Electronic drug alerts have been shown to reduce adverse drug events, resulting in fewer deaths, disabilities, hospitalizations, and lower health care costs.1 Drug alerts, however, are not always beneficial, and patient harm can occur when low-value or false-positive alerts appear. One study showed that 331 alerts were needed to prevent 1 adverse drug event.1 It is estimated that 90% of medication alerts are overridden by prescribing physicians,2 and more than half of overrides were due to alerts being deemed irrelevant.3 A large number of irrelevant alerts may result in alert fatigue, which has been defined as “declining physician responsiveness to a particular type of alert as the clinician is repeatedly exposed to that alert over a period of time . . . ,”4 which may result in critical warnings being missed. The ECRI Institute, a nonprofit medical safety organization, listed alert fatigue as a top technology hazard.5 The consequences are illustrated when a child received 38 times the normal dose of an antibiotic largely due to this information being overshadowed by a number of clinically inconsequential alerts.6
We conducted a quasi-experimental study to evaluate the extent of low-value alerts and reduce excessive warnings, while preserving critical alerts. All medication alerts for May and June 2014 at an academic dermatology clinic were assessed to determine their relative importance. Alerts were designated as a low, moderate, or high value based on the judgment of 3 dermatologists, and through Micromedex software (Truven Health Analytics, Ann Arbor, MI) to evaluate possible drug interactions. Criteria to determine the value of each alert included clinical utility, patient safety benefit, evidence-based warnings, and warning values already built into the EPIC electronic health record (EHR). Over 10 months, several meetings were conducted with information systems and EHR committee representatives to designate and suppress low-value alerts. These included unnecessary duplicate orders (eg, ketoconazole cream ordered for the face and ketoconazole shampoo for the scalp); duration of topical steroid use warnings (topical steroids used longer than the US Food and Drug Administration indication); and an inexplicable oral antibiotic “contraindication” with topical tretinoin. These categories made up the majority of low-value alerts. Medication alerts for May and June 2015 were analyzed for comparison.
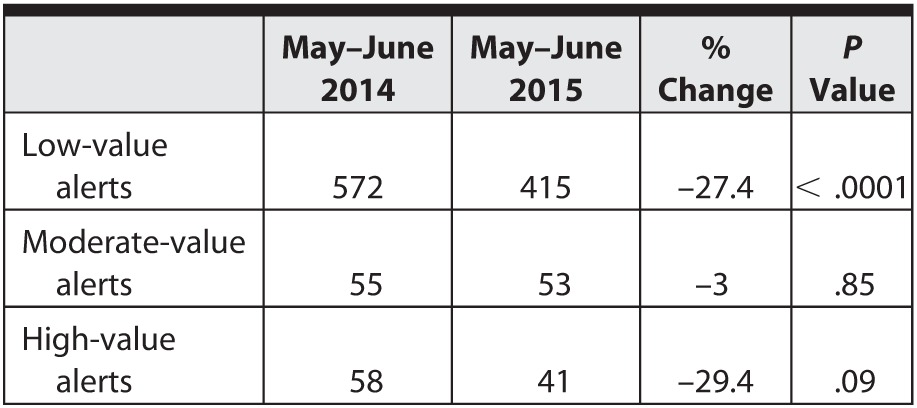
In May and June 2014, a total of 572 low-value alerts were recorded, in contrast to 415 low-value alerts for May and June 2015. The test of a single binomial proportion indicates a 27.4% reduction in low-value alerts with a 95% confidence interval (22.1%–32.8%), which was statistically significant. The data shown in the table demonstrate a desired outcome: a significant reduction in low-value alerts, while preserving the moderate- and high-value alerts.
Table.
Number of Alerts and Percentage Change Over 1 Year
We encountered several challenges, including considerable subjectivity in grading alerts, with dermatologists differing in their impression of the value of certain alerts. Physicians from different specialties also may need different alerts. While dermatologists are sensitized to the importance of not overusing topical steroids, primary care physicians may welcome this alert. If there is any concern over the usefulness of a particular alert, it is usually best to leave it alone and determine the value as it arises. Finally, EHR committees may be hesitant to modify warnings. However, a recent study showed that modifying alerts can decrease alert fatigue without increasing the risk of adverse event and subsequent litigation.7
We demonstrated a successful reduction in low-value alerts, representing a major contributor to alert fatigue. Training and culture change is needed to address flaws in the EHR that contribute to alert fatigue, and work in this area at our institution is ongoing.
References
- 1. Weingart SN, Simchowitz B, Padolsky H, et al. An empirical model to estimate the potential impact of medication safety alerts on patient safety, health care utilization, and cost in ambulatory care. Arch Intern Med. 2009; 169 16: 1465– 1473. [DOI] [PubMed] [Google Scholar]
- 2. Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009; 169 3: 305– 311. [DOI] [PubMed] [Google Scholar]
- 3. Topaz M, Seger DL, Slight SP, et al. Rising drug allergy alert overrides in electronic health records: an observational retrospective study of a decade of experience. J Am Med Inform Assoc. 2016; 23 3: 601– 608. [DOI] [PubMed] [Google Scholar]
- 4. Embi PJ, Leonard AC. Evaluating alert fatigue over time to EHR-based clinical trial alerts: findings from a randomized controlled study. J Am Med Inform Assoc. 2012; 19 e1: e145– e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ECRI Institute. Top 10 health technology hazards for 2015: a report from Health Devices . November 2014. https://www.ecri.org/Documents/White_papers/Top_10_2015.pdf. Accessed April 27, 2016. [Google Scholar]
- 6. Wachter R. The Digital Doctor: Hope, Hype, and Harm at the Dawn of Medicine's Computer Age. New York, NY: McGraw-Hill; 2015. [Google Scholar]
- 7. Kesselheim AS, Cresswell K, Phansalkar S, et al. Clinical decision support systems could be modified to reduce “alert fatigue” while still minimizing the risk of litigation. Health Aff (Millwood). 2011; 30 12: 2310– 2317. [DOI] [PubMed] [Google Scholar]


