ABSTRACT
As research, development, and manufacturing of biosimilar protein therapeutics proliferates, there is great interest in the continued development of a portfolio of complementary analytical methods that can be used to efficiently and effectively characterize biosimilar candidate materials relative to the respective reference (i.e., originator) molecule. Liquid phase separation techniques such as liquid chromatography and capillary electrophoresis are powerful tools that can provide both qualitative and quantitative information about similarities and differences between reference and biosimilar materials, especially when coupled with mass spectrometry. However, the inherent complexity of these protein materials challenges even the most modern one-dimensional (1D) separation methods. Two-dimensional (2D) separations present a number of potential advantages over 1D methods, including increased peak capacity, 2D peak patterns that can facilitate unknown identification, and improvement in the compatibility of some separation methods with mass spectrometry. In this study, we demonstrate the use of comprehensive 2D-LC separations involving cation-exchange (CEX) and reversed-phase (RP) separations in the first and second dimensions to compare 3 reference/biosimilar pairs of monoclonal antibodies (cetuximab, trastuzumab and infliximab) that cover a range of similarity/disimilarity in a middle-up approach. The second dimension RP separations are coupled to time-of-flight mass spectrometry, which enables direct identification of features in the chromatograms obtained from mAbs digested with the IdeS enzyme, or digestion with IdeS followed by reduction with dithiothreitol. As many as 23 chemically unique mAb fragments were detected in a single sample. Our results demonstrate that these rich datasets enable facile assesment of the degree of similarity between reference and biosimilar materials.
KEYWORDS: Biosimilars, cation-exchange, mass spectrometry, middle-up analysis, monoclonal antibodies, reversed-phase, two-dimensional liquid chromatography
Introduction
Biosimilars are biologics that are highly similar to the reference biological products, despite some minor differences in clinically inactive components.1 Biosimilar proteins such as haematopoietic growth factors (e.g., erythropoietin, filgrastim) and monoclonal antibodies (mAbs) (e.g., infliximab)2 are currently being used in clinical practice for the treatment of patients with cancer and immune-mediated disorders. In the next few years, a number of biologics will lose exclusivity, including trastuzumab, rituximab, cetuximab, bevacizumab and adalimumab. This loss of exclusivity allows biosimilar mAbs to be approved by regulatory authorities, and thus enter clinical use.3-6 MAbs and related therapeutics currently have global sales of over US$ 90 billion, but are starting to see competition from biosimilar products entering the marketplace.7 Many pharmaceutical companies have entered the rapidly growing biosimilar market. Remsima® (Celltrion) and Inflectra® (Hospira), which are both biosimilars of the blockbuster infliximab (Remicade®), were approved in the European Union in 2013,7,8 and, in 2016, the United States Food and Drug Administration (FDA) also approved Inflectra®.
The general purpose of the head-to-head comparison of biosimilar and reference products is to detect whether any differences between the biosimilar and its reference product are clinically meaningful (e.g., safety, purity, potency). The first step is always an extensive analytical characterization. Then, if differences are observed, further evaluation is required via a successive stepwise approach, including in vitro functionality comparisons and/or in vivo preclinical comparisons.1 Next, comparative pharmacokinetic and pharmacodynamic studies are undertaken to detect potential differences in these parameters. The extensive analytical and non-clinical comparisons allow the clinical efficacy studies to be more tailored and targeted to addressing whether any existing differences are clinically meaningful.1
Remsima (infliximab) is the world's first approved biosimilar mAb.9 Extensive physicochemical characterization of Remsima in relation to Remicade, the reference product, was conducted to demonstrate the highly similar properties between the 2 molecules. Remsima has identical primary and higher order structures compared to Remicade.9 Many different analytical tools and techniques were applied for the thorough characterization at different levels, including: amino acid analysis, peptide mapping, reduced intact mass analysis, free thiol analysis, Fourier transform infrared spectroscopy, differential scanning calorimetry, antibody conformational array, X-ray crystallography, various modes of chromatography, light scattering, analytical ultracentrifugation, electrophoresis, isoelectric focusing, monosaccharide analysis, sialic acid analysis, glycopeptide analysis, N-glycan analysis, enzyme-linked immunosorbent assay, and surface plasmon resonance.9
Biosimilar antibodies must have the same amino acid sequence as the reference marketed product.10 However, because mAbs are large molecules with highly complex structures, the products are sensitive to changes in host cells, media, and manufacturing process.11,12 Because even minor structural changes, including the glycosylation pattern, can affect the safety, purity or potency of the recombinant protein, it is important to characterize these differences.
A study by Xie et al. showed that state-of-the-art liquid chromatography (LC) and mass spectrometry (MS) can be used for rapid verification of identity and characterization of sequence variants and post-translational modifications (PTMs) for antibody products.13 A candidate biosimilar IgG1 mAb was compared in detail to a commercially available reference product. Intact mass measurement, primary sequence assessment, and identification and quantification of PTMs was performed. Although the products were very similar in terms of sequences and modifications, a 32 Da mass difference observed at the intact protein level using LC-MS indicated that they were not strictly identical. Peptide mapping located the mass difference between the biosimilar and the reference products to a 2 amino acid residue variation in the heavy chain. The peptide mapping technique was also used to comprehensively compare the differences in PTMs of the biosimilar and reference mAbs. Comprehensive glycosylation profiling confirmed that the proportion of individual glycans was also different, although the number and composition of glycans shared between the 2 mAbs were the same.
Beck et al. compared reference and biosimilar trastuzumab and cetuximab using cutting-edge MS techniques such as native MS and ion-mobility MS at different levels (top, middle and bottom).14 Investigation of intact mAbs or mAb subunits using LC-MS provided fast and accurate mass profiles at the top and middle levels, respectively. Fine structural characterization of mAbs was further performed by a classical ‘bottom-up’ approach after enzymatic digestion. Capillary electrophoresis coupled with MS has also recently been applied to the characterization of mAbs at the intact and subunit level, yielding information that is complementary to that obtained from chromatographic methods.15-19
Nevertheless, the inherent complexity of mAb materials resulting from complex glycosylation patterns and PTMs challenges the capabilities of modern one-dimensional LC. The combination of different modes of HPLC such as ion exchange (IEX), reversed phase (RP), hydrophobic interaction chromatography (HIC) or size exclusion (SEC) in 2-dimensional (2D) separation setups provides unique selectivity, and can provide a significant peak capacity improvement. Alvarez et al. first demonstrated the utility of a generic 2D format for the rapid characterization of mAb charge and size variants.20 Proteins contained in selected fractions of interest from IEX or SEC separations were identified by trapping and desalting the fractions using a series of trap cartridges containing a RP stationary phase, with subsequent online MS analysis. An online disulfide reduction step was successfully incorporated in the workflow, allowing more detailed characterization of modified mAbs. Recently, other combinations of separation modes and configurations for 2D-LC separations such as heartcutting and comprehensive analysis have been applied for mAb characterization at the intact protein and peptide levels.21-23 The implementation of online, selective comprehensive 2D-LC (sLCxLC) coupled with MS using a middle-up approach has also recently been used for the characterization of rituximab.24 In this setup, IEX and RP were used as the first and second separation dimensions, respectively. The combination of these 2 chromatographic modes allowed a direct assignment of the identities of CEX peaks using data from the MS detector because RPLC is directly compatible with MS detection, whereas CEX is not in cases where non-volatile buffers and/or salts are used as mobile phase additives.
Continued improvement of tools for the characterization of mAbs will be important as the number of biosimilars in development increases in the future. Approval of biosimilar products requires a comprehensive understanding of the similarities and differences between reference and biosimilar antibodies, which is derived from a wide array of cutting-edge analytical techniques.25 We recently demonstrated the use of selective comprehensive 2D-LC-MS to characterize the main isoforms and subunits of rituximab, a reference therapeutic mAb.24 The use of cation exchange and reversed phase separation modes in the first and second dimensions, respectively, provided highly orthogonal separation mechanisms, an improvement in peak capacity and resolution, and a way to indirectly couple cation-exchange separation with MS detection. The aim of the work described here was to extend our previous 2D-LC investigations to support the characterization and comparison of several biosimilar and reference products. The combination of CEX and RPLC has been used in the full comprehensive LCxLC mode of 2D separation, which enabled efficient determination of differences between reference and biosimilar mAbs, including cetuximab, infliximab and trastuzumab, in a middle-up approach. We developed a set of generic chromatographic conditions that were applied with small adjustments to 3 different reference/biosimilar pairs ranging from relatively dissimilar (trastuzumab/trastuzumab-B) to highly similar (infliximab/infliximab-B). Previous studies have reported on the characterization of the antibodies discussed here using LC, capillary electrophoresis, and mass spectrometry (cetuximab/cetuximab-B;14 trastuzumab/trastuzumab-B;14 infliximab26/infliximab-B9). To the best of our knowledge there have not been any reports describing in depth characterization of these materials using 2D-LC.
Results
Cetuximab
Cetuximab and its biosimilar, cetuximab-B, are an example of a reference/biosimilar pair of intermediate similarity. They have the same amino acid sequence in both the light chain and heavy chain, but have quite different glycosylation patterns, due to the fact that they are produced from different host cell lines.14 Like most antibodies, cetuximab is glycosylated in the Fc domain, but it also contains a second glycosylation site in the Fd domain. The glycosylation profile of the Fd domain is quite different between cetuximab and cetuximab-B, while the profile of the Fc domain is more similar. The 2D-LC separations of cetuximab and cetuximab-B following IdeS enzymatic digestion are shown in Fig. 2. The separations of the same samples, but following reduction with dithiothreitol (DTT), are shown in Fig. 3. For simplicity, the chromatograms obtained using fluorescent detection (FLD) and ultraviolet detection (UV) are only shown for the case of cetuximab:IdeS.
Figure 2.
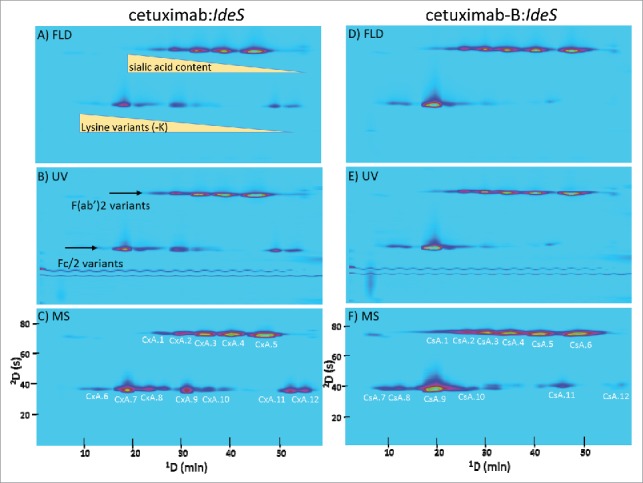
Comparison of 2D-LC separations of cetuximab and cetuximab-B following IdeS digestion, with 3 different detection modes. Variations such as glycosylation pattern and other post translational modifications were shown to significantly affect retention in the 1D CEX separation, particularly when these caused a change in the overall net charge of the protein, as is the case with lysine variants or glycosylation with sialic acid. The specific peak labels shown in (C) and (F) correspond to assignments listed in Tables S1 and S3.
Figure 3.
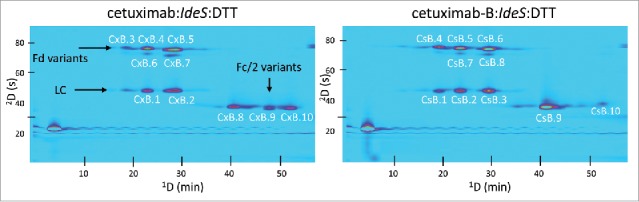
Comparison of 2D-LC separations (UV detection) of cetuximab and cetuximab-B following IdeS digestion and DTT reduction. Fragment assignments for labeled peaks are given in Table S1.
In the first dimension, CEX was very powerful for resolving charge variants. In all 4 cases (Ides only and IdeS:DTT, for both cetuximab and cetuximab-B) multiple peaks were observed, corresponding to variants of the F(ab’)2, Fd, and Fc/2 fragments. The 2D RP separations provided separation primarily by size, complementing the CEX separation mechanism. This demonstrated the power and benefits of 2D-LC, in which adding a second dimension separation mode enabled facile separation of fragments that co-eluted in the 1D CEX separation. For example, in Fig. 2 peaks CxA.2 and CxA.9 have very similar 1D retention times. In a 1D CEX separation of these fragments, they would appear as a single peak, thereby limiting the ability to quantify differences in the abundance of these fragments in comparable samples.
Generally, in the case of cetuximab, proteoforms that were missing the C-terminal lysine residue (−K) eluted prior to those that did not. Moreover, variants that contained sialic acid (N-acetylneuraminic acid [NANA] and N-glycolylneuraminic acid [NGNA]) as part of their glycan composition generally eluted prior to those that did not contain such moieties. A complete list of cetuximab and cetuximab-B fragments detected in these separations is shown in Tables S1–S4. Other glycan abbreviations used are as follows: H = hexose, N = N-acetylhexosamine, F = fucose.
Examples of deconvoluted mass spectra of cetuximab and cetuximab-B fragments are shown in Fig. 4. Differences in glycosylation patterns on both the Fd as well as the Fc/2 fragment were observed, as expected. Again, 2D-LC enabled direct identification of charge variants separated by the CEX column by indirect coupling of the separation to MS detection through the 2D RP separation. The fact that we identified as many as 23 different fragments (i.e., in the case of cetuximab:IdeS) suggests that conventional MS instrumentation (e.g., TOF) can be used for ‘in depth’ characterization, assuming optimized 2D-LC separation, minimizing the need for much more expensive high resolution MS instruments.14
Figure 4.

Deconvoluted mass spectra corresponding to peaks CxB.5 (A) and CsB.6 (B) (see Fig. 3, Tables S2–S3) from cetuximab and cetuximab-B, respectively, following IdeS digestion and DTT reduction. Clear differences in glycosylation patterns are observed on the Fd domain, as expected. Mass spectra in panels (C) and (D) correspond to peaks labeled CxA.7 and CsA.9 (see Fig. 2, Tables S1 and S3), respectively, from cetuximab and cetuximab-B following IdeS digestion only. Highly similar glycosylation assignments are observed for the originator and biosimilar in the Fc/2 domain, with slight variations in glycan abundance.
Trastuzumab
The trastuzumab/trastuzumab-B pair is an example of a reference/biosimilar pair of low similarity. They differ by one amino acid in the Fd domain (Lys -> Arg), leading to a 28 Da difference between the Fd fragments of the 2 mAbs.14 Both of them were, however, produced in CHO cell lines, and therefore have relatively similar glycosylation patterns.
Full 2D chromatograms showing the separation following IdeS digestion or IdeS digestion followed by DTT reduction are shown in Fig. 5. Again, charge variants of mAb fragments were well separated by the 1D CEX separation, particularly the Fd and F(ab’)2 fragments, while 2D RP conditions allowed for separation of co-eluting species. This result is particularly evident in the case of trastuzumab:IdeS:DTT (lower panels of Fig. 5). The large peak observed in the lower left corner (1tr = 5 min; 2tr = 10 s) of the bottom-right chromatogram warrants some discussion here, as it also appears in the UV chromatograms for replicates 2 and 3 of Fig. 9. Although we do not know what this compound is, it elutes in the dead volume of both columns, is probably not protein (as indicated by no MS signal; see UV and MS chromatograms in Fig. 9), and was only observed after several days of storage of IdeS digested samples. We do not believe this peak has any impact on the principal conclusions of this work.
Figure 5.
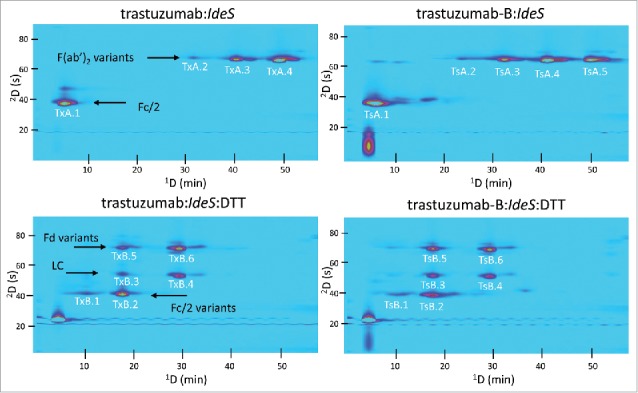
Comparison of 2D-LC separations (UV detection) of trastuzumab and trastuzumab-B following IdeS digestion or IdeS digestion and DTT reduction. Fragment assignments corresponding to the peak labels are given in Table S5.
Figure 9.

2D-LC chromatograms illustrating the repeatability of the IdeS digestion of cetuximab and 2D-LC separation with either UV detection (left) or MS detection (right).
Examples of deconvoluted mass spectra of trastuzumab and trastuzumab-B fragments are shown in Fig. 6. As is shown in Figs. 6a and 6b, the mass difference corresponding to the amino acid difference between reference and biosimilar is easily detected. In this case, the F(ab’)2 fragment of trastuzumab-B showed a 56 Da increase compared to the innovator molecule, consistent with the change of an lysine residue to arginine (+28 Da) on each Fd chain. Additionally, highly similar glycosylation patterns on the Fc/2 domain were observed between reference and biosimilar, as is expected in the case of trastuzumab; this is evident in Figs. 6c and 6d.
Figure 6.
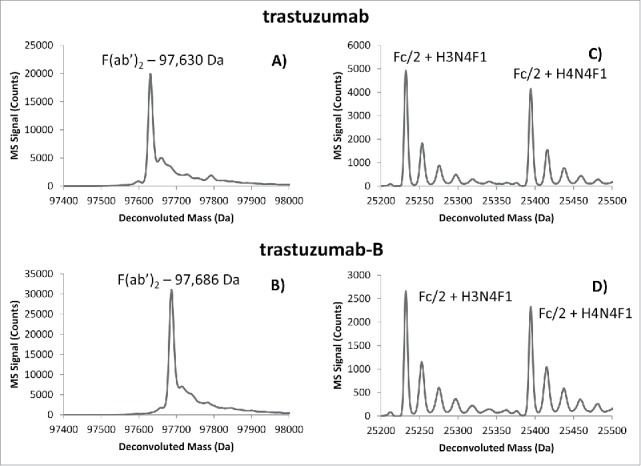
Deconvoluted mass spectra corresponding to TxA.3 (A) and TsA.4 (B) of the F(ab’)2 fragment of trastuzumab and trastuzumab-B. The indicated mass difference observed between originator and biosimilar corresponds to the one amino acid difference on the heavy chain (lysine217 -> arginine217). Panels (C) and (D) correspond to peaks TxA.1 and TxB.1, respectively, from Fig. 5.
Infliximab
The infliximab/infliximab-B pair is an example of reference/biosimilar molecules of high similarity because they share the same amino acid sequence and highly similar glycosylation patterns. As with trastuzumab, only the Fc domain is glycosylated. Jung et al. reported the physicochemical characterization of Remsima®, and observed H3N4F1 and H4N4F1 as the top 2 most abundant glycan compositions.9
The 2D separations of infliximab and infliximab-B following IdeS digestion or IdeS digestion followed by DTT reduction are shown in Fig. 7. In each case, several different peaks are observed for each mAb fragment (i.e., Fc/2, F(ab’)2), indicating the large number of proteoforms present. As was the case with cetuximab, a correlation was observed for the retention profile in CEX and the types of proteoforms identified. Generally, fragments that were missing a C-terminal lysine and/or contained a sialic acid molecule (NANA or NGNA) in their glycan composition were observed at earlier retention times in the 1D CEX separation.
Figure 7.
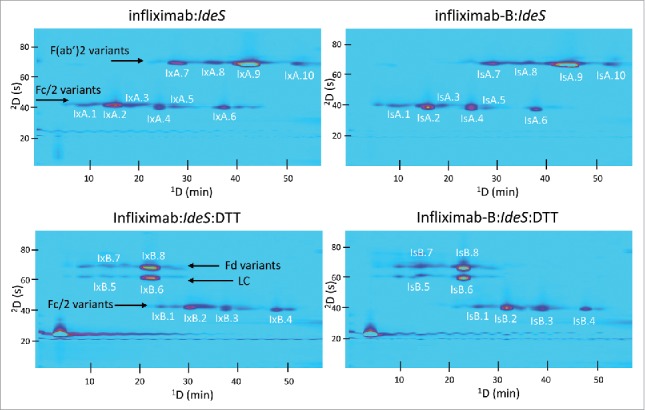
Comparison of 2D-LC separations (UV detection) of infliximab and infliximab-B following IdeS digestion or IdeS digestion and DTT reduction. Fragment assignments corresponding to the peak labels are given in Tables S7–S9.
Examples of deconvoluted mass spectra of infliximab and infliximab-B fragments are shown in Fig. 8. Consistent with the work of Jung et al.,9 the 2 most abundant glycan compositions identified were H3N4F1 and H4N4F1 (Fig. 8c and 8d). We identified a number of other glycoforms of both infliximab and infliximab-B, which are summarized in Tables S7–S9. As shown through this example, the 2D-LC-MS approach enables a detailed qualitative comparison of glycoforms with very little sample preparation needed.
Figure 8.
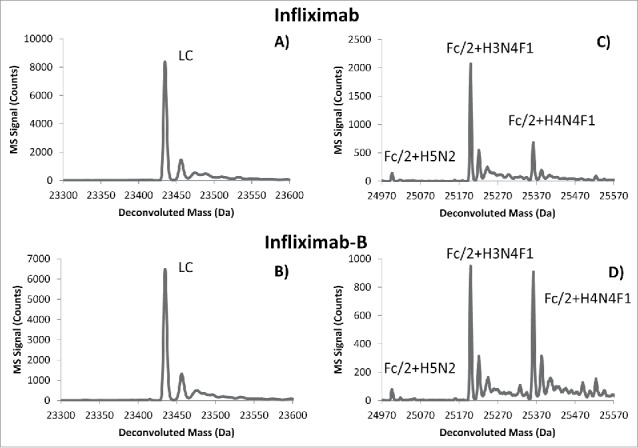
Deconvoluted mass spectra corresponding to peaks IxB.6 (A) and IsB.6 (B) of infliximab and infliximab-B, respectively (see Fig. 7, Tables S7 and S9). The primary structure of the light chain is identical between originator and biosimilar. Spectra in panels (C) and (D) correspond to peaks labeled IxB.2 and IsB.2, respectively (see Fig. 7, Tables S7 and S9). Very similar glycosylation patterns were observed on the Fc/2 domain between originator and biosimilar, with slight variations in glycan abundance.
Method repeatability
The repeatability of the 2D-LC methods described here, as well as of the IdeS digestion protocol, were investigated. Fig. 9 shows chromatograms obtained from 2D-LC separations of 3 different IdeS digestions of cetuximab that were carried out on different days over a 2-month period. Peak patterns and relative peak intensities were very similar across the 3 separations.
Discussion
Many factors can affect mAb fragment retention under CEX and RP separation conditions. We observed that, following IdeS digestion and DTT reduction, for all 3 originator/biosimilar pairs, at least 2 peaks with different CEX retention times were observed that corresponded to the light chain (e.g., peaks CxB.1 and CxB.2 in Fig. 3; their masses are indistinguishable as indicated in Table S2). This is observed despite the fact that there are no known variants of the light chain fragment in the molecules studied in this work. Thus, we would expect to observe the light chain as a single ‘spot’ in each 2D chromatogram. Our interpretation of this unexpected observation is that, even after reduction of the F(ab’)2 fragment by DTT, the Fd and light chain fragments remain associated through non-covalent interactions under the conditions (non-denaturing) of the CEX separations. Then, when these associated fragments are exposed to acetonitrile and low pH buffer upon injection into the 2D column, they dissociate and are subsequently separated into distinct Fd and light chain peaks. If this is the case, then we would expect to see a light chain spot for each F(ab’)2 variant having a different CEX retention time. Indeed, this is what we observe in all of the DTT reduced samples for all 6 mAbs studied. The fact that the intensities of the light chain peaks vary in proportion to the intensities of the Fd peaks is also consistent with this hypothesis.
The 1D CEX separations were very powerful for resolving proteoforms varying in charge state. We observed that the addition of sialic acid, either as NGNA or NANA, generally caused a decrease in retention under CEX conditions. This was particularly evident in the separation of cetuximab and infliximab and their biosimilar versions. These monosaccharides present a negative charge, effectively reducing the net positive charge of the fragments containing these moieties, and therefore reducing the retention in the CEX separation. Similarly, fragments without C-terminal lysines generally eluted prior to those that were intact. A lysine residue presents a net positive charge at pH 6; therefore, a loss of a lysine from the fragment should be less retained in the CEX separation. This is observed when analyzing the retention profile of the Fc/2 variants, particularly those of the cetuximab/cetuximab-B and infliximab/infliximab-B pairs (e.g., see Fig. 2 for indications of the retention trends for lysine and sialic acid variants). This kind of retention patterning in the 2D chromatograms, which becomes more clear upon resolution of F(ab’)2 or Fd fragments with Fc/2 fragments by the 2D RP separation, can be extremely helpful in identifying novel unknowns.27
A number of other factors can affect retention in CEX, most notably other PTMs. Deamidation is a commonly observed PTM that is known to contribute to the heterogeneity and stability of recombinant mAbs.28 Deamidation by itself results in an acidic charge variant wherein the amide group of asparagine is hydrolyzed to a carboxylate group, forming aspartate. This increases the net negative charge, affecting retention in CEX, and results in a +1 Da mass change. In the 2D-LC separations shown here, we believe this type of PTM was easily resolved under CEX conditions. The infliximab peaks IxA.7 and IxA.9 (Fig. 7, Table S7) and the trastuzumab peaks TxA.2 and TxA.3 (Fig. 5, Table S5) are examples of these deamidation variants. We see that both the CEX retention decreases (e.g., 42 min. for IxA.9 to 28 min. for IxA.7) and the mass increases by 1 Da (e.g., 98,150.9 Da for IxA.9 to 98,152.0 Da for IxA.7). The utility of the 2D-LC separation is evidenced by the fact that mass measurement of a 1 Da difference in a 100 kDa mAb fragment would be very difficult in the absence of chromatographic separation, given the resolution offered by most commercially available mass spectrometers. These observations are also consistent with the identification of mAb “hot spots,” for example on the Fd domain of trastuzumab, which are known to be prone to PTMs.14 Likewise, neutral glycan moieties can cause changes in solvation and structure of a protein, which can contribute to differences in retention, particularly in CEX.29,30 Lastly, cyclization of N-terminal glutamate to pyroglutamate (pyro-E) is a common PTM that results in a mass change of −18 Da and a decrease in positive charge.28 This is observed in the case of infliximab where the pyroE-modified F(ab’)2 fragment elutes much earlier than the unmodified F(ab’)2 fragment (see peaks IxA.8 and IxA.9 in Fig. 7, Table S7).
Interestingly, in some cases more proteoforms were identified while analyzing the MS data from IdeS-only samples, while in other cases more were identified in the IdeS:DTT samples. For example, in the case of the Fc/2 fragments of infliximab-B, 20 different variants were identified in the IdeS-only case, while only 7 variants were identified in the IdeS:DTT case (Table S9). These proteoforms included glycoforms and lysine variants. When we compared the IdeS-only and IdeS:DTT samples for each mAb pair, we observed that sometimes the charge variants of a given fragment type are more resolved in the IdeS-only samples than in the IdeS:DTT samples, and in other cases the reverse was observed. We believe this is caused by the different salt gradients used to maximize the CEX separation space for the different samples, and that the fragments of each mAb respond to differences in these gradients in different ways. This is observed most dramatically in the case of cetuximab, where the IdeS generated Fc/2 variants eluted over 10 to 58 min of the CEX separation, whereas they eluted over a much smaller 15 min window for the IdeS:DTT generated sample. In contrast, in the case of trastuzumab, we observed that the Fc/2 fragments were more spread out in the separation of the IdeS:DTT sample than they were in the separation of the IdeS-only sample. As the CEX salt gradients are optimized to ensure both retention and elution of all fragments during the separation, it is likely that the resolution of one type of fragment is often compromised to meet these objectives. This may in turn lead to increased overlap of the peaks of a particular fragment type (e.g., Fc/2 fragments of cetuximab, see Figs. 3 and 4), which may lead to ionization suppression of less abundant proteoforms.
Conclusions
We developed chromatographic conditions for the high resolution separation of middle-level fragments of reference and biosimilar mAbs using comprehensive 2D-LC with CEX and RP separations in the first and second dimensions, respectively. The 2D RP separation was directly coupled to TOF-MS detection, enabling the facile identification of tens of different fragments for each of the 6 mAbs studied, including those with different glycans and a variety of PTMs. Following are the 4 principal observations from our results.
First, with only minor modifications to the salt (first dimension) and ACN (second dimension) gradient elution conditions used, a single set of 2D-LC-MS conditions was compatible with the 6 mAbs studied in this work. These conditions were used both for mAbs digested with IdeS, as well as mAbs digested with IdeS followed by DTT reduction. This simplifies method development by providing a unified starting point for new mAb samples.
Second, systematic study of 3 reference/biosimilar pairs using the 2D-LC-MS method showed that very similar 2D chromatograms were obtained when the mAbs are expected to be very similar (e.g., infliximab/infliximab-B). In cases where the mAbs are expected to be slightly (e.g., cetuximab/cetuximab-B, differences in glycosylation) or significantly (trastuzumab/trastuzumab-B, one amino acid difference) different, clear differences in peak patterns and intensities are evident in the 2D chromatograms and deconvoluted masses derived from the TOF-MS data.
Third, there are recurring patterns in the organization of peaks in the 2D chromatograms according to chemical modifications of mAb fragments that are otherwise obscured when the same fragments are separated by either CEX or RP mechanisms using conventional 1D chromatography. For example, there is a clear trend of decreasing sialic acid content of the Fd fragments of cetuximab with increasing CEX retention time. This patterning in the 2D chromatograms may be very helpful for the identification of unknown mAb fragment modifications.
Our fourth principal observation was that comprehensive 2D-LC separations of middle level fragments of the mAbs, as studied in this work, are highly repeatable in terms of fragment retention time patterns. This repeatability will enable the use of retention time as a characteristic of mAb fragments to discern differences between originator and biosimilar mAbs, in addition to the highly reliable and informative mass information obtained from mass spectrometric detection.
Materials and methods
Reagents and sample preparation
All reagents were used as obtained from their respective manufacturers: acetonitrile (ACN, Chromasolv LC-MS grade), formic acid (FA; part no. 09676), and DTT (part no. 43815) were all from Sigma Aldrich (St. Louis, MO). Water was purified in-house using a Milli-Q water purification system (Billerica, MA). The IdeS protease enzyme was obtained from Genovis AB (part no. A0-FRI-008; Lund, Sweden). Trastuzumab (Herceptin®, Roche), cetuximab (Erbitux®, Eli Lilly and Company), infliximab (Remicade®, Johnson & Johnson), infliximab-B (Remsima®, Celltrion), and trastuzumab-B and cetuximab-B (Pierre-Fabre), were obtained from their respective manufacturers.
Partial digestion of intact mAbs was carried out by addition of 100 µg of mAb to a polypropylene tube containing 100 units of lyophilized IdeS enzyme, and the total volume was brought to 100 μL using IdeS digestion buffer (50 mM sodium phosphate, 150 mM sodium chloride, pH 6.6). This solution was incubated at 37°C for 1 hr. This solution was diluted 1:1 with Milli-Q water prior to analysis. Reduction of interchain disulfide bonds was carried out by taking a 25 µL aliquot of the IdeS digest solution and diluting 1:1 with 20 mM dibasic sodium phosphate, followed by addition of 5 µL of 100 mM DTT in water. The solution was incubated at 37°C for 30 min.
Instrumentation
A diagram of the instrument used for LCxLC-MS separations is shown in Fig. 1. All instrument modules were from the 1290 Infinity line from Agilent Technologies (Waldbronn, Germany): First (1D) and second (2D) dimension binary pumps (Model G4220A); autosampler (Model G4226A), thermostated column compartments (Model G1316C), diode-array UV detectors (DADs) (Model G4212A), and fluorescence detector (Model G1321B). The interface valve connecting the 2 dimensions of the system (p/n: 5067-4244) was set up with 2 nominally identical 160 μL sample loops made from 88-cm lengths of 0.020′ i.d. PEEK tubing. A pressure relief kit (p/n: G4236-60010) was installed between the outlet of the 1D detector and the inlet to the interface valve to minimize disturbances in the 1D detector baseline. The mass spectrometer was a time-of-flight (TOF-MS) instrument (Model 6230B) equipped with an Agilent JetStream electrospray ionization source. The mass analyzer was calibrated using a standard tuning compound mixture (Agilent, part no. G1969-85000). Hexakis(1H,1H,3H-perfluoropropoxy)phosphazene was used as a reference mass (m/z 922.0098) compound for calibration of mass spectra prior to deconvolution, sprayed continuously into the JetStream source from a secondary reference nebulizer at 30 μL/min.
Figure 1.

General schematic representation of the 2D-LC-MS setup, and the timing of events in methods employed for the separation of originator and biosimilar mAbs. A total analysis time of 60 min was used, with fractions of 1D column effluent injected onto the 2D column at 90 s intervals. The panel on the right shows a schematic diagram of the 1D and 2D gradient methods employed. The salt diversion valve was used to limit contamination of the mass spectrometer.
The 2D-LC instrument was controlled by OpenLab Chromatography Data System (C.01.07), with a 2D-LC Add-on (G2198AA) installed (Agilent Technologies). The TOF-MS was controlled using MassHunter (B.05.01, Agilent Technologies). Calculated masses of mAb fragments were obtained using BioConfirm software based on amino acid sequences published previously for each mAb. Protein mass spectra were deconvoluted also using BioConfirm software (B.06.00, Agilent Technologies). 2D chromatograms were generated using GC Image Version 2.5 LCxLC HRMS Edition software. The ‘phase’ of each 2D chromatogram was shifted by −15 s to avoid apparent wrap-around of 2D peaks.
Separation and detection conditions
1D cation-exchange separations were performed using a 250 mm × 2.1 mm i.d. Bio Mab NP5 column (Agilent Technologies, 5 µm nonporous particles). A salt gradient elution method was used in the first dimension, where solvent A contained 10 mM ammonium acetate (pH 6.0), and solvent B contained 0.25 M ammonium acetate (pH 6.0). The salt gradient used for each mAb sample is shown in Table 1. The column temperature was 40°C, the flow rate was 0.10 mL/min, and the injection volume was 10 µL. 2D reversed-phase separations were performed using a 50 mm × 2.1 mm i.d. PLRP-S 1000 Å column (Agilent Technologies, 5 µm particle size). Solvent A was 1.0% (v/v) formic acid in water, and solvent B was ACN. The ACN gradient used for each mAb sample is shown in Table 1. The column temperature was 75°C, the flow rate 0.6 mL/min, and 150 µL of 1D column effluent was injected into the 2D column at 90 s intervals (see Fig. 1). The different gradient elution programs were used to optimize the usage of the 2D separation space for each pair of mAbs. The effluent from the 2D column was diverted to waste for a period of 18 s, starting 9 s after injection of each fraction into the 2D to avoid salt contamination of the MS ionization source (see Fig. 1, Salt Valve).
Table 1.
Gradient elution programs used for each sample studied in this work.
|
1D %B |
||||||
|---|---|---|---|---|---|---|
| cetuximab/cetuximab-B |
trastuzumab/trastuzumab-B |
infliximab/infliximab-B |
||||
| Time (min) | IdeS | Ides:DTT | IdeS | Ides:DTT | IdeS | Ides:DTT |
| 0 | 20 | 6 | 28 | 20 | 20 | 10 |
| 46 | 28 | 28 | 44 | 32 | 32 | 32 |
| 55 | 80 | 80 | 80 | 80 | 80 | 80 |
| 55.01 | 20 | 6 | 28 | 20 | 20 | 10 |
| 60 | 20 | 6 | 28 | 20 | 20 | 10 |
|
2D %B |
||||||
| cetuximab/cetuximab-B |
trastuzumab/trastuzumab-B |
infliximab/infliximab-B |
||||
| Time (s) |
IdeS |
Ides:DTT |
IdeS |
Ides:DTT |
IdeS |
Ides:DTT |
| 0 | 25 | 25 | 25 | 25 | 25 | 25 |
| 54 | 34 | 34 | 32 | 32 | 32 | 32 |
| 72 | 50 | 50 | 50 | 50 | 50 | 50 |
| 73 | 25 | 25 | 25 | 25 | 25 | 25 |
| 90 | 25 | 25 | 25 | 25 | 25 | 25 |
UV absorbance data were collected at 280 nm. In the first dimension, the acquisition rate was 1.25 Hz; in the second dimension, the acquisition rate was 40 Hz. Fluorescence emission data were collected with excitation and emission wavelengths of 280 and 360 nm, respectively, at a sampling frequency of 37 Hz. When fluorescence data were collected, as in Fig. 3, the fluorescence and UV detectors were coupled in series, and MS detection was not used. Likewise, when MS detection was used, fluorescence was not. TOF-MS data were acquired from m/z 500 to m/z 8000 in positive ion mode at an acquisition rate of 3 Hz. The drying gas temperature and flow rate were 325°C and 8 L/min, respectively, while the sheath gas temperature and flow rate were 350°C and 11 L/min, respectively. The nebulizer gas pressure was 35 psi. The capillary and nozzle voltages were 5.5 and 1.0 kV, respectively, and the fragmentor voltages set to 300 and 350 V. Parameters used for deconvolution of protein mass spectra were as follows: m/z range 90,000–110,000 for F(ab’)2 fragments; step size 1.0 Da; top 25% of peak; 20,000–30,000 for Fd, Fc/2, and LC; step size 0.1 Da; top 25% of peak; and 20,000–60,000 for Fd-LC dimers; step size 0.1 Da; top 25% of peak. Mass spectra were re-calibrated prior to deconvolution using a reference mass of m/z 922.0098.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
M.S., D.C.H., and D.S. acknowledge financial support of a grant from Agilent Technologies University Relations program. All of the columns and instrumentation used in this work were provided by Agilent Technologies. D.G. wishes to thank the Swiss National Science Foundation for support through a fellowship to S.F. (31003A 159494). The authors also acknowledge Elsa Wagner, Olivier Colas, Marie-Clarie Janin-Bussat and Mélissa Excoffier (Center d’Immunologie Pierre Fabre, Saint-Julien en Genevois, France) for helpful discussions on mAbs analytical and structural characterization.
References
- 1.Macdonald JC, Hartman H, Jacobs IA. Regulatory considerations in oncologic biosimilar drug development. mAbs 2015; 7:653-61; PMID:25961747; http://dx.doi.org/ 10.1080/19420862.2015.1040973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelenetz AD, Ahmed I, Braud EL, Cross JD, Davenport-Ennis N, Dickinson BD, Goldberg SE, Gottlieb S, Johnson PE, Lyman GH, et al.. NCCN Biosimilars White Paper: regulatory, scientific, and patient safety perspectives. J Natl Compr Cancer Netw JNCCN 2011; 9 Suppl 4:S1-22; PMID:21976013 [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Chow SC. On the regulatory approval pathway of biosimilar products. Pharm Basel Switz 2012; 5:353-68; PMID:24281406; http://dx.doi.org/21175852 10.3390/ph5040353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niederwieser D, Schmitz S. Biosimilar agents in oncology/haematology: from approval to practice: Biosimilars in oncology/haematology. Eur J Haematol 2011; 86:277-88; PMID:21175852; http://dx.doi.org/ 10.1111/j.1600-0609.2010.01566.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mounho B, Phillips A, Holcombe K, Grampp G, Lubiniecki T, Mollerup I, Jones C. Global regulatory standards for the approval of biosimilars. Food Drug Law J 2010; 65:819-837, ii-iii; PMID:24479248 [PubMed] [Google Scholar]
- 6.Dranitsaris G, Amir E, Dorward K. Biosimilars of biological drug therapies: regulatory, clinical and commercial considerations. Drugs 2011; 71:1527-36; PMID:21861538; http://dx.doi.org/ 10.2165/11593730-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 7.Udpa N, Million RP. Monoclonal antibody biosimilars. Nat Rev Drug Discov 2015; 15:13-4; PMID:26678619; http://dx.doi.org/ 10.1038/nrd.2015.12 [DOI] [PubMed] [Google Scholar]
- 8.Beck A, Reichert JM. Approval of the first biosimilar antibodies in Europe: A major landmark for the biopharmaceutical industry. mAbs 2013; 5:621-3; PMID:23924791; http://dx.doi.org/ 10.4161/mabs.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung SK, Lee KH, Jeon JW, Lee JW, Kwon BO, Kim YJ, Bae JS, Kim DI, Lee SY, Chang SJ. Physicochemical characterization of Remsima®. mAbs 2014; 6:1163-77; PMID:25517302; http://dx.doi.org/ 10.4161/mabs.32221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck A, Sanglier-Cianférani S, Van Dorsselaer A. Biosimilar, biobetter, and next generation antibody characterization by mass spectrometry. Anal Chem 2012; 84:4637-46; PMID:22510259; http://dx.doi.org/ 10.1021/ac3002885 [DOI] [PubMed] [Google Scholar]
- 11.Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol 2007; 19:411-9; PMID:17872902; http://dx.doi.org/ 10.1093/annonc/mdm345 [DOI] [PubMed] [Google Scholar]
- 12.Vlasak J, Bussat MC, Wang S, Wagner-Rousset E, Schaefer M, Klinguer-Hamour C, Kirchmeier M, Corvaïa N, Ionescu R, Beck A. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal Biochem 2009; 392:145-54; PMID:19497295; http://dx.doi.org/ 10.1016/j.ab.2009.05.043 [DOI] [PubMed] [Google Scholar]
- 13.Xie H, Chakraborty A, Ahn J, Yu YQ, Dakshinamoorthy DP, Gilar M, Chen W, Skilton SJ, Mazzeo JR. Rapid comparison of a candidate biosimilar to an innovator monoclonal antibody with advanced liquid chromatography and mass spectrometry technologies. mAbs 2010; 2:379-94; PMID:20458189; http://dx.doi.org/ 10.4161/mabs.11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck A, Debaene F, Diemer H, Wagner-Rousset E, Colas O, Van Dorsselaer A, Cianférani S. Cutting-edge mass spectrometry characterization of originator, biosimilar and biobetter antibodies. J Mass Spectrom 2015; 50:285-97; PMID:25800010; http://dx.doi.org/ 10.1002/jms.3554 [DOI] [PubMed] [Google Scholar]
- 15.Gahoual R, Beck A, François YN, Leize-Wagner E. Independent highly sensitive characterization of asparagine deamidation and aspartic acid isomerization by sheathless CZE-ESI-MS/MS: Characterization of Asn and Asp PTMs by CZE-ESI-MS. J Mass Spectrom 2016; 51:150-8; PMID:26889931; http://dx.doi.org/ 10.1002/jms.3735 [DOI] [PubMed] [Google Scholar]
- 16.Gahoual R, Biacchi M, Chicher J, Kuhn L, Hammann P, Beck A, Leize-Wagner E, François YN. Monoclonal antibodies biosimilarity assessment using transient isotachophoresis capillary zone electrophoresis-tandem mass spectrometry. mAbs 2014; 6:1464-73; PMID:25484058; http://dx.doi.org/ 10.4161/mabs.36305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biacchi M, Gahoual R, Said N, Beck A, Leize-Wagner E, François YN. Glycoform separation and characterization of cetuximab variants by middle-up off-line capillary zone Electrophoresis-UV/Electrospray Ionization-MS. Anal Chem 2015; 87:6240-50; PMID:25970692; http://dx.doi.org/ 10.1021/acs.analchem.5b00928 [DOI] [PubMed] [Google Scholar]
- 18.François YN, Biacchi M, Said N, Renard C, Beck A, Gahoual R, Leize-Wagner E. Characterization of cetuximab Fc/2 dimers by off-line CZE-MS. Anal Chim Acta 2016; 908:168-76; http://dx.doi.org/ 10.1016/j.aca.2015.12.033 [DOI] [PubMed] [Google Scholar]
- 19.Redman EA, Batz NG, Mellors JS, Ramsey JM. Integrated microfluidic capillary electrophoresis-electrospray ionization devices with online ms detection for the separation and characterization of intact monoclonal antibody variants. Anal Chem 2015; 87:2264-72; PMID:25569459; http://dx.doi.org/ 10.1021/ac503964j [DOI] [PubMed] [Google Scholar]
- 20.Alvarez M, Tremintin G, Wang J, Eng M, Kao Y-H, Jeong J, Ling VT, Borisov OV. On-line characterization of monoclonal antibody variants by liquid chromatography–mass spectrometry operating in a two-dimensional format. Anal Biochem 2011; 419:17-25; PMID:21867674; http://dx.doi.org/ 10.1016/j.ab.2011.07.033 [DOI] [PubMed] [Google Scholar]
- 21.He Y, Friese OV, Schlittler MR, Wang Q, Yang X, Bass LA, Jones MT. On-line coupling of size exclusion chromatography with mixed-mode liquid chromatography for comprehensive profiling of biopharmaceutical drug product. J Chromatogr A 2012; 1262:122-9; PMID:22999205; http://dx.doi.org/ 10.1016/j.chroma.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 22.Mazur MT, Seipert RS, Mahon D, Zhou Q, Liu T. A platform for characterizing therapeutic monoclonal antibody breakdown products by 2D chromatography and top-down mass spectrometry. AAPS J 2012; 14:530-41; PMID:22581105; http://dx.doi.org/ 10.1208/s12248-012-9361-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanhoenacker G, Vandenheede I, David F, Sandra P, Sandra K. Comprehensive two-dimensional liquid chromatography of therapeutic monoclonal antibody digests. Anal Bioanal Chem 2015; 407:355-66; PMID:25410642; http://dx.doi.org/ 10.1007/s00216-014-8299-1 [DOI] [PubMed] [Google Scholar]
- 24.Stoll DR, Harmes DC, Danforth J, Wagner-Rousset E, Guillarme D, Fekete S, Beck A. Direct identification of rituximab main isoforms and subunit analysis by online selective comprehensive two-dimensional liquid chromatography – mass spectrometry. Anal Chem 2015; 87:8307-15; PMID:26145446; http://dx.doi.org/ 10.1021/acs.analchem.5b01578 [DOI] [PubMed] [Google Scholar]
- 25.Schiel JE, Davis DL, Borisov OV, editors. Chapter 2: Sequence variants and sequence variant analysis in biotherapeutic proteins [Internet] In: State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization Volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study. Washington, DC: American Chemical Society; 2015. [cited 2016February11]. page 63-6.Available from: http://pubs.acs.org/doi/book/10.1021/bk-2015-1201 [Google Scholar]
- 26.Beck A, Diemer H, Ayoub D, Debaene F, Wagner-Rousset E, Carapito C, Van Dorsselaer A, Sanglier-Cianférani S. Analytical characterization of biosimilar antibodies and Fc-fusion proteins. TrAC Trends Anal Chem 2013; 48:81-95; http://dx.doi.org/ 10.1016/j.trac.2013.02.014 [DOI] [Google Scholar]
- 27.Marriott PJ, Chin ST, Maikhunthod B, Schmarr HG, Bieri S. Multidimensional gas chromatography. TrAC Trends Anal Chem 2012; 34:1-21; http://dx.doi.org/ 10.1016/j.trac.2011.10.013 [DOI] [Google Scholar]
- 28.Schiel JE, Davis DL, Borisov OV, editors. Chapter 3: Structural Elucidation of post-Translational Modifications in Monoclonal Antibodies [Internet] In: State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization Volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study. Washington, DC: American Chemical Society; 2015. [cited 2016February11]. page 123-35.Available from: http://pubs.acs.org/doi/book/10.1021/bk-2015-1201 [Google Scholar]
- 29.Huhn C, Selman MHJ, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics 2009; 9:882-913; PMID:19212958; http://dx.doi.org/ 10.1002/pmic.200800715 [DOI] [PubMed] [Google Scholar]
- 30.Abès R, Teillaud JL. Impact of glycosylation on effector functions of therapeutic IgG. Pharmaceuticals 2010; 3:146-58; http://dx.doi.org/ 10.3390/ph3010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


