ABSTRACT
Infliximab is an anti-tumor necrosis factor monoclonal antibody approved in chronic inflammatory diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), Crohn's disease (CD) and ulcerative colitis (UC). Infliximab pharmacokinetics is variable between patients, but influence of the underlying disease was never assessed. This study aimed at assessing this influence using a cohort of patients monitored in a single center and with the same assay. Infliximab trough concentrations were determined on samples collected between weeks 0 and 22 after treatment initiation in 218 patients treated for RA, PsA, AS, CD or UC. Infliximab pharmacokinetics was analyzed by a one-compartment population model with first-order elimination rate constant. In AS patients, volume of distribution (V) and elimination clearance (CL) were 5.4 L and 0.24 L/day, respectively. In CD and UC patients, V was 49% and 52% higher than in AS, respectively, and CL was 47% and 60% higher than in AS, respectively. In RA patients, CL was 49% higher than in AS patients. Simulations showed that without methotrexate, a 3 mg/kg dosing regimen would lead only 16% of RA patients to reach the target concentration (2.5 mg/L) at week 22, whereas target concentrations would be reached in approximately half of RA patients cotreated with methotrexate, as well as half of CD (3.5 mg/L) and UC (3.7 mg/L) patients. The suboptimality of approved dosing regimens supports the development of dosing optimization based on concentration measurements.
KEYWORDS: Ankylosing spondylitis, Crohn's disease, infliximab, inflammatory bowel disease, monoclonal antibodies, pharmacokinetics, psoriatic arthritis, rheumatoid arthritis, therapeutic drug monitoring, ulcerative colitis
Abbreviations
- 2LL
minus 2 log-likelihood (objective function)
- AS
Ankylosing spondylitis
- ADA
Anti-drug antibodies
- AIC
Akaike's information criterion
- CAT
Binary covariate
- CD
Crohn's disease
- CL
Elimination clearance
- COV
Continuous covariate
- CRP
C-reactive protein
- DV
Observed concentrations
- ELISA
Enzyme-linked immunosorbent assay
- IBD
Inflammatory bowel disease
- IQR
Interquartile range
- IgG
Immunoglobulin G
- IPRED
Individual-predicted measurements
- IWRES
Individual weighted residuals
- K
Iteration Kernel
- LLOQ
Lower limit of quantification
- MTX
Methotrexate
- NPDE
Normalized prediction distribution error
- OFV
Objective function value
- PK
Pharmacokinetics
- PRED
Population-predicted measurements
- PsA
Psoriatic arthritis
- PWRES
Population weighted residuals
- RA
Rheumatoid arthritis
- RSE
Relative standard error
- SAEM
Stochastic expectation-maximization algorithm
- SF
Synovial fluid
- t½
Half-life
- TMDD
Target-mediated drug disposition
- TNF
Tumor necrosis factor
- UC
Ulcerative colitis
- ULOQ
Upper limit of quantification
- V
Volume of distribution
- WT
Weight
Introduction
Infliximab is a chimeric monoclonal immunoglobulin G1 (IgG1) targeting tumor necrosis factor (TNF). It is approved for the treatment of immuno-inflammatory diseases, including rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA), Crohn's disease (CD) and ulcerative colitis (UC). Despite the adjustment of dose based on patients' weight, large interindividual variability in infliximab serum concentrations is observed.1-3 Infliximab pharmacokinetics has been analyzed in RA,4 AS5,6 and inflammatory bowel disease (IBD)7-12 patients using population compartmental modeling. These studies showed that several individual characteristics such as body weight,4-6,9,10,12,13 sex,5,9,13 anti-drug antibodies,14,15 cotreatment with methotrexate4 or pre-infusion C-reactive protein (CRP)4 were related to infliximab pharmacokinetic parameters.
In addition to these factors, underlying disease itself may influence infliximab pharmacokinetics. Infliximab elimination half-life was reported to be ∼14 d on average,16 but with lower values in RA patients without methotrexate (9 days), than in RA patients cotreated with methotrexate (13 days),4 AS5,6 and in IBD9,11,13 patients (∼14 d for both conditions) (Table 1). The increase in infliximab clearance (CL) with pre-infusion CRP4,11 suggests an influence of target-antigen burden on infliximab pharmacokinetics, the elimination increasing with the target-antigen quantity. Therefore, a difference in TNF levels between immune-inflammatory diseases, as reported for RA and AS, could lead to differences in infliximab pharmacokinetics. Yet the hypothesis of an influence of the disease on this pharmacokinetics has never been assessed in an integrated pharmacokinetic study, i.e., using data obtained in a single center and with the same assay.
Table 1.
Infliximab pharmacokinetic parameters estimated by a population approach.
| Study | Disease | Patients (n) | Compartments (n) | Vss (L) | CL (L/day) | t1/2 (days) |
|---|---|---|---|---|---|---|
| Ternant 20067 | CD | 6 | 1 | 3 | 0.20 | 10.9 |
| Ternant 20088 | IBD | 33 | 2 | 5.50 | 0.29 | 20.3 |
| Fasanmade 20099 | UC | 482 | 2 | 7.30 | 0.38 | 13.4 |
| Fasanmade 201110 | CD | 692 | 2 | 4.90 | 0.38 | 11.5 |
| Ternant 201411 | CD | 111 | 1 | 5.80 | 0.29 | 14.0 |
| Dotan 201412 | IBD | 54 | 2 | 3.80 | 0.38 | 12.0 |
| Aubourg 201513 | CD | 133 | 2 | 7.10 | 0.34 | 15.7 |
| Present study | IBD | 79 | 1 | 7.95 | 0.35 | 15.5 |
| Xu, 20085 | AS | 274 | 2 | 6.00 | 0.27 | 15.8 |
| Ternant 20116 | AS | 25 | 2 | 4.10 | 0.21 | 13.8 |
| Present study | AS + PsA | 121 | 1 | 5.43 | 0.24 | 15.7 |
| Ternant 20144 | RA | 84 | 2 | 5.90 | 0.32 | 9.0* |
| Present study | RA | 18 | 1 | 5.43 | 0.30 | 10.5* |
Abbreviations are as follows: Vss, volume steady-state; CL, elimination clearance; t1/2, terminal elimination half-life; IBD; inflammatory bowel disease; AS, ankylosing spondylitis; PsA; psoriatic arthritis; RA, rheumatoid arthritis; CD, Crohn's disease; UC, ulcerative colitis.
without methotrexate cotreatment.
The clinical response increases with infliximab trough concentrations in RA,1,17,18 AS,2 IBD3,19,20 and psoriatic patients,21 and target concentrations predictive of good clinical response were proposed in RA (2.5 mg/L22), CD (3.5 mg/L23) and UC (3.7 mg/L24). The achievement of these targets depends on both dosage and infliximab pharmacokinetics. Recommended infliximab infusion dose ranges from 3 mg/kg (initiation dose in RA) to 10 mg/kg.25,26 However, to our knowledge, neither the relationship between dose and proportion of patients achieving the proposed target concentration nor the influence of dose increase on this proportion were reported.
The aims of this study were therefore to quantify the influence of the underlying disease on both infliximab pharmacokinetic parameters and on resulting patients' potential exposure to this anti-TNF biopharmaceutical.
Results
Patients
Two-hundred and eighteen patients were retrospectively included in this study (Table 2). Median infliximab starting dose was 5 mg/kg (IQR: 4.5 – 5.2 mg/kg) and 870 infliximab serum concentrations were available. Median treatment follow-up was 23 weeks (IQR: 15.6 – 25.0 weeks) and median delay between 2 infliximab infusions was 5.1 weeks (IQR: 3.1–6.3 weeks).
Table 2.
Patients and infliximab treatment characteristics.
| Characteristics | Patients (n = 218) |
|---|---|
| Women | 141 (65) |
| > 15 y old | 207 (95) |
| Weight (kg) | 67 [28.2–125] |
| AS/RA/PsA/CD/UC | 91/18/30/63/16 |
| Follow-up (weeks) | 23 [2–28.6] |
| Starting dose (mg/kg) | 5.0 [2.5–8.9] |
| Dosing interval (weeks) | 5.1 [1.4–17.9] |
Results are presented as the median [interquartile range] or as the number (percentage).
Pharmacokinetic analysis
Base model
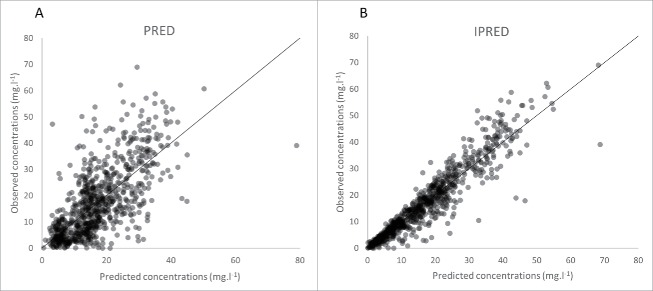
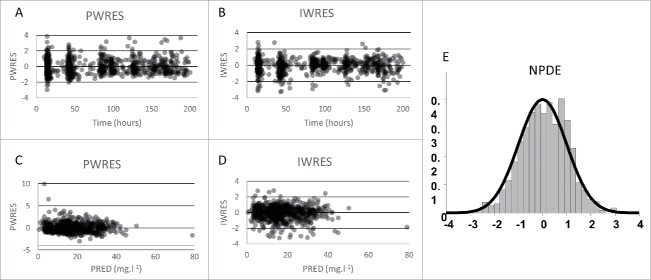
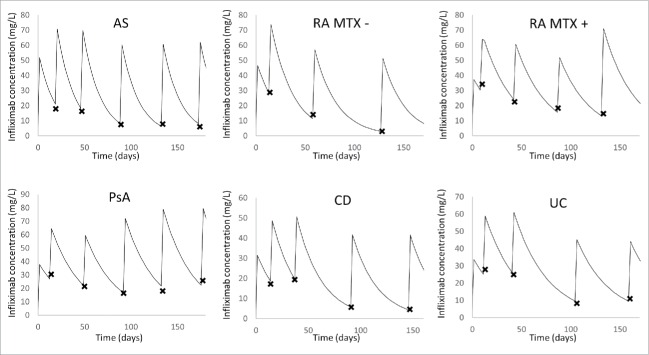
Infliximab concentrations were best described using a one compartment model with first-order elimination rate. Estimated infliximab pharmacokinetic parameters were volume of distribution (V, in L) and elimination clearance (CL in L/day). Parameters describing a peripheral compartment were not identifiable. The best residual error model was mixed additive-proportional. Plots of predicted vs. observed concentrations showed that infliximab concentration data were satisfactorily described by the pharmacokinetic model (Fig. 1). Even if some concentrations were over-predicted, population (PWRES) and individual (IWRES) residuals and normalized prediction distribution error (NPDE) plots showed that there was no major bias or model misspecification (Fig. 2). Even though only trough concentrations were available, our one-compartment model described satisfactorily the observed concentrations for each underlying disease (Fig. 3). The pharmacokinetic parameters and interindividual variance were estimated with satisfactory precision (Table 3).
Figure 1.
Observed vs. predicted concentrations of infliximab. Population predicted (PRED, A) and individual predicted values (IPRED, B).
Figure 2.
Population (top) and individual (bottom) residuals vs. time (A, B) and vs. predictions (C, D) and normalized population distribution error vs. Gaussian law (NPDE, E).
Figure 3.
Individual fits of observed and model-predicted infliximab concentrations. A representative patient is figured for each underlying disease: ankylosing spondylitis (AS), rheumatoid arthritis (RA) with (MTX +) and without (MTX -) methotrexate cotreatment, psoriatic arthritis (PsA), Crohn's disease (CD) and ulcerative colitis (UC). The observed concentrations are represented by crosses and the model-predicted concentrations over time is represented by the curve.
Table 3.
Infliximab pharmacokinetic parameters estimates.
| Parameter (unit) | Estimate | RSE (%) | ΔLL |
|---|---|---|---|
| V (L) | 5.2 | 4 | — |
| WT on V | 0.277 | 45 | 5.57 |
| Male sex on V | 0.209 | 30 | 12.66 |
| ≤ 15 y.o. on V | −0.396 | 37 | 8.65 |
| CD on V | 0.399 | 17 | 26.79 |
| UC on V | 0.417 | 27 | 21.21 |
| CL (L/day) | 0.23 | 4 | — |
| WT on CL | 0.603 | 18 | 30.96 |
| SEX on CL | 0.181 | 30 | 13.57 |
| CD on CL | 0.384 | 15 | 35.55 |
| UC on CL | 0.472 | 21 | 11.52 |
| RA on CL | 0.392 | 32 | 10.62 |
| MTX on CL | −0.336 | 52 | 4.78 |
| ωV | 0.224 | 12 | — |
| ωCL | 0.304 | 7 | — |
| σadd (mg/L) | 0.72 | 19 | — |
| σprop (%) | 0.223 | 7 | — |
The RSE (%) was obtained as follows: RSE = (estimates/standard error) × 100. Abbreviations are as follows: CD, Crohn's disease; CL, elimination clearance; LL, log-likelihood; MTX, methotrexate; RA, rheumatoid arthritis; RSE, relative standard error; UC, ulcerative colitis; V, volume of distribution; WT, body weight; ω, interindividual standard deviation; σadd, additive error standard deviation; σprop, proportional error standard deviation.
Final model with covariates
During the univariate step, weight, sex, CD and UC significantly influenced both V and CL; pediatric population and PsA significantly influenced V; and RA combined with methotrexate treatment significantly influenced CL. During the multivariate step, weight, sex, CD and UC significantly influenced both V and CL; pediatric population significantly influenced V and RA combined with methotrexate treatment significantly influenced CL. All these covariates remained statistically significant during the backward step analysis (Table 3).
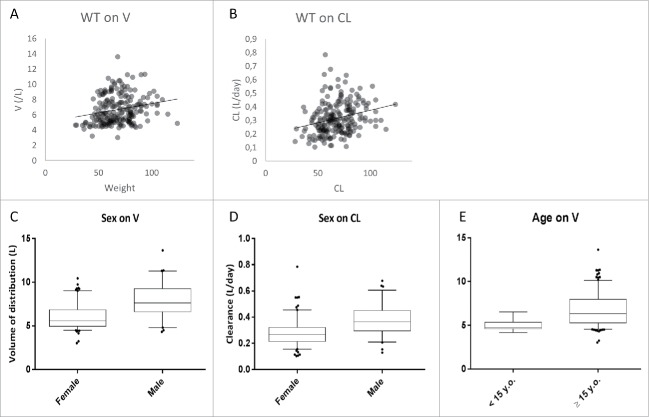
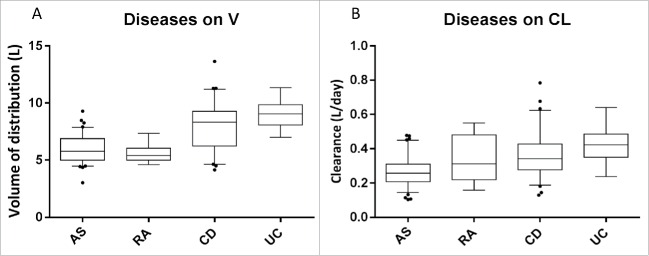
Infliximab V and CL increased with weight; a gain of 25 Kg was associated with an 11% increase in V (ΔOFV = 5.57; p = 0.018) and a 25% increase in CL (ΔOFV = 30.96; p < 0.00001) (Fig. 4). Men had a 23.2% (ΔOFV = 12.7; p = 0.00037) higher V and a 19.8% (ΔOFV = 13.6; p = 0.0002) higher CL than women. Volume of distribution was 32.7% lower in pediatric CD patients than in adults (ΔOFV = 8.65; p = 0.0032). Infliximab V (ΔOFV = 26.79; p < 0.00001) and CL (ΔOFV = 35.55; p < 0.00001) were higher in patients with CD than in those with AS. Infliximab V (ΔOFV = 21.21; p < 0.00001) and CL (ΔOFV = 44.98; p < 0.00001) were higher in patients with UC than in those with AS. Clearance was higher in patients with RA than in those with AS (ΔOFV = 10.62; p = 0.0011) (Fig. 5). Methotrexate was associated with a 28.5% decrease in infliximab CL in RA patients (ΔOFV = 4.78; p = 0.029).
Figure 4.
Influence of continuous (A, B) and binary (C, D, E) demographic covariates on pharmacokinetic parameters of infliximab: volume of distribution (A) and clearance (B) vs. body weight, and association of sex with volume of distribution (C) and clearance (D), and association of age with volume of distribution (E). From bottom to top, horizontal lines of boxes represent 5th, 25th, 50th, 75th and 95th percentiles (bottom).
Figure 5.
Influence of treated disease on the pharmacokinetic parameters of infliximab. Compared to ankylosing spondylitis (AS), there was an influence of inflammatory bowel disease (IBD) on volume of distribution (A) and on clearance (B), and of rheumatoid arthritis (RA) on clearance (C). From bottom to top, horizontal lines of boxes represent 5th, 25th, 50th, 75th and 95th percentiles.
Simulations
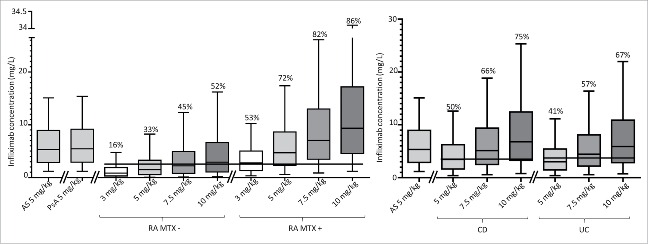
Doses of 3, 5, 7.5 and 10 mg/kg at weeks 0, 2, 8 and 14 were simulated using estimated structural, covariate, interindividual and residual parameters, which provided 90% prediction intervals of infliximab trough concentrations at steady state for AS, RA (with and without methotrexate), CD, UC and PsA. With infusion doses of 5 mg/kg, predicted infliximab concentrations were similar between AS and PsA patients, but were lower in RA, CD and UC patients than in AS patients (Fig. 6). The lower predicted infliximab concentrations in RA patients were due to an increase in CL and the lower predicted concentrations in CD and UC patients were due to an increase of both V and CL. For RA patients without methotrexate, simulations showed that with 3 mg/kg, 5 mg/kg, 7.5 mg/kg and 10 mg/kg infusions, proportion of patients above the concentration predictive of good clinical response using the threshold of 2.5 mg/L22 would be 16%, 33%, 45% and 52%, respectively (Fig. 6). With methotrexate cotreatment, these proportions would be 53%, 72%, 82% and 86%, respectively. Regarding CD patients, simulations with doses of 5 mg/kg, 7.5 mg/kg and 10 mg/kg showed that the proportion of patients above the concentration predictive of good clinical response (3.5 mg/L23) would be 50%, 66% and 75%, respectively (Fig. 6). Regarding UC patients, these proportions would be 41%, 57% and 67% for a target concentration of 3.7 mg/L.24
Figure 6.
Boxplots representing 90% prediction intervals of simulated trough concentrations of infliximab at week 22 following 3, 5 and 7.5 mg/kg doses infused at weeks 0, 2, 6 and 14 in ankylosing spondylitis (AS), psoriatic arthritis (PsA), rheumatoid arthritis (RA), and inflammatory bowel diseases (IBD). Horizontal dotted or solid lines represent reported concentrations predictive of good clinical response for RA (2.5 mg/kg), Crohn's disease (3.5 mg/L) and ulcerative colitis (3.7 mg/L). Percentages are proportion of patients above the target concentration for the corresponding disease. From bottom to top, horizontal lines of boxes represent 5th, 25th, 50th, 75th and 95th percentiles.
Discussion
Although differences of infliximab pharmacokinetics according to the underlying disease are suggested in literature (Table 1), our study is the first to quantify its influence. Moreover, by using the same validated ELISA technique to analyze all samples, we ensured that there were no assay-related differences in estimated pharmacokinetic parameters, as previously observed when different ELISA techniques were compared.27 Since only trough concentrations were available, we used a one-compartment model. Because most studies used a 2-compartmental model to describe infliximab pharmacokinetics,4-6,8-10,12,13 there is a potential risk of increased bias and decreased precision of PK parameter estimation. However, this limitation was reported for compounds with poorly known pharmacokinetics, which is not the case of infliximab.28 Two main aspects have been suggested to lead to bias in infliximab pharmacokinetic parameter estimates: 1) the large intra-individual variability observed during follow-ups longer than 6 months, and/or 2) the inclusion of patients both with and without anti-drug antibodies (ADA).13 In this study, we assessed patients for whom infliximab concentrations were available at treatment initiation and up to 25 weeks, and in whom ADA was not detected during this period. Overall elimination half-life of infliximab was 15 days, a value that is in agreement with most published studies (Table 3).
Our study demonstrated that infliximab pharmacokinetics was significantly influenced by the underlying disease. First, to our knowledge, this is the first study that describes the pharmacokinetics of infliximab in PsA patients, and shows the estimated parameters are not different from those of AS patients. In RA, infliximab CL was higher (+ 48 %) than in AS. This may be explained by target-mediated drug disposition (TMDD), as target-antigen burden is likely to be high in RA patients: 1) infliximab CL increases with pre-infusion CRP4 and TNF levels increase with CRP concentrations,29 2) systemic inflammation (i.e., TNF burden) is higher in RA than in AS,30 and 3) high levels of soluble TNF were reported in blood31 and synovial fluid32 of RA patients. Methotrexate cotreatment was associated with decreased infliximab CL in RA patients, leading to a longer elimination half-life in patients with than without methotrexate cotreatment (14.8 vs. 10.5 days, respectively), a difference similar to values previously reported (13 and 9 d with and without methotrexate cotreatment, respectively4). The effect of methotrexate was kept in the final model to avoid misevaluation of the influence of the underlying disease on infliximab pharmacokinetics. The lower anti-infliximab antibodies prevalence in RA patients with methotrexate than in those without methotrexate was previously reported.33 Nevertheless, we conducted our study only in patients without detected anti-infliximab antibodies. Our results consequently suggest an influence of methotrexate on infliximab pharmacokinetics, independent of ADA influence.
Both V and CL were higher in CD and UC patients than in AS patients. Similarly to what was reported in RA, we previously showed that infliximab CL increases with pre-infusion CRP in CD patients, an observation that may also be explained by TMDD.11 In addition, studies reported loss of infliximab in stools of patients with CD or UC.34,35 This fecal loss may lead to lower blood concentrations of infliximab and consequently to an overestimation of V.
In IBD, data suggest that infliximab acts at least partly by antibody-dependent cell-meditated cytotoxicity.36 Infliximab binds to transmembrane TNF tightly, notably compared to etanercept.37 Inflammatory bowel diseases are characterized by substantial leucocyte infiltration38 with various cells expressing transmembrane TNF.39 Increase in V may therefore be explained by retention of the monoclonal antibody by cellular TNF. A similar phenomenon was reported for another monoclonal antibody, rituximab (an anti-CD20 monoclonal antibody): its volume at steady-state (Vss) was 6.6 L, 5.6 L and 13.5 L, in RA,40 follicular non-Hodgkin lymphomas41 and diffuse large-cell lymphomas,42 respectively. In all, the similar elimination half-life of infliximab observed in IBD and in AS (Table 3) may result from opposite effects of TNF, the higher CL estimated in IBD being compensated by a higher V as compared with AS.
Of note, our study was conducted on only 18 RA and 16 UC patients. Even though the effect on volume of distribution of these diseases was satisfactorily estimated (RSE = 32% and 20%, respectively), it would be of interest to confirm these results on a larger cohort of patients.
The results of this study confirm the previously reported increase in V and CL with body weight and their higher values in men than in women.5,9,13 Unexpectedly, we found a higher V in pediatric CD patients than in adults. As for the influence of methotrexate on CL, this effect of age on V was kept in the final model. To our knowledge, the influence of age on infliximab PK was not reported previously. Very few studies reported the influence of age on a pharmacokinetic parameter of a monoclonal antibody. A study on prematurely born children under 37 months showed that apparent V and apparent CL of an anti-respiratory syncytial virus monoclonal antibody were higher in younger patients.43 Regarding elimination, we previously reported that children receiving adalimumab for a focal segmental glomerulosclerosis had a faster elimination than adults (elimination half-lives of 1.7 and 5.7 days, respectively).44 A recent review suggests that differences between adults and children may be explained by specific factors (such as target-antigen burden), a hypothesis which deserves further investigation.45 Of note, the influence of age on absorption was reported for an anti-interleukin 4 receptor monoclonal antibody administered subcutaneously, the absorption rate decreasing with age.46
The infliximab pharmacokinetic parameters estimated in our study were used to predict the distribution of infliximab trough concentrations at steady-state and compare them with cut-off concentrations predictive of good clinical response. In RA, the published cut-off value is 2.5 mg/L.22 Simulations highlighted the effect of methotrexate cotreatment on the proportion of patients reaching target infliximab concentration. For initial 3 mg/kg infusions every 8 weeks, this proportion would be more than 3 times higher for patients receiving methotrexate than for those without methotrexate (53% vs 16%, respectively). According to our simulations, the proportion of patients receiving 10 mg/kg infusions of infliximab without methotrexate with infliximab concentration above the target value would be similar to that of patients receiving 3 mg/kg infusions of infliximab with methotrexate. This low proportion of patients with concentrations above the target is consistent with the dose intensification reported in 30–50% of RA patients.47,48 In addition to the increase in the proportion of patient above the threshold for response, increased trough concentrations may decrease the risk of developing ADA.14,49 For IBD, approximately half of patients are above target concentrations (3.5 mg/L and 3.7 mg/L for CD and UC, respectively), meaning that half of patients would need dose intensification, a figure which is consistent with the fact that infliximab dosing regimen is intensified in 45% of CD patients.50 This suboptimality of dosing regimen advocates the development of infliximab concentration monitoring to adjust the dose of the monoclonal antibody individually.
In conclusion, our study is the first to quantify the influence of underlying disease on infliximab pharmacokinetics. Compared to AS patients, RA patients have a higher CL, whereas CD and UC patients both have a higher CL and a higher V. Furthermore, RA patients without methotrexate may benefit from initiation infusion doses higher than 3 mg/kg.
Materials and methods
Study design
The study was conducted on a retrospective cohort of 363 patients of daily practice treated and followed up in the University Hospital of Tours, France, between 2006 and 2012. To allow a robust estimation of PK parameters, we assessed patients for which infliximab trough serum concentrations were available before the first infusion and at each visit, and in whom no ADA was detected during the follow-up. Of 363 patients, 218 were selected for the present study. Ninety-one patients had AS, 63 had CD, 16 had UC, 18 had RA and 30 had PsA. (Table 1). Among CD patients, 10 were pediatric patients (< 15 y.o.). One AS patient was pediatric. Among RA patients, 9 were also treated with methotrexate. As part of routine therapeutic drug monitoring of patients treated with infliximab, blood samples were collected to measure infliximab serum trough concentrations. Individual results were sent to the prescriber and discussed during clinic-biological rounds. The samples were therefore not drawn specifically for this study, which was performed retrospectively. Part of these data were used in previously published studies.13,14
Blood samples were collected immediately before a next infusion of infliximab. Trough serum infliximab concentrations were measured in the laboratory of Pharmacology-Toxicology of the University Hospital of Tours, France, using a validated enzyme-linked immunosorbent assay.7 The limit of detection was 0.014 mg/L and the lower (LLOQ) and upper limits of quantification (ULOQ) were 0.04 mg/L (between-assay accuracy coefficient of variation: 9.8%) and 4.5 mg/L (between-assay accuracy coefficient of variation: 5.3%), respectively.
Pharmacokinetic analysis
Software
Pharmacokinetic data were analyzed with a population approach using the nonlinear mixed-effects modeling software Monolix 4.3.3. (Lixoft, Orsay, France), which combines the stochastic expectation-maximization (SAEM) algorithm and a Markov chain Monte-Carlo procedure for likelihood maximization. To ensure the best possible convergence, a large number of iterations (1000 for K1, 300 for K2) were performed, with K1 and K2 being “iteration kernels” referring to the SAEM procedure of Monolix. During K1, the sequence of step sizes is constant, which allows exploration of the parameter space. During K2, the step sizes decrease to ensure convergence. Simulated annealing was applied to improve the convergence of the SAEM algorithm toward the global maximum of the likelihood. The Fisher Information Matrix and likelihood were computed using stochastic approximation and importance sampling, respectively. These techniques are longer to compute than linearization, but provide more reliable results. The random seed was changed between each run.
Structural models
Infliximab concentrations were analyzed using compartmental pharmacokinetic modeling. One and 2 mammillary models were tested. Structural models were compared using Akaike's information criterion (AIC) defined as follows: AIC=−2LL + 2p, where −2LL is −2 x ln-likelihood and p is the number of model parameters to estimate. The model with the lowest AIC was selected.
Interindividual and residual error models
The interindividual variability of pharmacokinetic parameters was described using the following exponential model: θi = θTV+exp(ηi), where θi is the estimated individual parameter, θTV is the typical value of the parameter and ηi is the random effect for the ith patient. The values of ηi were assumed to be normally distributed, with mean 0 and variance ω2. The variance ω2 of a given parameter was fixed to 0 if its ηi could not be estimated properly. Additive, proportional and mixed additive-proportional error models were tested. For example, the combined additive-proportional model was implemented as follows: Yo,ij = YP,ij×(1+εprop,ij) + εadd,ij, where YO,ij and YP,ij are observed and predicted jth measurements for the ith patient, respectively, and εadd,ij and εprop,ij are additive and proportional errors, with a mean 0 and respective variances σadd2 and σprop2.
Covariates
Several potential sources of interindividual variability were tested as covariates. Body weight (WT) was tested as a continuous covariate after it was power-transformed and centered on its median as follows: θi = θ0 × (COV/med(WT))βweight, where θ0 is the value of θ for a median subject, βweight is the influence of WT on θ and med(WT) is the median WT of the population. Sex, age and the underlying disease (CD, UC, RA, PsA) were tested as binary covariates. Age was dichotomized into 2 categories: pediatrics (<15 years) and adult (≥15 years) patients. The pediatric AS patient was not included in the pediatric group. Adult (≥15 y old) female patients with an AS were considered as reference. The influence of a binary covariate (CT) on θTV was implemented as ln(θTV) = ln(θCAT=0)+βCAT=1, where θCAT=0 is the value of θ for the reference category and βCAT=1 provides value of θTV for the other category. Because methotrexate cotreatment was reported to decrease infliximab CL, methotrexate cotreatment was tested as a covariate in RA patients.
Model comparison and covariate selection
Interindividual, residual and covariate models were compared using the −2 log-likelihood (−2LL) as objective function value (OFV) and AIC. From pairs of nested models, the model with the lowest −2LL was chosen. This was assessed by a likelihood ratio test (LRT), in which the difference in OFV between 2 models is assumed to follow a Χ2 distribution. The influence of patient covariates was assessed with a 2 steps approach defined as follows:
Univariate step. The influence of each covariate on pharmacokinetic or pharmacodynamic parameters associated with interindividual variability was tested by including them separately in the base model. Covariates showing a significant influence (α < 0.1) were included in the full model.
Multivariate step. A forward stepwise multivariable analysis was performed where all covariates were added one by one. Covariates whose addition resulted in a statistically significant decrease in the OFV (α < 0.02) were retained for the backward stepwise elimination. In this step, the covariates of the full model were removed one by one. Covariates whose removal resulted in a statistically significant increase in the OFV (α < 0.02) were retained in the final model.
Model goodness of fit and evaluation
The goodness of fit was assessed for each model by plotting population-predicted (PRED) and individually predicted (IPRED) concentrations vs. observed concentrations (DV) and IPRED and DV vs. time. Population predictions were obtained using typical parameters, which include explained variability (i.e., population estimates and covariates), whereas individually predicted concentrations were obtained using individual parameters, which include both explained and unexplained variability (i.e., the random effects ηi for each parameter). In addition, the distribution of residuals was evaluated by graphical inspection of population (PWRES) and individual weighted residual distributions (IWRES), and normalized prediction distribution errors (NPDE). These residuals should follow a standard normal distribution to confirm a satisfactory fit of the model to the data and to allow a Χ2 distribution for LRT tests.
Simulation process
Simulation of infliximab concentrations in AS, IBD, RA and PsA patients given different infliximab doses (3, 5, 7.5 and 10 mg/kg at weeks 0, 2, 6, and 14) were performed. A virtual population of patients was created using each patient's individual characteristics (age, body weight, sex, underlying disease and age) and infliximab infusions at weeks 0, 2, 6 and 14. Tested doses were 5 mg/kg for all diseases, 3, 7.5 and 10 mg/kg for RA and 7.5 and 10 mg/kg for IBD patients since 10 mg/kg is the highest approved infusion dose. Simulations were made using typical parameters and interindividual variances of the final model (Table 2). The data set was simulated 1000 times. The 90% confidence interval of simulated steady-state infliximab trough concentrations at 22 weeks was compared to infliximab trough concentrations predictive of good clinical response in RA (2.5 mg/L,22), Crohn's disease (3.5 mg/L,23) and ulcerative colitis (3.7 mg/L,24).
Disclosure of potential conflicts of interest
Gilles Paintaud has been a consultant for Laboratoire Français du Fractionnement et des Biotechnologies (LFB) and Pierre Fabre Laboratories. His research team has received grants from Roche Pharma, Chugai, Pfizer, Novartis, and Janssen. Denis Mulleman participated on behalf of his institution in clinical trials sponsored by Abbott, Roche, BMS, Pfizer, UCB and MSD; his hospital received a grant for research from Abbott in 2004 and from Nordic Pharma in 2012; he has acted as a consultant and given lectures on behalf of his institution for MSD and Pfizer; he has been invited to attend international congresses by MSD, Roche, BMS, Abbott and Janssen-Cilag. Philippe Goupille declares no support from any organization of the submitted work; participated on behalf of his institution in clinical trials sponsored by Abbott, Roche, BMS, Lilly, Novartis, Pfizer, UCB and MSD; he has been a consultant and given lectures on behalf of his institution for Abbott, BMS, MSD, Pfizer, UCB; he has been invited to attend international congresses by MSD, Roche, BMS and Abbott. Stephanie Willot received fees from Abbvie for a symposium. Alexandre Aubourg has been a consultant for MSD and Biogaran. David Ternant has given lectures for Amgen and Sanofi. Theodora Bejan-Angoulvant, Thierry Lecomte, Laurence Picon and Christophe Passot declare no conflict of interest.
Acknowledgments
The authors would like to thank Céline Desvignes for analytical help, Anne-Claire Duveau and Caroline Guerineau for technical assistance with infliximab assays, nurses and medical staff from the Rheumatology department, the Gastroenterology department and the Pediatrics department. Measurement of infliximab serum concentrations was carried out within the CePiBAc platform, cofinanced by the European Union. Europe is committed to the region Center with the European Regional Development Fund. CNRS UMR 7292 participates in the Consortium “Monitoring of monoclonal Antibodies Group in Europe” (MAGE) for inflammatory diseases. The MAGE Consortium is supported by LE STUDIUM Loire Valley Institute for Advanced Studies (http://www.lestudium-ias.com/).
Funding
This work was partly supported by the French Higher Education and Research Ministry under the program “Investissements d'avenir” Grant Agreement: LabEx MAbImprove ANR-10-LABX-53-01.
Ethical statement
Ethical approval and informed consent were not sought in this retrospective analysis of routine patients, which is in accordance with institutional guidelines.
References
- 1.St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, Keystone EC. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002; 46:1451-9; PMID:12115174; http://dx.doi.org/ 10.1002/art.10302 [DOI] [PubMed] [Google Scholar]
- 2.Krzysiek R, Breban M, Ravaud P, Prejean MV, Wijdenes J, Roy C, Henry YD, Barbey C, Trappe G, Dougados M, et al.. Circulating concentration of infliximab and response to treatment in ankylosing spondylitis: results from a randomized control study. Arthritis Rheum 2009; 61:569-76; PMID:19405015; http://dx.doi.org/ 10.1002/art.24275 [DOI] [PubMed] [Google Scholar]
- 3.Karmiris K, Paintaud G, Noman M, Magdelaine-Beuzelin C, Ferrante M, Degenne D, Claes K, Coopman T, Van Schuerbeek N, Van Assche G, et al.. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology 2009; 137:1628-40; PMID:19664627; http://dx.doi.org/ 10.1053/j.gastro.2009.07.062 [DOI] [PubMed] [Google Scholar]
- 4.Ternant D, Ducourau E, Perdriger A, Corondan A, Le Goff B, Devauchelle-Pensec V, Solau-Gervais E, Watier H, Goupille P, Paintaud G, et al.. Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis. Br J Clin Pharmacol 2014; 78:118-28; PMID:24354889; http://dx.doi.org/ 10.1111/bcp.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, Seitz K, Fasanmade A, Ford J, Williamson P, Xu W, Davis HM, Zhou H. Population pharmacokinetics of infliximab in patients with ankylosing spondylitis. J Clin Pharmacol 2008; 48:681-95; PMID:18401017; http://dx.doi.org/ 10.1177/0091270008316886 [DOI] [PubMed] [Google Scholar]
- 6.Ternant D, Mulleman D, Lauféron F, Vignault C, Ducourau E, Wendling D, Goupille P, Paintaud G. Influence of methotrexate on infliximab pharmacokinetics and pharmacodynamics in ankylosing spondylitis. Br J Clin Pharmacol 2012; 73:55-65; PMID:21692827; http://dx.doi.org/ 10.1111/j.1365-2125.2011.04050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ternant D, Mulleman D, Degenne D, Willot S, Guillaumin J-M, Watier H, Goupille P, Paintaud G. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther Drug Monit 2006; 28:169-74; PMID:16628126; http://dx.doi.org/ 10.1097/01.ftd.0000189901.08684.4b [DOI] [PubMed] [Google Scholar]
- 8.Ternant D, Aubourg A, Magdelaine-Beuzelin C, Degenne D, Watier H, Picon L, Paintaud G. Infliximab pharmacokinetics in inflammatory bowel disease patients. Ther Drug Monit 2008; 30:523-9; PMID:18641542; http://dx.doi.org/ 10.1097/FTD.0b013e318180e300 [DOI] [PubMed] [Google Scholar]
- 9.Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, Davis HM, Zhou H. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 2009; 65:1211-28; PMID:19756557; http://dx.doi.org/ 10.1007/s00228-009-0718-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM. Pharmacokinetic properties of infliximab in children and adults with Crohn's disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther 2011; 33:946-64; PMID:21741088; http://dx.doi.org/ 10.1016/j.clinthera.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 11.Ternant D, Berkane Z, Picon L, Gouilleux-Gruart V, Colombel J-F, Allez M, Louis E, Paintaud G. Assessment of the influence of inflammation and FCGR3A Genotype on Infliximab pharmacokinetics and time to relapse in patients with Crohn's disease. Clin Pharmacokinet 2015; 54:551-62; PMID:25516415; http://dx.doi.org/ 10.1007/s40262-014-0225-3 [DOI] [PubMed] [Google Scholar]
- 12.Dotan I, Ron Y, Yanai H, Becker S, Fishman S, Yahav L, Ben Yehoyada M, Mould DR. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis 2014; 20:2247-59; PMID:25358062; http://dx.doi.org/ 10.1097/MIB.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 13.Aubourg A, Picon L, Lecomte T, Bejan-Angoulvant T, Paintaud G, Ternant D. A robust estimation of infliximab pharmacokinetic parameters in Crohn's disease. Eur J Clin Pharmacol 2015; 71(12):1541-2; PMID:26369535; http://dx.doi.org/ 10.1007/s00228-015-1942-8 [DOI] [PubMed] [Google Scholar]
- 14.Ducourau E, Mulleman D, Paintaud G, Miow Lin DC, Lauféron F, Ternant D, Watier H, Goupille P. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther 2011; 13:R105; PMID:21708018; http://dx.doi.org/ 10.1186/ar3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Bemt BJ, den Broeder AA, Wolbink GJ, Hekster YA, van Riel PL, Benraad B, van den Hoogen FH. Anti-infliximab antibodies are already detectable in most patients with rheumatoid arthritis halfway through an infusion cycle: an open-label pharmacokinetic cohort study. BMC Musculoskelet Disord 2011; 12:12; PMID:21232150; http://dx.doi.org/ 10.1186/1471-2474-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ternant D, Bejan-Angoulvant T, Passot C, Mulleman D, Paintaud G. Clinical pharmacokinetics and pharmacodynamics of monoclonal antibodies approved to treat rheumatoid arthritis. Clin Pharmacokinet 2015; 54:1107-23; PMID:26123705; http://dx.doi.org/ 10.1007/s40262-015-0296-9 [DOI] [PubMed] [Google Scholar]
- 17.Mulleman D, Chu Miow Lin D, Ducourau E, Emond P, Ternant D, Magdelaine-Beuzelin C, Valat JP, Paintaud G, Goupille P. Trough infliximab concentrations predict efficacy and sustained control of disease activity in rheumatoid arthritis. Ther Drug Monit 2010; 32:232-6; PMID:20216124; http://dx.doi.org/ 10.1097/FTD.0b013e3181cc6fef [DOI] [PubMed] [Google Scholar]
- 18.Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, Dijkmans BA, Aarden L. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005; 64:704-7; PMID:15485995; http://dx.doi.org/ 10.1136/ard.2004.030452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baert F, Noman M, Vermeire S, Van Assche G, D'Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003; 348:601-8; PMID:12584368; http://dx.doi.org/ 10.1056/NEJMoa020888 [DOI] [PubMed] [Google Scholar]
- 20.Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol 2006; 4:1248-54; PMID:16931170; http://dx.doi.org/ 10.1016/j.cgh.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 21.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CE. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet (London, England) 2005; 366:1367-74; PMID:16226614; http://dx.doi.org/ 10.1016/S0140-6736(05)67566-6 [DOI] [PubMed] [Google Scholar]
- 22.van den Bemt BJ, den Broeder AA, Wolbink GJ, van den Maas A, Hekster YA, van Riel PL, Benraad HB, van den Hoogen FH. The combined use of disease activity and infliximab serum trough concentrations for early prediction of (non-)response to infliximab in rheumatoid arthritis. Br J Clin Pharmacol 2013; 76:939-45; PMID:23601129; http://dx.doi.org/ 10.1111/bcp.12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornillie F, Hanauer SB, Diamond RH, Wang J, Tang KL, Xu Z, Rutgeerts P, Vermeire S. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014; 63:1721-7; PMID:24474383; http://dx.doi.org/ 10.1136/gutjnl-2012-304094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adedokun OJ, Sandborn WJ, Feagan BG, Rutgeerts P, Xu Z, Marano CW, Johanns J, Zhou H, Davis HM, Cornillie F, et al.. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014; 147:1296-307.e5; PMID:25173754; http://dx.doi.org/ 10.1053/j.gastro.2014.08.035 [DOI] [PubMed] [Google Scholar]
- 25.FDA Infliximab label information. Accessed at http://http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf Accessed 07January2016 [Internet]. [cited 2014August21]; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf [Google Scholar]
- 26.EMA Infliximab. Summary of product characteristics. Accessed at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdf Accessed 26January2016 [Internet]. [cited 2016January7]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdf [Google Scholar]
- 27.Fischer SK, Yang J, Anand B, Cowan K, Hendricks R, Li J, Nakamura G, Song A. The assay design used for measurement of therapeutic antibody concentrations can affect pharmacokinetic parameters: Case studies. MAbs 2012; 4:623-31; PMID:22820463; http://dx.doi.org/ 10.4161/mabs.20814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Booth BP, Gobburu JV. Considerations in analyzing single-trough concentrations using mixed-effects modeling. J Clin Pharmacol 2003; 43:1307-15; PMID:14615466; http://dx.doi.org/ 10.1177/0091270003258670 [DOI] [PubMed] [Google Scholar]
- 29.Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan YX, Jiang YF. Plasma levels of IL-37 and correlation with TNF-α, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS One 2014; 9:e95346; PMID:24788826; http://dx.doi.org/ 10.1371/journal.pone.0095346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gratacós J, Collado A, Filella X, Sanmartí R, Cañete J, Llena J, Molina R, Ballesta A, Muñoz-Gómez J. Serum cytokines (IL-6, TNF-α, IL-1 β and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol 1994; 33:927-31; PMID:7921752; http://dx.doi.org/8457224 10.1093/rheumatology/33.10.927 [DOI] [PubMed] [Google Scholar]
- 31.Manicourt DH, Triki R, Fukuda K, Devogelaer JP, Nagant de Deuxchaisnes C, Thonar EJ. Levels of circulating tumor necrosis factor α and interleukin-6 in patients with rheumatoid arthritis. Relationship to serum levels of hyaluronan and antigenic keratan sulfate. Arthritis Rheum 1993; 36:490-9; PMID:8457224; http://dx.doi.org/ 10.1002/art.1780360409 [DOI] [PubMed] [Google Scholar]
- 32.Manicourt DH, Poilvache P, Egeren A Van, Devogelaer JP, Lenz ME, Thonar EJ. Synovial fluid levels of tumor necrosis factor α and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum 2000; 43:281; PMID:10693867; http://dx.doi.org/ 10.1002/1529-0131(200002)43:2%3c281::AID-ANR7%3e3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- 33.Thomas SS, Borazan N, Barroso N, Duan L, Taroumian S, Kretzmann B, Bardales R, Elashoff D, Vangala S, Furst DE. Comparative immunogenicity of TNF inhibitors: Impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. Bio Drugs 2015; 29:241-58; PMID:26280210; http://dx.doi.org/25917786 10.1007/s40259-015-0134-5 [DOI] [PubMed] [Google Scholar]
- 34.Brandse JF, van den Brink GR, Wildenberg ME, van der Kleij D, Rispens T, Jansen JM, Mathôt RA, Ponsioen CY, Löwenberg M, D'Haens GR. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015; 149:350-5.e2; PMID:25917786; http://dx.doi.org/ 10.1053/j.gastro.2015.04.016 [DOI] [PubMed] [Google Scholar]
- 35.Brandse JF, Wildenberg ME, de Bruyn JR, Wolbink G, Lowenberg M, Ponsioen CY, van den Brink GR, D'Haens GR. M. P500 Fecal loss of infliximab as a cause of lack of response in severe inflammatory bowel disease. J Crohn's Colitis 2013; 7:S210; PMID:25917786; http://dx.doi.org/23358932 10.1016/S1873-9946(13)60521-8 [DOI] [Google Scholar]
- 36.Moroi R, Endo K, Kinouchi Y, Shiga H, Kakuta Y, Kuroha M, Kanazawa Y, Shimodaira Y, Horiuchi T, Takahashi S, et al.. FCGR3A-158 polymorphism influences the biological response to infliximab in Crohn's disease through affecting the ADCC activity. Immunogenetics 2013; 65:265-71; PMID:23358932; http://dx.doi.org/ 10.1007/s00251-013-0679-8 [DOI] [PubMed] [Google Scholar]
- 37.Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002; 301:418-26; PMID:11961039; http://dx.doi.org/ 10.1124/jpet.301.2.418 [DOI] [PubMed] [Google Scholar]
- 38.Leitner GC, Vogelsang H. Pharmacological- and non-pharmacological therapeutic approaches in inflammatory bowel disease in adults. World J Gastrointest Pharmacol Ther 2016; 7:5-20; PMID:26855808; http://dx.doi.org/ 10.4292/wjgpt.v7.i1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010; 49:1215-28; PMID:20194223; http://dx.doi.org/ 10.1093/rheumatology/keq031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol 2005; 45:792-801; PMID:15951469; http://dx.doi.org/ 10.1177/0091270005277075 [DOI] [PubMed] [Google Scholar]
- 41.Iacona I, Lazzarino M, Avanzini MA, Rupolo M, Arcaini L, Astori C, Lunghi F, Orlandi E, Morra E, Zagonel V, et al.. Rituximab (IDEC-C2B8): validation of a sensitive enzyme-linked immunoassay applied to a clinical pharmacokinetic study. Ther Drug Monit 2000; 22:295-301; PMID:10850396; http://dx.doi.org/ 10.1097/00007691-200006000-00010 [DOI] [PubMed] [Google Scholar]
- 42.Müller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, Wenger M, Nickenig C, Peter N, Lengfelder E, et al.. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood 2012; 119:3276-84; PMID:22337718; http://dx.doi.org/ 10.1182/blood-2011-09-380949 [DOI] [PubMed] [Google Scholar]
- 43.Meissner HC, Groothuis JR, Rodriguez WJ, Welliver RC, Hogg G, Gray PH, Loh R, Simoes EA, Sly P, Miller AK, et al.. Safety and pharmacokinetics of an intramuscular monoclonal antibody (SB 209763) against respiratory syncytial virus (RSV) in infants and young children at risk for severe RSV disease. Antimicrob Agents Chemother 1999; 43:1183-8; PMID:10223933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ternant D, Paintaud G, Trachtman H, Gipson DS, Joy MS. A possible influence of age on absorption and elimination of adalimumab in focal segmental glomerulosclerosis (FSGS). Eur J Clin Pharmacol 2015; 2016 Feb; 72(2):253-5; PMID:26521258; http://dx.doi.org/25516414 10.1007/s00228-015-1973-1 [DOI] [PubMed] [Google Scholar]
- 45.Edlund H, Melin J, Parra-Guillen ZP, Kloft C. Pharmacokinetics and pharmacokinetic-pharmacodynamic relationships of monoclonal antibodies in children. Clin Pharmacokinet 2015; 54:35-80; PMID:25516414; http://dx.doi.org/ 10.1007/s40262-014-0208-4 [DOI] [PubMed] [Google Scholar]
- 46.Kakkar T, Sung C, Gibiansky L, Vu T, Narayanan A, Lin SL, Vincent M, Banfield C, Colbert A, Hoofring S, et al.. Population PK and IgE pharmacodynamic analysis of a fully human monoclonal antibody against IL4 receptor. Pharm Res 2011; 28:2530-42; PMID:21604075; http://dx.doi.org/ 10.1007/s11095-011-0481-y [DOI] [PubMed] [Google Scholar]
- 47.Berger A. Dose intensification with infliximab in patients with rheumatoid arthritis. Ann Pharmacother 2005; 39:2021-5; PMID:16288073; http://dx.doi.org/ 10.1345/aph.1G264 [DOI] [PubMed] [Google Scholar]
- 48.Rahman MU, Strusberg I, Geusens P, Berman A, Yocum D, Baker D, Wagner C, Han J, Westhovens R. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis 2007; 66:1233-8; PMID:17392352; http://dx.doi.org/ 10.1136/ard.2006.065995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor α inhibitor infliximab. Arthritis Rheum 2006; 54:3782-9; PMID:17133559; http://dx.doi.org/ 10.1002/art.22214 [DOI] [PubMed] [Google Scholar]
- 50.Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, Simoens S, Rutgeerts P, Gils A, Vermeire S. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015; 148:1320-9.e3; PMID:25724455; http://dx.doi.org/ 10.1053/j.gastro.2015.02.031 [DOI] [PubMed] [Google Scholar]