32 confirmed, two probable); Staphylococcus spp. in nine (3%) cases; Streptococcus spp. in nine (3%) cases; influenza A virus in three (1%) cases; and one case each with Neisseria meningitidis, Burkholderia pseudomallei, Proteus spp., Clostridium perfringens, Cryptococcus neoformans, Klebsiella pneumoniae, and an unidentified Gram-positive coccus.
Characteristics of Fatal Laboratory-Positive Dengue Cases
Of the 58 fatal laboratory-positive dengue cases, 53 (91%) were DENV RT-PCR positive in tissue, serum or both; four (7%) were anti-DENV IgM positive in a single serum specimen; and one (2%) demonstrated anti-DENV IgM seroconversion in paired specimens (Table 2). Autopsies were performed on 26 (45%) of the 58 fatal laboratory-positive dengue cases and DENV was most commonly identified by IHC or RT-PCR in liver (18/26, 69%), lung (15/22, 68%), and kidney (9/24, 38%) tissue. Of 43 cases with rRT-PCR positive serum specimen, 26 (60%) were DENV-1, 16 (37%) DENV-4, and one (2%) DENV-2; similar to DENV-type distribution in PDSS during the same time period [18]. Among the 36 fatal laboratory-positive dengue cases with an available acute serum specimen, 30 (83%) had a secondary DENV infection and 6 (17%) had primary infection. Five of the DENV RT-PCR positive cases had co-infection with another pathogen, including Leptospira species (4 cases) [46] and Streptococcus species (1 case).
Table 2. Diagnostic laboratory results for fatal laboratory-positive dengue cases detected by the Enhanced Fatal Acute Febrile Illness Surveillance System, Puerto Rico 2010–2012.
| Diagnostic Result | 2010–2012 (N = 58) | 2010 (N = 39) | 2011 (N = 6) | 2012 (N = 13) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| Tissue RT-PCR and IHC positive, and serum RT-PCR positive with or without IgM positive | 13 | 22.4 | 11 | 28.2 | 0 | 0.0 | 2 | 15.4 |
| Tissue RT-PCR positive with or without IHC positive only | 10 | 17.2 | 8* | 20.5 | 0 | 0.0 | 2 | 15.4 |
| Tissue IHC positive and serum RT-PCR and IgM positive | 1 | 1.7 | 0 | 0.0 | 0 | 0.0 | 1 | 7.7 |
| Serum RT-PCR positive with or without IgM positive only | 29 | 50.0 | 18 | 46.2 | 6† | 100 | 5‡ | 38.5 |
| Seroconversion by IgM | 1 | 1.7 | 1 | 2.6 | 0 | 0.0 | 0 | 0.0 |
| Serum IgM positive only | 4 | 6.9 | 1 | 2.6 | 0 | 0.0 | 3 | 23.1 |
* Three were dual infections; two had DENV and Leptospira spp. bacteria identified and one had DENV and Streptococcus pneumonia identified in tissue.
† One dual infection with DENV and Leptospira spp. bacteria identified.
‡ One dual infection with DENV and Leptospira spp. bacteria identified.
Epidemiology of Fatal Laboratory-Positive Dengue Cases
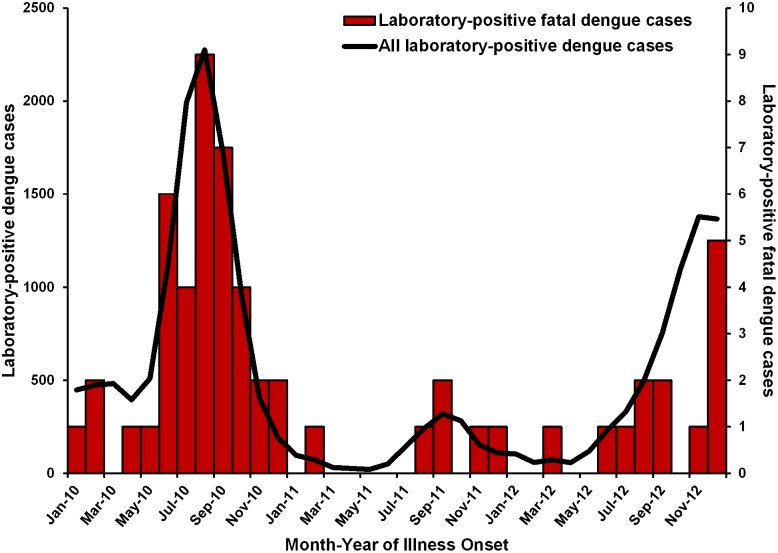
Fatal laboratory-positive dengue cases occurred in the months with increased PDSS dengue reporting (Fig 1). The dengue mortality rate was 1.05 per 100,000 population in 2010, 0.16 in 2011 and 0.36 in 2012; the 3-year average mortality rate was 0.52 dengue deaths per 100,000. The overall CFR for the three-year period was 0.32% and varied from 0.38% during the 2010 epidemic [18],; to 0.39% in 2011, a non-epidemic year, to 0.22% in 2012, an epidemic year.
Fig 1. Number of laboratory-positive dengue cases reported to the Passive Dengue Surveillance System and fatal laboratory-positive dengue cases detected by the Enhanced Fatal AFI Surveillance System by month of illness onset, Puerto Rico 2010–2012.
The median age of fatal laboratory-positive dengue case-patients was 46 years (range: 5 months–89 years). Six (10%) case-patients were <20 years old, 39 (67%) 20–64 years old, and 13 (22%) ≥65 years old. EFASS case-patients were significantly older than laboratory-positive dengue case-patients reported to PDSS (median 46 vs. 18 years, P < 0.001) and a higher proportion of fatal laboratory-positive dengue case-patients were adults (90% vs. 49%, respectively, P < 0.001).
The majority of fatal laboratory-positive dengue case-patients were female (Table 3), and were significantly more likely to be female than laboratory-positive dengue cases reported to PDSS during the same period (59% vs. 45%, P <0.05). In 2010, rates of fatal laboratory-positive dengue were 1.3 times higher among females than males; 1.19 and 0.90 cases per 100,000 population, respectively (female-to-male IRR = 1.3; 95% confidence interval [CI] = 0.70–2.50, P = 0.20). Slightly less than half (27, 47%) of fatal laboratory-positive dengue case-patients were obese, similar to that reported in Puerto Rico [47]. Most fatal laboratory-positive dengue case-patients had more than one pre-existing medical condition (35, 60%). The most common included: diabetes mellitus (23, 40%), asthma (13, 22%), cardiovascular disease (9, 16%), thyroid disease (8, 14%), psychiatric disease (8, 14%), and rheumatologic conditions (8, 14%).
Table 3. Characteristics of all fatal laboratory-positive dengue case-patients detected by the Enhanced Fatal AFI Surveillance System, Puerto Rico, 2010–2012.
| Lab-positive dengue cases (n = 58) | Children and adolescents (n = 6) | Adults ≥ 20 years old | ||
|---|---|---|---|---|
| Dengue only (n = 47) | DENV co-infections (n = 5) | |||
| Demographics, no. (%) | ||||
| Female | 34 (59) | 5 (83) | 29 (62) | 0 (0) |
| Born in Puerto Rico | 53 (91) | 5 (83) | 44 (94) | 4 (80) |
| Medical history, no. (%) | ||||
| Obese | 27 (47) | 1 (17) | 22 (47) | 4 (80) |
| No chronic disease | 10 (17) | 3 (50) | 7 (15) | 0 (0) |
| One chronic disease | 13 (22) | 2 (33) | 10 (21) | 1 (20) |
| More than one chronic disease | 35 (60) | 1 (17) | 30 (64) | 4 (80) |
| Diabetes | 23 (40) | 0 (0) | 21 (45) | 2 (40) |
| Asthma | 13 (22) | 2 (33) | 10 (21) | 1 (20) |
| Cardiovascular disease | 9 (16) | 0 (0) | 8 (17) | 1 (20) |
| Psychiatric disease | 8 (14) | 0 (0) | 7 (15) | 1 (20) |
| Thyroid disease | 8 (14) | 0 (0) | 8 (17) | 0 (0) |
| Rheumatologic condition | 8 (14) | 0 (0) | 7 (15) | 1 (20) |
| Neurologic disease | 6 (10) | 0 (0) | 6 (13) | 0 (0) |
| Gastrointestinal disease | 5 (9) | 1(17) | 4 (9) | 0 (0) |
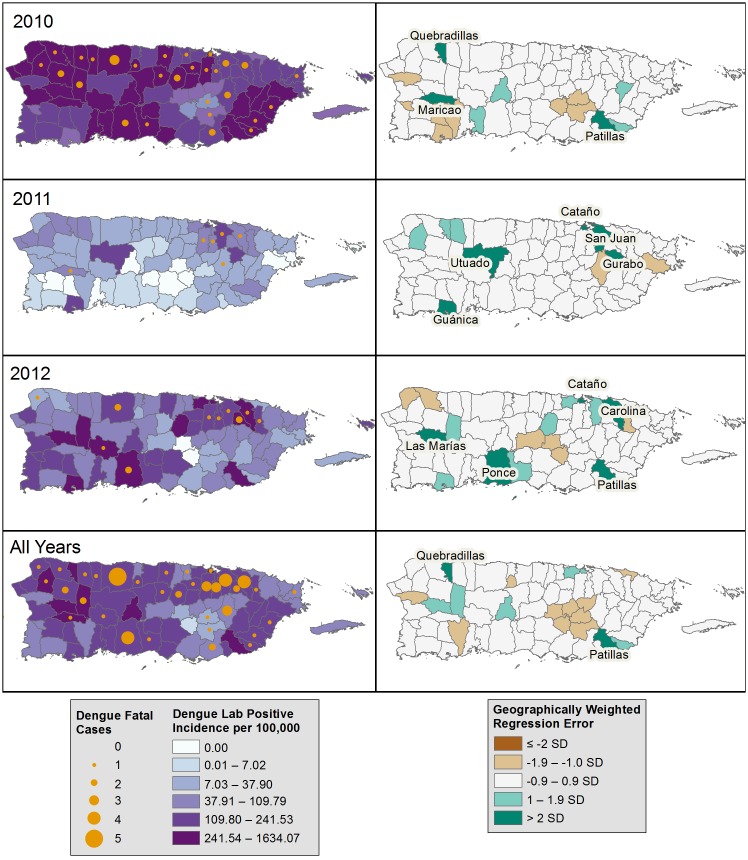
Fatal laboratory-positive dengue case-patients were residents of 35 of the 78 Puerto Rico municipalities, and most (53, 91%) were born in Puerto Rico (Table 3). Fatal laboratory-positive dengue rates were highest in 2010 in Maunabo (0.82 per 10,000 residents), Maricao in 2011 (1.59), and Adjuntas in 2012 (0.51). In all years, there was a positive correlation between the number of PDSS laboratory-positive dengue cases and the number of fatal laboratory-positive dengue cases in a municipality (R = 0.56, 0.59, 0.62, and 0.73 in 2010, 2011, 2012, and overall, respectively) (Fig 2). The number of fatal laboratory-positive dengue cases detected was no more than expected based on municipality-specific, laboratory-positive dengue incidence rates. However, there were fewer than expected fatal laboratory-positive dengue cases detected in 11 of 78 municipalities in individual years. Only Quebradillas and Patillas had fewer than expected fatalities in all years.
Fig 2. Incidence of laboratory-positive dengue, and observed and expected number of fatal laboratory-positive dengue cases by municipality of residence and year, Puerto Rico, 2010–2012.
Left panels: Incidence per 100,000 population of non-fatal, laboratory-positive dengue cases reported to the Passive Dengue Surveillance System, and number of fatal laboratory-positive dengue cases identified by the Enhanced Fatal AFI Surveillance System. Right panels: the standard deviation (SD) of the standard residuals are displayed. Differences >2 SD denotes significantly fewer than expected fatal laboratory-positive dengue cases, while ≤2 SD denotes significantly more than expected fatal laboratory-positive dengue cases.
Healthcare Seeking Behavior of Fatal Laboratory-Positive Dengue Case-Patients
All 58 fatal laboratory-positive dengue case-patients sought care at least once during their illness; 25 (43%) had two healthcare visits, and seven (12%) had three or more visits. The median interval between fever onset and arrival at the first visit was 3.5 days (range: 0–8.5 days). Of the 49 (84%) cases with a medical record for review, at the first visit, most (71%) had dengue fever, while some had severe dengue (35%) or DHF (18%) (Table 4); the majority (67%) had warning signs for severe dengue, the most common being persistent vomiting (21 of 33, 64%), abdominal pain (15, 45%), and mucosal bleeding (14, 42%). The leading diagnoses during the first visit were dengue, viral syndrome, gastroenteritis, and urinary tract infection. At the first visit, 17 (35%) were admitted to the hospital, 7 (14%) were transferred to another hospital, 7 (14%) died in the ED, and 18 (37%) were discharged home. Of those transferred to another hospital, six (86%) were admitted and died in the ICU, and one died in the ED.
Table 4. Clinical features and outcomes for fatal laboratory-positive dengue case-patients detected by the Enhanced Fatal AFI Surveillance System, Puerto Rico, 2010–2012.
| First outpatient healthcare visit* | At time of death in the hospital | |||
|---|---|---|---|---|
| N = 49 | N = 55 | |||
| Days post onset, median (range) | 3.5 | (0–8.5) | 4.5 | (0.5–13.0) |
| No. prior visits, median (range) | NA | 2 | (1–5) | |
| Clinical diagnosis,† no. (%) | ||||
| Dengue | 25 | (51.0) | 34 | (61.8) |
| Viral syndrome | 7 | (14.3) | 2 | (3.6) |
| Gastroenteritis | 6 | (12.2) | 3 | (5.5) |
| Urinary tract infection | 3 | (6.1) | 1 | (1.8) |
| Leptospirosis | 2 | (4.1) | 4 | (7.3) |
| Multi-organ failure | 2 | (4.1) | 4 | (7.3) |
| Pancytopenia | 1 | (2.0) | 3 | (5.5) |
| Dengue and leptospirosis | 1 | (2.0) | 1 | (1.8) |
| Respiratory tract infection | 1 | (2.0) | 0 | --- |
| Dehydration | 1 | (2.0) | 0 | --- |
| Meningitis with shock | 0 | --- | 2 | (3.6) |
| Myocarditis | 0 | --- | 1 | (1.8) |
| Signs and symptoms, no. (%) | ||||
| Fever measured at facility | 28 | (57.1) | 32 | (58.2) |
| Headache | 21 | (42.9) | 27 | (49.1) |
| Eye pain | 6 | (12.2) | 10 | (18.2) |
| Muscle pain | 31 | (63.3) | 33 | (60.0) |
| Joint pain | 14 | (28.6) | 23 | (41.8) |
| Bone pain | 9 | (18.4) | 15 | (27.3) |
| Rash | 9 | (18.4) | 19 | (34.6) |
| Any bleeding manifestation | 14 | (28.6) | 47 | (85.5) |
| Vomiting | 21 | (42.9) | 34 | (61.8) |
| Abdominal pain | 15 | (30.6) | 31 | (56.4) |
| Diarrhea | 12 | (24.5) | 26 | (47.3) |
| Cough | 7 | (14.3) | 21 | (38.2) |
| Sore throat | 6 | (12.2) | 6 | (10.9) |
| Clinical laboratory findings | ||||
| White blood cells (109/L), median (range) | 4.6 | (0.94–19.1) | 4.9 | (0.94–18.6) |
| Leukopenia, no. (%) | 25 | (51.0) | 29 | (52.7) |
| Platelet count (109/L), median (range) | 79 | (8–367) | 55 | (7–269) |
| Thrombocytopenia, no. (%) | 29 | (59.2) | 46 | (83.6) |
| Hemoconcentrated, no. (%) | 11 | (22.5) | 8 | (14.6) |
| Aspartate aminotransferases (U/L), median (range) | 212 | (29–5,733) | 284 | (22–15,481) |
| Alanine aminotransferases (U/L), median (range) | 187 | (23–22,046) | 176 | (23–22,046) |
| Aminotransferases ≥1000 U/L, no. (%) | 9 | (18.4) | 18 | (32.7) |
| Serum sodium ≤125 mEq/L, no. (%) | 2 | (4.1) | 8 | (14.6) |
| Clinical syndrome, no. (%) | ||||
| Dengue fever | 35 | (71.4) | 47 | (85.5) |
| Dengue hemorrhagic fever | 9 | (18.4) | 27 | (49.1) |
| Dengue shock syndrome | 5 | (10.2) | 19 | (34.6) |
| Had warning sign(s) | 33 | (67.3) | 53 | (96.4) |
| Severe dengue | 17 | (34.7) | 45 | (81.8) |
| Disposition, no. (%) | ||||
| Discharged to home | 18 | (37.0) | NA | --- |
| Transferred to another healthcare facility | 7 | (14.0) | NA | --- |
| Died in Emergency Department | 7 | (14.0) | 12 | (22.0) |
| Died on inpatient ward | 2 | (4.0) | 10 | (18.0) |
| Died in intensive care unit | 15 | (31.0) | 33 | (60.0) |
*First healthcare visit may have been at a private clinic or a hospital emergency department. Data does not include that collected after admission to the hospital or transfer to another hospital.
† Case-patients could have had more than one diagnosis listed as a discharge, admission or transfer diagnosis.
Among the 18 case-patients discharged home after their first visit, seven (39%) had at least one recorded warning sign, including compensated shock or hemorrhagic manifestations. Median age was 36.6 years (range 0.6–74.9), 14 (78%) were female, and 16 (89%) had at least one chronic medical condition. Two died at home after being discharged; diagnoses of dengue and moderate dehydration, and acute gastroenteritis. The remaining 16 returned to a hospital on average 2 days (range 0.0–3.5 days) after discharge. Nine (56%) died during their second visit, four (25%) were transferred to another facility, and three (19%) were again discharged home.
Overall, the median interval between illness onset and death was 7.1 days (range: 1.2–28.4 days); in six (10%) cases the interval exceeded 14 days. Most (43, 74%) case-patients died as an inpatient in a hospital; however, 12 (21%) died in the Emergency Department prior to hospital admission, and three (5%) died at home: one after a 2-day hospitalization, and two after being seen in an ED (Table 4). Of the 43 case-patients who died after being admitted, 33 (57%) died in the intensive care unit, and 10 (17%) died in an inpatient ward. The median interval from hospital admission to death was 1.9 days (range: 0.1–28.8 days).
Only 25 of 58 (43%) fatal laboratory-positive dengue case-patients had dengue, DHF, DSS, or dengue-like syndrome listed as primary (17 cases) or contributing (8 cases) cause of death on their death certificate. The five most common primary causes of death included dengue (29%), viral syndrome (16%), cardiorespiratory failure (12%), sepsis (9%), and thrombocytopenia (5%).
Clinical Outcomes Among Fatal Laboratory-Positive Dengue Case-Patients
Most of the 55 laboratory-positive dengue case-patients who died in hospital had signs and symptoms consistent with dengue (86%) or severe dengue (82%), but only 62% had that clinical diagnosis (Table 4). Of those who met criteria for severe dengue by the time of death, 38 of 45 (84%) had severe plasma leakage, 25 (56%) had severe bleeding, and 22 (49%) had severe organ impairment. Of note, hemoconcentration was documented in only a few case-patients even when hematocrits were performed over time. However, 34 (62%) case-patients who died in hospital had an effusion and most (62%) case-patients with effusions had acute respiratory failure or ARDS (Table 5).
Table 5. Demographic characteristics, medical history and clinical outcomes for fatal laboratory-positive dengue case-patients detected by the Enhanced Fatal AFI Surveillance System who died in a hospital, Puerto Rico, 2010–2012.
| Adults ≥20 years old | ||||
|---|---|---|---|---|
| All cases (n = 55) | Children and adolescents (n = 6) | Dengue only (n = 44) | Co-infections* (n = 5) | |
| Female, no. (%) | 32 (58.2) | 5 (83.3) | 27 (61.4) | 0 (0.0) |
| Obese, no. (%) | 24 (43.6) | 1 (16.7) | 19 (43.2) | 4 (80.0) |
| No chronic disease, no. (%) | 10 (18.2) | 3 (50.0) | 7 (15.9) | 0 (0.0) |
| One chronic disease, no. (%) | 12 (21.8) | 2 (33.3) | 9 (20.5) | 1 (20.0) |
| More than one chronic disease, no. (%) | 33 (60.0) | 1 (16.7) | 28 (63.6) | 4 (80.0) |
| Clinical outcomes | ||||
| Days post-illness onset at admission, median (range) | 4.5 (0.5–13.0) | 4.6 (0.5–8.9) | 4.5 (0.5–13.0) | 4.7 (3.6–7.2) |
| Length of hospital stay, median (range) | 2.7 (1.1–29.2) | 8.6 (8.6–8.6) | 2.7 (1.1–29.2) | 2.3 (2.3–2.3) |
| Admitted to ICU, no. (%) | 37 (67.3) | 4 (66.7) | 29 (65.9) | 4 (80.0) |
| ICU length of stay, median (range) | 1.1 (0.1–25.8) | 2.5 (0.4–4.9) | 1.1 (0.1–25.8) | 1.1 (0.7–5.2) |
| Clinical laboratory findings† | ||||
| Leukopenia, no. (%) | 29 (52.7) | 3 (50.0) | 24 (54.6) | 2 (40.0) |
| Thrombocytopenia, no. (%) | 46 (83.6) | 4 (66.7) | 37 (84.1) | 5 (100.0) |
| Hematocrit increase by ≥ 20%, no. (%) | 7 (12.7) | 0 (0.0) | 7 (15.9) | 0 (0.0) |
| Hemoconcentration by age, no. (%) | 1 (1.8) | 0 (0.0) | 1 (2.3) | 0 (0.0) |
| Days post onset of max HCT, median (range) | 4 (0–13) | 4 (0–9) | 4 (1–13) | 4 (3–7) |
| Effusion, no. (%)‡ | ||||
| Any effusion | 34 (61.8) | 6 (100.0) | 26 (59.1) | 2 (40.0) |
| Pleural effusion | 24 (43.6) | 4 (66.7) | 19 (43.2) | 1 (20.0) |
| Ascites | 14 (25.5) | 4 (66.7) | 10 (22.7) | 0 (0.0) |
| Pericardial effusion | 9 (16.4) | 1 (16.7) | 7 (15.9) | 1 (20.0) |
| Effusion with respiratory failure or ARDS | 21 (38.2) | 4 (66.7) | 16 (36.4) | 1 (20.0) |
| Bleeding Manifestation, no. (%) | ||||
| Any bleeding** | 47 (85.5) | 6 (100.0) | 38 (86.4) | 3 (60.0) |
| Severe bleed | 25 (45.5) | 4 (66.7) | 18 (40.9) | 3 (60.0) |
| Other Clinical Features, no. (%) | ||||
| Acute hepatitis | 42 (76.4) | 5 (83.3) | 32 (72.7) | 5 (100.0) |
| Acute liver failure | 5 (9.1) | 2 (33.3) | 3 (6.8) | 0 (0.0) |
| Cholecystitis | 5 (9.1) | 1 (16.7) | 3 (6.8) | 1 (20.0) |
| Myocarditis | 2 (3.6) | 0 (0.0) | 2 (4.6) | 0 (0.0) |
| Acute renal failure | 10 (18.2) | 1(16.7) | 7 (15.9) | 2 (40.0) |
| Medical complications, no. (%) | ||||
| Prolonged shock | 29 (52.7) | 2 (33.3) | 23 (52.3) | 4 (80.0) |
| Metabolic acidosis | 36 (65.5) | 5 (83.3) | 27 (61.4) | 4 (80.0) |
| Fluid overload | 17 (30.9) | 4 (66.7) | 11 (25.0) | 2 (40.0) |
| Abdominal compartment syndrome | 2 (3.6) | 2 (33.3) | 0 (0.0) | 0 (0.0) |
| Acute respiratory failure | 30 (54.6) | 5 (83.3) | 21 (47.7) | 4 (80.0) |
| Acute respiratory distress syndrome | 11 (20.0) | 4 (66.7) | 5 (11.4) | 2 (40.0) |
| Coma | 10 (18.2) | 2 (33.3) | 8 (18.2) | 0 (0.0) |
| Seizure | 8 (14.6) | 1 (16.7) | 7 (15.9) | 0 (0.0 |
| Hospital acquired infection | 16 (29.1) | 2 (33.3) | 11 (25.0) | 3 (60.0) |
| Disseminated intravascular coagulation | 5 (9.1) | 2 (33.3) | 3 (6.8) | 0 (0.0) |
| Treatment given, no. (%) | ||||
| Intravenous colloid | 16 (29.1) | 3 (50.0) | 11 (25.0) | 2 (40.0) |
| Blood transfusion | 13 (23.6) | 2 (33.3) | 10 (22.7) | 1 (20.0) |
| Platelet transfusion | 26 (47.3) | 2 (33.3) | 22 (50.0) | 2 (40.0) |
| Fresh frozen plasma | 12 (21.8) | 2 (3.33) | 9 (20.5) | 1 (20.0) |
| Inotropes | 38 (69.1) | 4 (66.7) | 30 (68.2) | 4 (80.0) |
| Diuretics | 22 (40.0) | 3 (50.0) | 16 (36.4) | 3 (60.0) |
| Corticosteroid | 25 (45.5) | 0 (0.0) | 22 (50.0) | 3 (60.0) |
* Co-infections include: Four DENV/Leptospira spp. bacteria, and one DENV/Streptococcus pneumonia.
† Number and percent presented unless otherwise stated.
‡ Most (44, 80%) of the 55 case-patients who died in hospital had a chest x-ray and/or an ultrasound done. Several case-patients (30, 55%) had at least one other imaging study done including an echocardiogram (17 done) and/or a computed tomography (CT) scan (2 abdominal and 23 brain CT scans done).
** Any bleeding was defined by the presence of any of the following: petechiae, purpura, ecchymosis, epistaxis, gingival bleeding, hematuria, menorrhagia, hemoptysis, hematemesis, melena, or an intracranial bleed.
The majority of case-patients who died in a hospital (86%) were bleeding, and 25 (46%) had severe bleeding: 19 (35%) gastrointestinal, 14 (26%) pulmonary, six (11%) vaginal, and six (11%) intracranial (Table 5). Of those with severe bleeding, 14 (56%) received a platelet transfusion while 11 (44%) received a blood transfusion; blood transfusion was more likely to occur with increased hospital stay (median 5.2 vs. 1.3 days, respectively; P <0.001). Of the 26 case-patients who received a platelet transfusion, three (12%) had no recorded bleeding. The best predictor of platelet transfusion was low platelet count [median 13,000 (range: 7,000–37,000) vs. 55,000 (range: 8,000–224,000) cells/mm3, recipients versus non-recipients, respectively; P <0.001].
Other severe clinical outcomes among the 55 laboratory-positive dengue case-patients who died in hospital included acute hepatitis (76%), acute renal failure (18%), cholecystitis (9%), acute liver failure (9%), myocarditis (4%), metabolic acidosis (66%), prolonged shock (53%), and acute respiratory failure (55%) (Table 5). All received intravenous crystalloids, 40% received a diuretic, and 29% received intravenous colloids. Twenty-five (45%; all adults) received intravenous corticosteroids. Those given steroids had a lower median platelet count than those not given steroids (16,000 vs. 47,000 cells/mm3; respectively, P <0.01), and were three times more likely to have a hospital acquired infection (75% vs. 25%, P <0.05).
Discussion
Enhanced surveillance for dengue deaths showed the majority were not reported to the standard dengue surveillance system and most did not have “dengue” coded on the death certificate. Identification of these unrecognized deaths resulted in a 2 to 3-fold higher dengue mortality rate than previously reported [14, 17, 20, 21, 48, 49]. EFASS demonstrated the importance of appropriate diagnostic testing of tissue and serum to make the correct diagnosis in deaths from a dengue-like acute febrile illness. In addition, EFASS showed its ability to identify unrecognized deaths from other pathogens of public health importance.
The EFASS estimated age-specific annual dengue mortality rates were comparable to those from other infectious diseases in the US, including influenza [50, 51]. However, in contrast to influenza, most dengue deaths occurred among adults 19–64 years of age. The estimated average annual influenza-associated US death rate is 2.4 per 100,000 residents (range: 0.4–5.1). In most years, 88% of these deaths are among persons aged ≥65 years [51, 52]; 17.0 deaths per 100,000 (range: 2.4–36.7). Influenza death rates among persons <19 years and 19–64 years are 0.1 (range: 0.1–0.3) and 0.4 (range: 0.1–0.8) per 100,000, respectively. In comparison, EFASS estimated that dengue mortality in 2010 was 0.42, 1.17, and 1.66 per 100,000 persons aged <19 years, 19–64 years and ≥65 years, respectively.
Most fatal laboratory-positive dengue case-patients appeared to have timely access to healthcare. However, many (~40%) were sent home after their first ED visit with warning signs of severe dengue. Although the majority sought care again within 48 hours, two died at home. Most case-patients who died in a hospital had severe plasma leakage, severe bleeding, or both, and most received inotropes and half received a platelet transfusion. Although bleeding was present in the majority who received platelets, half of those with severe bleeding did not receive red blood cells. A large proportion of case-patients received corticosteroids, which are not considered of benefit in dengue [2, 53]. As reported by others, we found an increased risk of hospital-acquired infections in these patients [54, 55].
Dengue deaths often occur among patients with comorbidities [14, 19]. Nearly half of case-patients were obese and over half had more than one chronic medical condition; prevalences similar to those found in the Puerto Rican adult population [47, 56], with the exception of diabetes and asthma. The prevalence of diabetes in case-patients was nearly four times that of the adult population, and asthma was twice as prevalent. Adult diabetics have been over-represented in other fatal case series [19, 57], and a recent meta-analysis found diabetes was associated with increased risk of severe dengue [58]. As many endemic areas have reported a substantial proportion of dengue cases in adults, healthcare providers should be attentive to dengue patients with these comorbidities [2].
Some patients developed acute liver or renal failure or had atypical presentations [19, 57, 59, 60]. Acute renal failure (ARF) affected ~20% of case-patients though none had pre-existing renal disease and 80% were non-elderly (median age 49 years). However, dengue patients with severe dengue, diabetes or secondary infections are known to be at risk for developing acute kidney injury [61]. Six of the ten ARF cases had at least one risk factor and two were co-infected with Leptospira spp. One of the four ARF case-patients without risk factors was an infant with abdominal compartment syndrome and multiple organ dysfunction.
While more sensitive than PDSS, EFASS may not have detected all fatal laboratory-positive dengue cases. For example, a few rural municipalities had fewer deaths than expected. In the case of Patillas, this may have been due to higher dengue case-reporting to PDSS because of an enhanced dengue surveillance project conducted prior to EFASS [62]. Alternatively, individuals in rural municipalities who died at home and were not known to have an AFI would not have been identified. These factors may have led to lower case ascertainment and estimated dengue mortality. Although we increased the proportion of suspect cases with an etiologic diagnosis by obtaining tissue and convalescent serum specimens, about one quarter of cases were dengue laboratory-indeterminate, and were not counted as fatal laboratory-positive dengue cases even if dengue was listed on their death certificate. Hence, our final dengue mortality estimate should be considered conservative.
EFASS demonstrated the feasibility and importance of enhanced surveillance for dengue deaths, and found a previously unrecognized high dengue mortality in Puerto Rico that was higher than rates observed in other dengue endemic regions during this time period [9–14]. Establishment of EFASS-like systems in selected dengue endemic countries would go a long way towards obtaining robust estimates of the global burden of deaths due to dengue, and identify areas for improvement in clinical care of patients with severe dengue.
Supporting Information
(PDF)
(PDF)
(DOCX)
Acknowledgments
We thank the staff members at the Demographic Registry of Puerto Rico for facilitating the review of death certificates including Zulma L. Escalera, Ivonne Vasallo, Wanda Llovet, Alejita Santos, and Nancy Vega. In addition, we would like to acknowledge the medical management information offices from hospitals in Puerto Rico for working with us to access medical records from the deceased case-patients. Last, we would like to acknowledge the support of Dr. María Conte, CEO of the PRIFS during the planning phases of our project, Daniel López, Manager at PRIFS, and all the pathologists at PRIFS and Centro Médico. Without their interest in and support of this project, EFASS would never have been possible.
Data Availability
The Centers for Disease Control and Prevention has determined that the data cannot be made publicly available for ethical reasons. Specifically, public availability of data would compromise patient privacy and be detrimental to surviving family members. Contact: Laura Youngblood, Human Subjects Advisor, Division of Vector Borne Diseases, NCEZID, CDC. Email: lyoungblood@cdc.gov | Office: (404) 639-6394
Funding Statement
The authors received CDC Dengue Branch programmatic funding for this project. No additional sources of funding were received. The funders had no role in the system design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Simmons CP, Farrar JJ, Nguyen V, Wills B. Dengue. N Engl J Med. 2012;366(15):1423–32. 10.1056/NEJMra1110265 . [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: WHO; 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. [PubMed] [Google Scholar]
- 3.Lim SP, Wang QY, Noble CG, Chen YL, Dong H, Zou B, et al. Ten years of dengue drug discovery: progress and prospects. Antiviral research. 2013;100(2):500–19. 10.1016/j.antiviral.2013.09.013 . [DOI] [PubMed] [Google Scholar]
- 4.Lam PK, Tam DT, Diet TV, Tam CT, Tien NT, Kieu NT, et al. Clinical characteristics of Dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57(11):1577–86. 10.1093/cid/cit594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha C, Silva S, Gordon A, Hammond SN, Elizondo D, Balmaseda A, et al. Improvement in hospital indicators after changes in dengue case management in Nicaragua. The American journal of tropical medicine and hygiene. 2009;81(2):287–92. . [PubMed] [Google Scholar]
- 6.Mayurasakorn S, Suttipun N. The impact of a program for strengthening dengue hemorrhagic fever case management on the clinical outcome of dengue hemorrhagic fever patients. The Southeast Asian journal of tropical medicine and public health. 2010;41(4):858–63. . [PubMed] [Google Scholar]
- 7.Thomas SJ, Rothman AL. Trials and Tribulations on the Path to Developing a Dengue Vaccine. American journal of preventive medicine. 2015;49(6 Suppl 4):S334–44. 10.1016/j.amepre.2015.09.006 . [DOI] [PubMed] [Google Scholar]
- 8.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira MG, Siqueira JB Jr., Ferreira GL, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. PLoS neglected tropical diseases. 2013;7(12):e2520 10.1371/journal.pntd.0002520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar LA, Rojas DP, Besada-Lombana S, Sarti E. Epidemiological trends of dengue disease in Colombia (2000–2011): a systematic review. PLoS neglected tropical diseases. 2015;9(3):e0003499 10.1371/journal.pntd.0003499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karyanti MR, Uiterwaal CS, Kusriastuti R, Hadinegoro SR, Rovers MM, Heesterbeek H, et al. The changing incidence of dengue haemorrhagic fever in Indonesia: a 45-year registry-based analysis. BMC infectious diseases. 2014;14:412 10.1186/1471-2334-14-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mia MS, Begum RA, Er AC, Abidin RD, Pereira JJ. Trends of dengue infections in Malaysia, 2000–2010. Asian Pacific journal of tropical medicine. 2013;6(6):462–6. 10.1016/S1995-7645(13)60075-9 . [DOI] [PubMed] [Google Scholar]
- 13.Limkittikul K, Brett J, L'Azou M. Epidemiological trends of dengue disease in Thailand (2000–2011): a systematic literature review. PLoS neglected tropical diseases. 2014;8(11):e3241 10.1371/journal.pntd.0003241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomashek KM, Gregory CJ, Rivera Sanchez A, Bartek MA, Garcia Rivera EJ, Hunsperger E, et al. Dengue deaths in Puerto Rico: lessons learned from the 2007 epidemic. PLoS neglected tropical diseases. 2012;6(4):e1614 10.1371/journal.pntd.0001614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leo YS, Thein TL, Fisher DA, Low JG, Oh HM, Narayanan RL, et al. Confirmed adult dengue deaths in Singapore: 5-year multi-center retrospective study. BMC infectious diseases. 2011;11:123 10.1186/1471-2334-11-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumbiganon P, Kosalaraksa P, Thepsuthammarat K, Sutra S. Dengue mortality in patients under 18 years old: an analysis from the health situation analysis of Thai population in 2010 project. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2012;95 Suppl 7:S108–13. . [PubMed] [Google Scholar]
- 17.Tomashek KM, Rivera A, Munoz-Jordan JL, Hunsperger E, Santiago L, Padro O, et al. Description of a large island-wide outbreak of dengue in Puerto Rico, 2007. The American journal of tropical medicine and hygiene. 2009;81(3):467–74. . [PubMed] [Google Scholar]
- 18.Sharp TM, Hunsperger E, Santiago GA, Munoz-Jordan JL, Santiago LM, Rivera A, et al. Virus-specific differences in rates of disease during the 2010 Dengue epidemic in Puerto Rico. PLoS neglected tropical diseases. 2013;7(4):e2159 10.1371/journal.pntd.0002159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigau-Perez JG, Laufer MK. Dengue-related deaths in Puerto Rico, 1992–1996: diagnosis and clinical alarm signals. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2006;42(9):1241–6. 10.1086/501355 . [DOI] [PubMed] [Google Scholar]
- 20.Rigau-Perez JG, Vorndam AV, Clark GG. The dengue and dengue hemorrhagic fever epidemic in Puerto Rico, 1994–1995. The American journal of tropical medicine and hygiene. 2001;64(1–2):67–74. . [DOI] [PubMed] [Google Scholar]
- 21.Rigau-Perez JG, Ayala-Lopez A, Garcia-Rivera EJ, Hudson SM, Vorndam V, Reiter P, et al. The reappearance of dengue-3 and a subsequent dengue-4 and dengue-1 epidemic in Puerto Rico in 1998. The American journal of tropical medicine and hygiene. 2002;67(4):355–62. . [DOI] [PubMed] [Google Scholar]
- 22.Santiago GA, Vergne E, Quiles Y, Cosme J, Vazquez J, Medina JF, et al. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS neglected tropical diseases. 2013;7(7):e2311 10.1371/journal.pntd.0002311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. Journal of clinical microbiology. 2000;38(5):1823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. Journal of clinical microbiology. 2000;38(5):1827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, et al. Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. Journal of clinical microbiology. 2000;38(11):4066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dikken H, Kmety E. In: Bergan T, Norris JR, editors. Methods in Microbiology. London: Acadmic Press; 1978. p. 259–307. [Google Scholar]
- 27.Shu B, Wu KH, Emery S, Villanueva J, Johnson R, Guthrie E, et al. Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 A (H1N1) pandemic influenza virus. Journal of clinical microbiology. 2011;49(7):2614–9. 10.1128/JCM.02636-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatnagar J, Blau DM, Shieh WJ, Paddock CD, Drew C, Liu L, et al. Molecular detection and typing of dengue viruses from archived tissues of fatal cases by rt-PCR and sequencing: diagnostic and epidemiologic implications. The American journal of tropical medicine and hygiene. 2012;86(2):335–40. 10.4269/ajtmh.2012.11-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagnostic microbiology and infectious disease. 2009;64(3):247–55. 10.1016/j.diagmicrobio.2009.03.014 . [DOI] [PubMed] [Google Scholar]
- 30.Miagostovich MP, Nogueira RM, dos Santos FB, Schatzmayr HG, Araujo ES, Vorndam V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 1999;14(3):183–9. 10.1016/S1386-6532(99)00059-1 . [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Geneva: WHO; 1997. Available from: http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/. [Google Scholar]
- 32.The Harriet Lane Handbook, A Manual for Pediatric House Officers. 16th ed Philadelphia: Mosby, Inc; 2002. [Google Scholar]
- 33.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C, et al. Hematological and iron-related analytes—reference data for persons aged 1 year and over: United States, 1988–94. Vital and health statistics Series 11, Data from the national health survey. 2005;(247):1–156. . [PubMed] [Google Scholar]
- 34.Tietz Textbook of Clinical Chemistry. 2nd ed St. Louis: W.B. Saunders; 1999. [Google Scholar]
- 35.Pediatric Reference Ranges. 4th ed Washington, D.C.: AACC Press; 1999. [Google Scholar]
- 36.Lockitch G, Halstead AC, Quigley G, MacCallum C. Age- and sex-specific pediatric reference intervals: study design and methods illustrated by measurement of serum proteins with the Behring LN Nephelometer. Clin Chem. 1988;34(8):1618–21. . [PubMed] [Google Scholar]
- 37.Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–98. . [PubMed] [Google Scholar]
- 38.Huffman JL, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol. 2010;8(1):15–22. 10.1016/j.cgh.2009.08.034 . [DOI] [PubMed] [Google Scholar]
- 39.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative w. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care. 2004;8(4):R204–12. 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraut JA, Madias NE. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol. 2010;6(5):274–85. 10.1038/nrneph.2010.33 . [DOI] [PubMed] [Google Scholar]
- 41.Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32(11):1722–32. 10.1007/s00134-006-0349-5 . [DOI] [PubMed] [Google Scholar]
- 42.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. 10.1164/ajrccm.149.3.7509706 . [DOI] [PubMed] [Google Scholar]
- 43.Coffin SE Z T. Healthcare-associated infections In: Long SS P L, Prober CG., editor. Principles and Practice of Pedatric Infectious Diseases. 3rd ed: Churchill Livingstone; 2008. [Google Scholar]
- 44.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33. 10.1111/j.1365-2141.2009.07600.x . [DOI] [PubMed] [Google Scholar]
- 45.American Fact Finder [Internet]. 2015 [cited July 20, 2015]. Available from: http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml.
- 46.Rodriguez NMP, Galloway R, Blau DM, Traxler R, Bhatnagar J, Zaki SR, et al. Case Report: Case Series of Fatal Leptospira spp./Dengue Virus Co-Infections-Puerto Rico, 2010–2012. American Journal of Tropical Medicine and Hygiene. 2014;91(4):760–5. 10.4269/ajtmh.14-0220. WOS:000342957600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez CM, Sanchez H, Ortiz AP. Prevalence of overweight and obesity and their cardiometabolic comorbidities in Hispanic adults living in Puerto Rico. Journal of community health. 2013;38(6):1140–6. 10.1007/s10900-013-9726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rigau-Perez JG, Ayala-Lopez A, Vorndam AV, Clark GG. Dengue activity in Puerto Rico during an interepidemic period (1995–1997). The American journal of tropical medicine and hygiene. 2001;64(1–2):75–83. . [DOI] [PubMed] [Google Scholar]
- 49.Dietz V, Gubler DJ, Ortiz S, Kuno G, Casta-Velez A, Sather GE, et al. The 1986 dengue and dengue hemorrhagic fever epidemic in Puerto Rico: epidemiologic and clinical observations. Puerto Rico health sciences journal. 1996;15(3):201–10. . [PubMed] [Google Scholar]
- 50.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;61(4):1–117. . [PubMed] [Google Scholar]
- 51.Centers for Disease C, Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morbidity and mortality weekly report. 2010;59(33):1057–62. . [PubMed] [Google Scholar]
- 52.Charu V, Simonsen L, Lustig R, Steiner C, Viboud C. Mortality burden of the 2009–10 influenza pandemic in the United States: improving the timeliness of influenza severity estimates using inpatient mortality records. Influenza and other respiratory viruses. 2013;7(5):863–71. 10.1111/irv.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panpanich R, Sornchai P, Kanjanaratanakorn K. Corticosteroids for treating dengue shock syndrome. The Cochrane database of systematic reviews. 2006; (3):CD003488. 10.1002/14651858.CD003488.pub2 . [DOI] [PubMed] [Google Scholar]
- 54.Zhang F, Kramer CV. Corticosteroids for dengue infection. The Cochrane database of systematic reviews. 2014;7:CD003488 10.1002/14651858.CD003488.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajapakse S. Corticosteroids in the treatment of dengue illness. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103(2):122–6. 10.1016/j.trstmh.2008.07.022 . [DOI] [PubMed] [Google Scholar]
- 56.Serrano R AA. Puerto Rico Behavioral Risk Factor Surveillance Survey Annual Report, 2011. San Juan, Puerto Rico: Puerto Rico Department of Health, 2014 January 2014. Report No.
- 57.Lahiri M, Fisher D, Tambyah PA. Dengue mortality: reassessing the risks in transition countries. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(10):1011–6. 10.1016/j.trstmh.2008.06.005 . [DOI] [PubMed] [Google Scholar]
- 58.Htun NS, Odermatt P, Eze IC, Boillat-Blanco N, D'Acremont V, Probst-Hensch N. Is diabetes a risk factor for a severe clinical presentation of dengue?—review and meta-analysis. PLoS neglected tropical diseases. 2015;9(4):e0003741 10.1371/journal.pntd.0003741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong A, Sandar M, Chen MI, Sin LY. Fatal dengue hemorrhagic fever in adults during a dengue epidemic in Singapore. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2007;11(3):263–7. 10.1016/j.ijid.2006.02.012 . [DOI] [PubMed] [Google Scholar]
- 60.Campos KB, Amancio FF, de Araujo VE, Carneiro M. Factors associated with death from dengue in the state of Minas Gerais, Brazil: historical cohort study. Tropical medicine & international health: TM & IH. 2015;20(2):211–8. 10.1111/tmi.12425 . [DOI] [PubMed] [Google Scholar]
- 61.Mallhi TH, Khan AH, Adnan AS, Sarriff A, Khan YH, Jummaat F. Incidence, Characteristics and Risk Factors of Acute Kidney Injury among Dengue Patients: A Retrospective Analysis. PloS one. 2015;10(9):e0138465 10.1371/journal.pone.0138465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramos MM, Arguello DF, Luxemburger C, Quinones L, Munoz JL, Beatty M, et al. Epidemiological and clinical observations on patients with dengue in Puerto Rico: results from the first year of enhanced surveillance—June 2005-May 2006. The American journal of tropical medicine and hygiene. 2008;79(1):123–7. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(DOCX)
Data Availability Statement
The Centers for Disease Control and Prevention has determined that the data cannot be made publicly available for ethical reasons. Specifically, public availability of data would compromise patient privacy and be detrimental to surviving family members. Contact: Laura Youngblood, Human Subjects Advisor, Division of Vector Borne Diseases, NCEZID, CDC. Email: lyoungblood@cdc.gov | Office: (404) 639-6394