ABSTRACT
Although the recently concluded CLEOPATRA trial showed clinical benefits of combining trastuzumab and pertuzumab for treating HER2-positive metastatic breast cancer, trastuzumab monotherapy is still the mainstay in adjuvant settings. Since trastuzumab resistance occurs in over half of these cancers, we examined the mechanisms by which treatment of intrinsically trastuzumab-resistant and -sensitive tumors can benefit from the combination of these antibodies. F(ab′)2 of both trastuzumab and pertuzumab were generated and validated in order to separately analyze antibody-dependent cell-mediated cytotoxicity (ADCC)-based and direct biological effects of the antibodies. Compared to monotherapy, combination of the two antibodies at clinically permitted doses enhanced the recruitment of natural killer cells responsible for ADCC, and significantly delayed the outgrowth of xenografts from intrinsically trastuzumab-resistant JIMT-1 cells. Antibody dose-response curves of in vitro ADCC showed that antibody-mediated killing can be saturated, and the two antibodies exert an additive effect at sub-saturation doses. Thus, the additive effect in vivo indicates that therapeutic tissue levels likely do not saturate ADCC. Additionally, isobole studies with the in vitro trastuzumab-sensitive BT-474 cells showed that the direct biological effect of combined treatment is additive, and surpasses the maximum effect of either monotherapy. Our results suggest the combined therapy is expected to give results that are superior to monotherapy, whatever the type of HER2-positive tumor may be. The combination of both antibodies at maximum clinically approved doses should thus be administered to patients to recruit maximum ADCC and cause maximum direct biological growth inhibition.
KEYWORDS: Antibody-dependent cell-mediated cytotoxicity (ADCC), combination antibody therapy, Herceptin resistance, HER2, pertuzumab, resistance, trastuzumab
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- DAPI
diamidino-2-phenylindole
- EC50
half maximal effective concentration
- F(ab’)2
F(ab’)2, bivalent antibody fragment of 2 Fab domains connected by disulfide bonds, lacking the Fc domain
- FCS
fetal calf serum
- HER2
human epidermal growth factor receptor 2, (a.k.a. ErbB2)
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NK
natural killer
- SCID
severe combined immunodeficiency
Introduction
Overexpression of HER2 is found in different types of human malignancies, including 20–25% of breast cancers1,2 and 17–22% of gastric cancers,3 and it is associated with aggressive growth and poor clinical outcomes. The breakthrough finding that some anti-HER2 antibodies can inhibit the growth of cancer cells overexpressing HER2 on their surface led to the development of several antibodies against the extracellular domain of the protein.4 Trastuzumab (Herceptin®), a humanized anti-HER2 monoclonal antibody, was the first antibody approved as a treatment for HER2-positive metastatic breast cancer,5 and, due to its remarkable success, it is currently a first-line treatment. Its ability to inhibit in vitro and in vivo tumor growth6,7 is attributed to internalization and down-regulation of cell surface HER2,8 inhibition of the PI3K/Akt pathway,9 cell cycle arrest in G1, inhibition of angiogenesis10 and antibody-dependent cell-meditated cytotoxicity (ADCC).11,12
Despite encouraging clinical results, about half of the HER2-positive cancers are primarily resistant to trastuzumab or become resistant during prolonged treatment.6,7 Many potential mechanisms by which resistance to targeted antibody therapy may develop have been described, including steric hindrance by the extracellular matrix (ECM) proteins,13 increased signaling from the insulin-like growth factor-I receptor,14 constitutive activation PI3K/Akt pathways15 or impaired ADCC response.16,17
We previously showed that HER2-positive JIMT-1 human breast cancer cells are intrinsically resistant to trastuzumab in vitro due to steric hindrance caused by ECM components, such as MUC413 and CD44-bound hyaluronic acid.18 However, trastuzumab treatment can delay the outgrowth of JIMT-1 xenografts in severe combined immunodeficiency (SCID) mice, if treatment is started at the time of tumor inoculation, but not if treatment starts after the tumor has reached a few hundred mm3.12 The antitumor effect is the result of ADCC mediated by trastuzumab, and cells that have little or no ECM, such as freshly trypsinized and injected tumor cells, as well as circulating and disseminated tumor cells,19 are the primary targets.
Pertuzumab (Perjeta®), another humanized monoclonal antibody, targets the dimerization arm of HER2, which is distinct from the binding site of trastuzumab.20 Through blocking HER2 dimerization with other HER (ErbB) family members, it inhibits downstream mitogenic signaling processes.20 Because trastuzumab and pertuzumab bind to distinct epitopes on HER2, it has been hypothesized that a combination of the 2 agents might provide a more effective inhibition of tumor growth than either agent alone. In fact, combining 2 HER2-targeting antibodies against xenografted, in vitro trastuzumab-sensitive N87 gastric cancer cells synergistically decreased tumor growth, reciprocating the in vitro antiproliferative effect of the combination.20,21 Since trastuzumab binds to HER2 close to the cell membrane, while pertuzumab binds to the dimerization arm, which is more distal from the membrane,20,22 it is possible that, in tumors where ECM components interfere with trastuzumab binding, pertuzumab can still bind to HER2.
Treating HER2-positive malignancies with the combination of trastuzumab and pertuzumab has been explored in the preclinical setting,23,24 and pertuzumab was approved for clinical use in combination with trastuzumab and docetaxel as neoadjuvant treatment for patients with HER2-positive locally advanced, inflammatory, or early-stage breast cancer in the USA25,26 and metastatic or locally recurrent unresectable breast cancer in Europe.25,26 Final overall survival analysis from the CLEOPATRA clinical trial showed that patients with metastatic HER2-positive breast cancer treated with this combination lived 15.7 months longer than those who received trastuzumab and chemotherapy alone, with a median overall survival of 56.5 vs. 40.8 months.27 For postoperative adjuvant therapy of HER2-positive breast cancers, however, trastuzumab in combination with chemotherapy is still the current mainstay. The benefit of combination therapy is currently being further tested in the Phase 3 APHINITY trial (ClinicalTrials.gov identifier NCT01358877), to determine if the combination of pertuzumab and trastuzumab is better than trastuzumab alone, in terms of efficacy, for patients who have had surgery for HER2-positive breast cancer.
Because trastuzumab resistance in HER2-overexpressing breast cancers remains a central problem,28 we aimed to explore the mechanisms by which the combination of trastuzumab and pertuzumab could provide benefits in treating HER2-positive tumors. We used the intrinsically trastuzumab-resistant breast cancer cell line JIMT-1 as in vitro ADCC target, and as xenograft tumors to show that, at tissue concentrations expected in the clinical setting, neither of the antibodies saturates ADCC, and consequently the combination of the 2 antibodies significantly delays tumor outgrowth by additively enhancing the recruitment of ADCC. In addition, isobole studies with the in vitro trastuzumab-sensitive BT-474 cells showed that the direct biological effect of combined treatment is additive, and surpasses the maximum effect of either monotherapy. Consequently, we recommend that the 2 antibodies should be used in combination at the currently approved clinical dosage regimes to achieve maximum effect both in ADCC and in direct biological growth inhibition.
Results
In order to distinguish between direct biological effects of the antibodies and their ability to recruit ADCC, F(ab’)2 of trastuzumab and pertuzumab were produced using pepsin digestion. Since the digested F(ab’)2 still has some affinity for protein A and protein G, the F(ab’)2 were purified using size-exclusion chromatography. The product was a homogeneous band in non-reducing SDS-PAGE, and bound with high affinity to native HER2 on cell surfaces, but did not bind anti-Fc polyclonal antibodies (Expanded View Fig. 1), and did not mediate ADCC in vitro (Expanded View Fig. 2).
Figure 1.
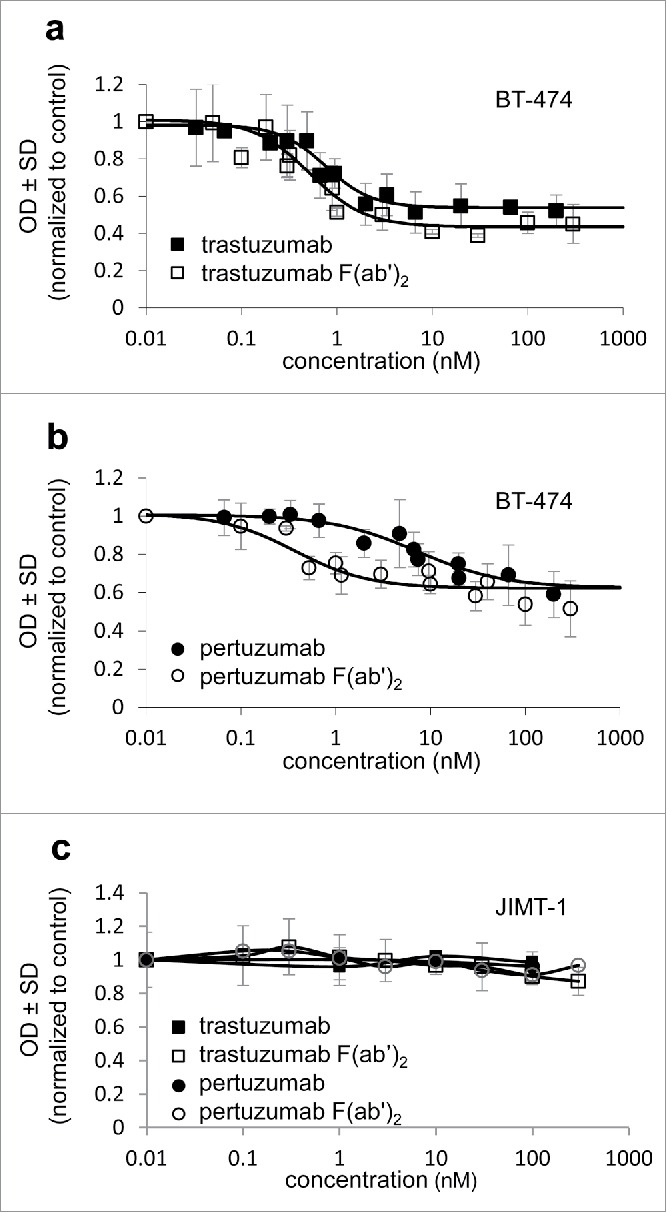
In vitro effect of anti-Her2 antibodies and F(ab’)2 on the proliferation of HER2+ tumors. Proliferation inhibition of the trastuzumab-sensitive BT-474 cell line (a,b) and the trastuzumab-resistant cell line JIMT-1 (c) was measured by an MTT-based colorimetric assay. All measured points are average ± SD of triplicates from ≥3 independent experiments normalized to untreated control. Half maximal effective inhibitory concentration (EC50) was calculated from the fitted dose-response curves.
Figure 2.
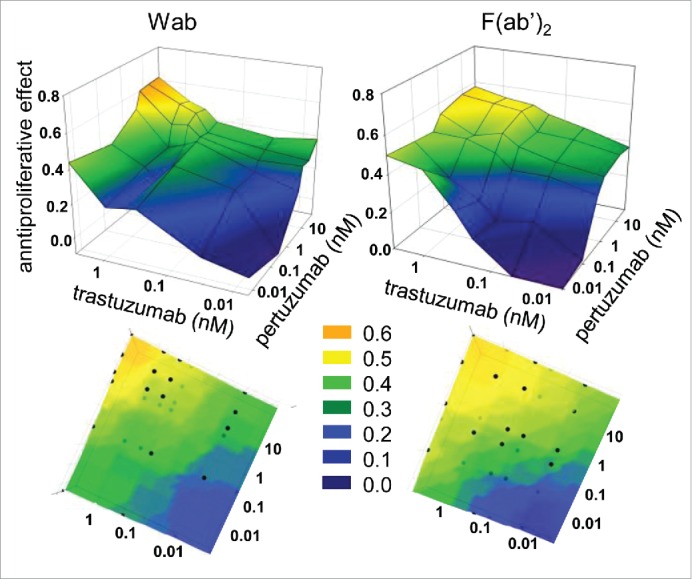
Isobole analysis of in vitro biological growth inhibitory effect of trastuzumab and pertuzumab combinations. Proliferation of BT-474 cells was assessed with MTT assay. Data are from ≥3 replicates from ≥3 independent experiments. The vertical axis of the 3D coordinate systems show how much the proliferation rate decreased compared to the untreated control. The x and y axes represent trastuzumab and pertuzumab concentrations, or that of their F(ab’)2 variants. Doses are centered around the EC50 values determined in Fig. 1. Bottom images show the top-view, smoothed images of the corresponding upper figures.
The in vitro effect of trastuzumab and pertuzumab, as well as their F(ab’)2, on the proliferation of HER2-positive breast tumor cell lines was tested with an MTT-based colorimetric assay (Fig. 1). Both trastuzumab IgG and trastuzumab F(ab’)2 decreased the proliferation of the trastuzumab sensitive BT-474 cell line to a similar extent, with similar half maximal effective concentrations of 0.5–0.8 nM (SEM = 0.1, Fig. 1a). Pertuzumab IgG was less efficient in decreasing the proliferation of BT-474 (EC50 = 7.2 nM, SEM = 2.2); however, the EC50 of its F(ab’)2 was nearly 20-times smaller (EC50 = 0.4 nM, SEM = 0.1) (Fig 1b). This can be explained by its ability to more effectively disrupt the interactions of HER2 with other epidermal growth factor receptor (EGFR) family members owed to its smaller size.29 The trastuzumab-resistant JIMT-1 cell line showed in vitro trastuzumab and pertuzumab resistance, and the removal of the Fc region did not alter this (Fig. 1c).
Next, isoboles were measured, also using MTT-based proliferation assay, to assess whether combining trastuzumab and pertuzumab IgGs, or their F(ab’)2 had an additive or synergistic effect on the proliferation of the sensitive BT-474 cells in vitro (Fig 2). Doses were gradated in logarithmic steps symmetrically upwards and downwards of the half effective dose determined in Fig. 1. Combining either trastuzumab and pertuzumab IgGs, or their F(ab’)2s, resulted in stronger inhibition of proliferation than using either whole antibody or F(ab’)2 alone. The isobole bands corresponding to equal growth inhibitory effects (color coded for better viewing) run straight across between equivalent concentrations of the singly applied antibodies. This suggests that the combination of trastuzumab and pertuzumab exert an additive, but not a synergistic effect in decreasing cell proliferation in vitro, regardless of being present as whole antibodies or F(ab’)2. In the case of the trastuzumab-resistant JIMT-1 cell line, none of the antibody combinations offered any in vitro proliferation inhibition (data not shown).
We therefore tested both trastuzumab and pertuzumab, as well as their F(ab’)2, either alone, or in combination, for their ability to inhibit the growth of JIMT-1 tumor xenografts in SCID mice. Fig. 3 shows that both trastuzumab and pertuzumab whole antibodies inhibited tumor outgrowth for a period of a month after inoculation, similarly to our earlier findings. However, there was no conceivable difference between the efficacy of trastuzumab and pertuzumab. When the 2 antibodies were administered at the same dose, but together, tumor outgrowth was delayed compared to the effect of either antibody alone, and was significantly slowed throughout the experiment. At the same time, no effect on tumor growth was detected for either the F(ab’)2 or their combination compared to the solvent-treated control. This observation is coherent with JIMT-1 cells being in vitro resistant to the biochemical effects of trastuzumab and pertuzumab, and emphasizes the importance of ADCC during in vivo antibody therapy.
Figure 3.
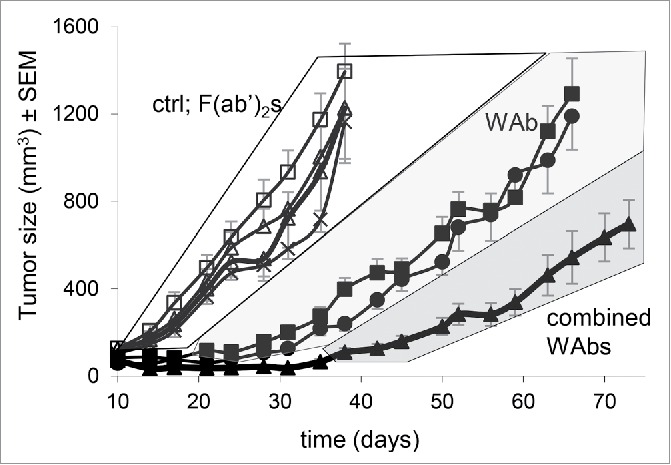
Effect of anti-HER2 antibodies and their F(ab’)2 on in vivo tumor growth. Antiproliferative effect of the antibodies on JIMT-1 was examined in subcutaneously xenotransplanted SCID mice. Each group consisted of 6–8 mice. Compared to untreated control, neither trastuzumab F(ab’)2, pertuzumab F(ab’)2, nor their combination inhibited tumor growth in SCID mice. Trastuzumab or pertuzumab whole antibodies significantly reduced tumor growth compared to control and to all F(ab’)2 treatments from day 21 onwards (p = 0.05) and did not differ from each other significantly. Combination of the 2 whole antibodies achieved greater tumor growth inhibition, the difference from trastuzumab and pertuzumab alone was significant from day 38 and 42 onwards, respectively (p = 0.05). Tumor sizes in the shaded areas (white, light gray, dark gray) significantly differed between the areas, but not within the areas (p < 0.05).
To explore the mechanism behind improved ADCC mediated by the combination of trastuzumab and pertuzumab, we quantitatively assessed in vitro ADCC using an ECIS ZΘ real time adherent cell analyzer. The CD16.176V.NK-92 immortalized effector cell line was used to make the ADCC measurements reproducible and independent of quality of donor peripheral blood mononuclear cells (PBMCs), which might fluctuate from experiment to experiment. To visually verify NK cell immune synapse formation at saturating antibody concentration, confocal microscopy of eGFP-tagged CD16.NK-92 cells targeting JIMT-1 cells was performed, after labeling CD16 on the NK cells and HER2 on the target cells (Fig. 4a). Next, we determined the range of antibody concentration where ADCC was evoked but was not saturated (Figs. 4b-d). At 6.6 pM, the antibody has already caused a considerable extent of cell killing, while this concentration applied without NK cells had no effect on cell proliferation (Figs. 1a and 1b). The extent of cell killing by 67 pM of either trastuzumab or pertuzumab was almost 90%, close to saturation (Figs. 4b, 4c), and concentrations above that caused maximum killing (Fig 4d).
Figure 4.
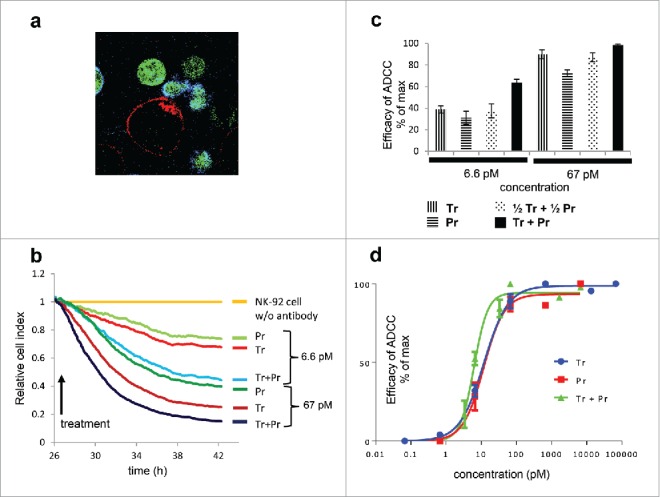
In vitro ADCC mediated by trastuzumab and pertuzumab. Confocal microscopy visualizes in vivo synapse formation induced by trastuzumab and pertuzumab. Red: HER2, green: eGFP expressing NK-92 cells, blue: CD16, FOV 60 µm × 60 µm. Quantitative, population level in vitro ADCC of JIMT-1 target cells with CD16.176V.NK-92 effector cell line was measured on ECIS ZΘ real-time cell analyzer. Traces from one experiment are show in (b). Effector/target cell ratio was 2.5:1 in all cases. Cell indices of antibody-free samples with NK-92 cells present were the same as double negative (NK-92 and antibody free) control and were used as reference for normalization. Reduction of cell number (impedance) at the end-point of each trace, averaged for ≥2 replicates per ≥ 3 independent experiments is shown in (c). Dose response curves fitted to the Hill equation are presented in (d).
In order to define how the combined effect of trastuzumab and pertuzumab relates to the ADCC evoked by their individual application, concentrations for single treatment were set to 6.6 pM and 67 pM, and compared to combinations using the same concentrations of the each antibody (Fig. 4b, 4c), as well as combinations using half of these concentrations, 3.3 pM and 33 pM for each antibody (Fig. 4c). The F(ab’)2 were not studied extensively in this system because none of them decreased the cell index; neither alone nor in combination did they induce ADCC (Supplementary Fig. 2). Our data reveal that both trastuzumab and pertuzumab IgG antibodies induced ADCC, and thus decreased the cell index in a dose-dependent manner, pertuzumab being slightly less efficient. Using combination treatments where the total antibody concentration (3.3 pM + 3.3 pM, or 33 pM + 33 pM) was equal to the comparable single treatment (6.6 pM or 67 pM), we detected very similar degrees of cytotoxicity that were statistically identical. Also, for the nearly saturating concentrations, combination of the two antibodies, to reach twice the concentration of singly applied antibodies, could not significantly increase the efficacy of killing. However, for the non-saturating antibody concentrations, the combination yielding twice the concentration of single applications resulted in doubling the average efficacies of the single treatments (Fig. 4b, 4c). Accordingly, the EC50 value for combined treatment determined from Hill-plots (Fig 4d) was 6.1 pM, as compared to 12.0 pM and 11.5 pM for trastuzumab- and pertuzumab-mediated ADCC, which suggests an additive effect.
To verify that such an additive effect could also exist in vivo, we quantitated the density of penetrating NK cells as a function of penetration depth in frozen sections of the tumors removed at the end of the in vivo experiment. NK cells were defined as 7–10 µm CD45-positive, HER2-negative cells, containing unanimously identifiable DAPI stained nuclei. We imaged the central 10 µm part of 14 µm thick tissue sections divided into 3 confocal slices to detect and evaluate the small, moderately fluorescent murine NK cells. Images of vehicle-treated control and combined antibody-treated tumors are shown in Fig. 5a. HER2 of the tumor is shown in green, nuclei in blue, and CD45 on murine NK cells in red (or, when it overlaps along the z axis with the nucleus, purple). NK cells were counted and their density plotted as a function of penetration depth (Fig. 5b). NK cell concentration was higher at the margins of the section, and decreased toward the center of the tumor. Both trastuzumab- and pertuzumab-treated tumors showed elevated numbers of NK cells compared to control. The tumors receiving combination treatment exhibited an even higher density of NK cells in the deeper areas. We also observed that NK cells could penetrate the tumor (stroma and matrix) deeper in the case of IgG combination treatment, creating longer and larger fissures along which they could attack antibody-binding tumor cells.
Figure 5.
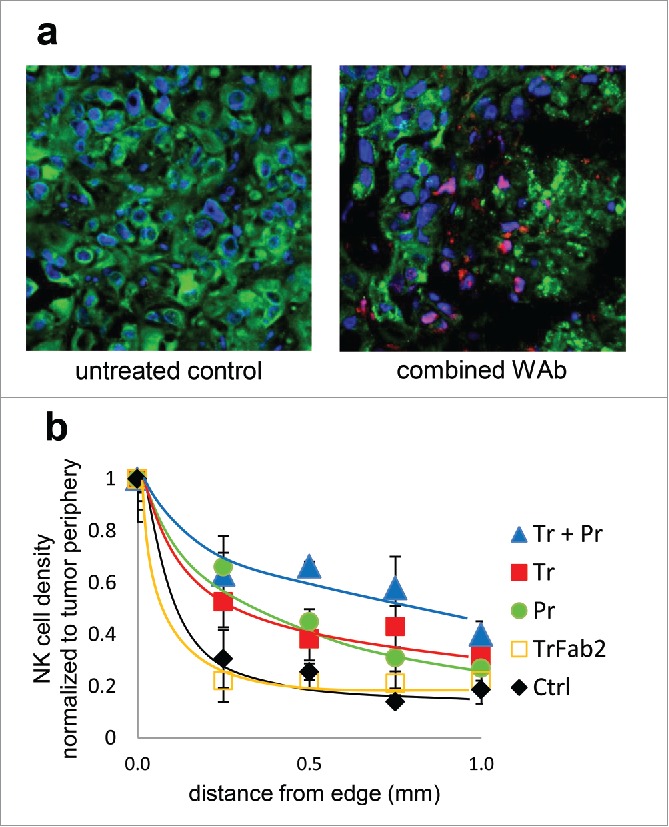
NK cell infiltration of JIMT-1 tumor xenografts. Frozen sections of excised tumors were fluorescently labeled for HER2 (green), NK cells (CD45, red/magenta), and nucleic DNA (blue). Sections from an untreated control (left) and one treated with trastuzumab plus pertuzumab combination (right) are shown in (a). Treatment with antibodies enhances NK cell invasion, which results in extensive tumor lesion and a less dense tumor tissue structure. NK cell penetration into the tumor was quantitatively analyzed by counting CD45+ nucleated cells in 0.05 mm2 areas starting from the side of the tumor toward the center (b).
A closer inspection also revealed that NK cells belong to 2 distinct morphological groups: flattened NK cells embracing HER2-positive tumor cells are likely functionally active, while round NK cells distant from tumor cells are not engaged. In addition to the differences of absolute NK cell densities, it is interesting to note the ratio of flattened to round NK cells. This was 0.48 in F(ab’)2 treated tumors, increased to 1.17 in single IgG treated ones, and was even higher, 1.68 in the case of tumors treated with antibody combination, suggesting that the NK cells in anti-HER2 IgG treated tumors were not only present in higher numbers, but also taking an active role.
Discussion
This study was undertaken to examine if the combination of trastuzumab and pertuzumab provides any benefit in treating HER2-positive tumors that are in vitro resistant to trastuzumab, and to examine the mechanisms behind the possible beneficial effect. To distinguish between direct biological (signaling-based) effects of the antibodies and ADCC mediated by them in vivo, we generated F(ab’)2 of both trastuzumab and pertuzumab. We verified that these F(ab’)2 do not bind anti-Fc polyclonal antibodies, do not mediate ADCC in vitro, but exert equal or greater antiproliferative effect in vitro on the trastuzumab sensitive BT-474 cells. This model, reflecting the direct biological effect of the antibodies, also allowed us to study quantitatively the interaction between trastuzumab and pertuzumab when applied in combination. Isoboles revealed that the combination of the two whole antibodies, as well as that of their F(ab’)2, exerted an additive effect, with no evidence for synergism. The maximum effect of the combined administration of the two antibodies is higher than the maximum effect of either antibody alone. This reflects the distinct mechanism by which each of them affects HER2 signaling, downregulation of HER2 by trastuzumab8 and inhibition of its dimerization with EGFR family members by pertuzumab,20 which can independently inhibit cell growth.
While neither of the antibodies or their F(ab’)2, nor their combination had any direct biological effect on the JIMT-1 cells, the whole antibodies could mediate ADCC against these cells in vitro. This ADCC showed an additive effect for non-saturating concentrations of the antibodies; however, under saturating concentration of each antibody, the maximum ADCC did not increase when both antibodies were applied together. This is explained by the single mechanisms of ADCC, which can be made more efficacious by engaging twice as many Fcγ receptors on the same target, with two antibodies binding to different epitopes, but is saturated when enough Fcγ receptors are collected into the immune synapse of the NK cell.
In vivo growth of trastuzumab-resistant JIMT-1 tumors was not affected by the F(ab’)2 of either trastuzumab, pertuzumab, or their combination, consistent with the lack of their effect in vitro and their inability to recruit ADCC. Trastuzumab prevented the outgrowth of tumors as established earlier.12 Also, pertuzumab exerted a similar antitumor effect. Combination of the two antibodies prolonged the tumor-free period and significantly slowed tumor growth. This implies that ADCC is a valuable therapeutic mechanism of action in the case of in vitro trastuzumab-resistant HER2-positive tumors, and that combining two distinct monoclonal antibodies, which can both mediate ADCC against the same molecular target, can be highly beneficial. In fact, we observed that the combination treatment increased the number of invading NK cells, as well as the proportion of NK cells actively engaged in killing, and this enhanced recruitment negatively correlated with tumor progression and size.
To analyze how the combination of the two antibodies enhances ADCC, we measured ADCC efficacy in vitro as well, as a function of antibody concentration. Using the same JIMT-1 cells as targets, we found that ADCC was saturable with increasing doses, and the combination of the 2 antibodies enhanced ADCC additively only in non-saturating concentrations. Since the same combination in vivo also produced an antitumor effect that was at least additive, it is likely that in vivo doses used in these experiments do not saturate ADCC either. One obvious possibility is that the in vivo administered antibody doses of 5 mg/kg resulted in non-saturating tissue concentrations in the tumor.30 Although it is highly challenging to give a correct estimate of antibody tissue levels,30,31 since the doses are comparable to those in clinical application but administration in mice was done even more frequently (twice per week) than the clinical administration (bi-weekly or even less frequently), tissue levels in the clinical setting might even be lower than those we achieved in mice.
Other factors to consider are the lower NK cell to target ratios in vivo, and the lower affinity FcγRIII receptors on native NK cells compared to those in CD16.NK92 cells. These factors together can all contribute to a decreased saturation of ADCC in vivo, which implies that if the two antibodies are applied in combination, each at their usual therapeutic dose, in vivo ADCC can be additively enhanced.
From these observations, we can conclude that ADCC mediated by anti-HER2 antibodies is able to efficiently combat even those HER2-positive tumors that are resistant to trastuzumab, which in the case of breast cancer can be over half of the cases.6,7 Since both ADCC and the direct biological effects are additive at the maximum tolerated/clinically permitted doses, combination of trastuzumab and pertuzumab is the best choice for all HER2-positive tumors. Specifically, if the tumor is trastuzumab resistant, the higher total tissue concentration of the antibodies, and possibly their sterically distinct attachment sites can improve the efficacy of the patient's own NK cells. If the tumor is trastuzumab sensitive, the antitumor effects of the distinct molecular mechanisms are also added to the effect of ADCC. These two additive mechanisms underpin the findings of the clinical biomarker analysis conducted during the CLEOPATRA trial, which revealed that “HER2 is the only marker suited for patient selection” and “only HER2, HER3, and PIK3CA were relevant prognostic factors.”32
Once the principle of additively applying the maximum tolerable doses has been established, it seems evident to look for additional agents with similar molecular targeting specificity. In the case of HER2-positive tumors, the dual (HER2 and EGFR) tyrosine kinase inhibitor lapatinib has successfully been applied in combination with chemotherapy and approved by the Food and Drug Administration (FDA).33 It has recently (March 2016) been announced at the Official 10th European Breast Cancer Conference that in the EPHOS-B randomized trial, the combination of lapatinib and trastuzumab shrunk HER2-positive breast cancer significantly in 11 d after diagnosis, providing an advantage over trastuzumab alone: pathologically complete response was observed in 11% vs 0%, and minimal residual disease in 17% vs 3% of the patients.34 The beneficial effect of the combination is probably based on providing an alternative blocking of HER2 (and EGFR) function through kinase inhibition also in those cells that do not bind trastuzumab well, or do not respond with a downregulation of HER2. This complementarity appears to be also true for the reverse situation, wherein addition of trastuzumab to lapatinib monotherapy was found to improve median overall survival by 4.5 months.35 In light of this, the triple combination of lapatinib, trastuzumab and pertuzumab could be even more efficacious than combining trastuzumab and pertuzumab, although the tolerability of kinase inhibition in the long run, especially that of EGFR, could be an issue.36
Overall, it appears safe and highly beneficial to use combination antibody therapy against HER2-positive tumors from the start, which is currently being tested in the Phase 3 APHINITY clinical trial. Switching to combination in the case of ongoing trastuzumab monotherapy is also supported both by clinical and preclinical data. On the one hand, the combined use of trastuzumab and pertuzumab was beneficial in cases where the tumor has progressed following trastuzumab monotherapy.24 On the other hand, we observed earlier that circulating and disseminated tumor cells are still sensitive to ADCC when the primary tumor has already become resistant,19 probably owed to their lack of a well-developed ECM.13,18 Consequently, ADCC enhanced by combination antibody therapy could better prevent the dissemination of tumor cells even when the primary tumor or larger inoperable metastases become resistant to the combination. Swain et al.27 conclude their account of the CLEOPATRA trial by stating: “Finally, the question of when, if ever, therapy with pertuzumab plus trastuzumab for metastatic breast cancer should be stopped remains unanswered.” Considering the additive effect of this combined treatment in mediating ADCC and the importance of ADCC in preventing tumor dissemination, including that from previously dormant tumor cells, we propose that in the adjuvant setting combination antibody therapy of HER2-positive breast cancer should be the first choice, and possibly it should not be stopped.
Collectively, in any clinical setting the combination of both antibodies at maximum clinically approved doses should be applied to recruit maximum ADCC and cause maximum direct biological growth inhibition. This is the mechanistic background, based on which the combined therapy is expected to give results that are superior to monotherapy, whatever the type of HER2-positive tumor may be.
Materials and methods
All materials were from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated.
Cells
BT-474 (HTB-20™) and SKBR-3 (HTB-30™) cells derived from trastuzumab-sensitive, HER2-positive invasive ductal adenocarcinomas were obtained from the American Type Culture Collection. The cells were grown in RPMI (R6504) that was supplemented with 20% FCS, insulin, glutamin and antibiotics and in DMEM (D7777) that was supplemented with 10% FCS (F9665), glutamin and antibiotics in a 5% CO2 humidified incubator with glutamine antibiotics and passaged 3 times weekly. JIMT-1 is a HER2-positive, trastuzumab-resistant human breast cancer cell line, established from pleural metastasis in the laboratory of Cancer Biology, University of Tampere, Finland.37 JIMT-1 cells were cultured in 1:1 ratio of Ham's F-12 (N6760) and DMEM supplemented with 20% FCS, 300 U/L insulin (I9278), glutamine and antibiotics and split every 3–4 d. For in vitro ADCC, the cell line CD16.176V.NK-92, here abbreviated NK92 (from Dr. Kerry S. Campbell, the Fox Chase Cancer Center, Philadelphia, PA) was used. These cells are derived from a human NK-like phenotype non-Hodgkin's lymphoma,38 and have been transduced to express a high affinity variant (176V) of FcγRIIIA receptor (CD16).39,40 A variant of this cell line co-expresses eGFP with CD16. The cell lines were cultured in special NK medium of α-MEM (M0644) containing 10% FCS and 10% horse serum (Hyclone SH 30070), supplemented with glutamine, non-essential amino acids, Na-pyruvate (S8636), antibiotics and IL-2 at 100 IU/ml (Proleukin 18 × 106 NE, Novartis). All cell cultures were routinely checked for the absence of mycoplasma contamination.
F(ab’)2 preparation from whole immunoglobulins
Trastuzumab (Herceptin®, Roche) and pertuzumab (Perjeta®, Roche) antibodies were dissolved in 20 mM acetate buffer (pH 4.5), dialyzed thrice against the same buffer and concentrated using Amikon Ultra filters (Millipore, UFC 801096.). The Fc domain was digested by immobilized pepsin (ThermoScientific, 20343). The reaction was stopped by 10 ml 2 M TRIS-HCl, pH 8.2. Immobilized pepsin was cleared by 5 min centrifugation at 300 xg, and the supernatant was filtered sterile and concentrated on Amikon Ultra filters. Sephacryl S300 and Superdex 200 (GE Health Care Life Science, 17-0438-09 and 17-1043-99) gel filtration columns were used for size-exclusion chromatography purification of trastuzumab F(ab’)2 and pertuzumab F(ab’)2, respectively. Absorbance of fractions was measured with a NanoDrop ND 1000 (Thermo Science), protein content was assessed with non-reducing SDS-PAGE, and fractions contaminated with smaller fragments and partially digested or undigested antibodies were pooled and re-chromatographed. F(ab’)2 were tested for lack of the Fc, for preserved HER2 binding affinity and for inability to mediate ADCC (see supplementary data).
Flow cytometric assessment of F(ab’)2 quality
SKBR-3 cells were grown in DMEM supplemented with 10% FCS in a 5% CO2 humidified incubator with antibiotics and passaged twice weekly. Freshly detached SKBR-3 cells were treated with trastuzumab, pertuzumab, or their F(ab’)2 at 10 µg/ml concentration for 10 min at RT, then labeled with Alexa Fluor 488 conjugated goat-anti-human Fc or Alexa Fluor 488 (A11013) conjugated goat anti-human (H+L) antibodies at 10 µg/ml for 10 min. HER2-positivity of SKBR-3 cells was verified with Alexa Fluor 647 conjugated 76.5 antibody. Measurements were performed on a BD FACSCalibur flow cytometer, 20,000 events were collected and the median of histograms is displayed.
In vitro cell proliferation
The effect of the antibodies on in vitro proliferation of HER2-expressing, trastuzumab sensitive and resistant cell lines were tested using an MTT-based colorimetric cell proliferation assay (EZ4U, Biomedica GmbH, BI-5000). Cells were incubated in 96-well plates for 3 d in the presence or absence of antibodies or their F(ab’)2 at the given concentrations. After incubation with EZ4U, absorption (λ = 488 nm, corrected with absorption at λ = 620 nm) of the metabolic substrate was measured on Synergy HT Multi-Detection microplate Reader (BioTek). Replicates (n ≥ 3) were averaged, and growth inhibition was expressed as normalized to untreated control.
In vitro ADCC
Inability of the F(ab’)2 to recruit ADCC was tested with PBMC as effectors using the xCELLigence (Roche) real time cell analyzer. Cell index was calculated by the analyzer based on the measured impedance. BT-474 cells were seeded in 8-well chambers (Applied Bioscience, 8W10E) with gold electrodes and allowed to proliferate until 80% confluence (50 hours). Mononuclear leukocytes freshly separated from human PBMC were added at a 5:1 ratio to the BT-474 cells and impedance was monitored for 24 h. Data from 3 wells were averaged and traces normalized to impedance measured at the start of ADCC.
For quantitative analysis of ADCC efficiency in vitro, an ECIS ZΘ real-time cell analyzer was used. JIMT-1 target cells were grown in 8W10E PET 8well arrays with gold electrodes at the bottom. The complex impedance spectrum of cells adhered to the electrodes was assessed in a the range of 1 Hz to 100,000 Hz. Effector/target ratio was set at 2.5:1, considering that NK cells constitute around 15% of the freshly separated PBMC, and both in our previous experiments and in the literature a 15:1 PBMC to target cell ratio was determined to be highly efficient. NK92 effector cells or antibodies at defined concentration were administered after impedance of the target cells has reached a plateau.
Xenograft tumors and in vivo antibody treatment
SCID (C.B-17/Icr-Prkdcskid/IcrIcoCrl, Fox-Chase) mice were purchased from Charles River Laboratories, and housed in a specific-pathogen-free environment. All animal experiments were performed in accordance with FELASA guidelines and recommendations and DIN EN ISO 9001 standards. Only non-leaky SCID mice with murine IgG levels below 100 ng/ml were used. Each seven-week-old female SCID mouse participating in the study was given a subcutaneous injection of 5 × 106 JIMT-1 cells suspended in 100 µl Hanks' A buffer and mixed with an equal volume of Matrigel (BD Matrigel, BD Biosciences, 356237) in both flanks. Tumor volumes were derived as the product of the length, width and height of the tumor measured 2 times a week with a caliper. Trastuzumab (n = 8), trastuzumab F(ab’)2 (n = 7), pertuzumab (n = 8), pertuzumab F(ab’)2 (n = 7), trastuzumab+pertuzumab (n = 8), trastuzumab F(ab’)2 + pertuzumab F(ab’)2 (n = 7), and, as a control, HEPES buffer (n = 7) was injected intraperitoneally in a volume of 100 µl HEPES buffer twice weekly. The injected dose was 100 µg of each antibody or F(ab’)2 used. Treatment commenced at the time of JIMT-1 inoculation and was continued until the end of the experiment. At the end of the experiment the animals were euthanized by cervical dislocation. The experiments were done with the approval of the National Ethical Committee for Animal Research (# 4/2012/DE MÁB).
In vitro immune synapse formation by CD16.NK-92 Cells
JIMT-1 cells were seeded in Poly-L-Lysine (81804) coated 2 × 9 well µ-slides (Ibidi) at 50,000 cells/cm2 density and incubated for 2 d in JIMT-1 medium. After that, cells were treated with trastuzumab and pertuzumab whole antibodies at 10µg/ml concentration in 20 µl indicator-free NK medium at 37°C, 5% CO2, for 10 minutes. After 10 minutes, 120,000 NK92 eGFP cells/well were added in 20 µl medium and coincubated at 37°C, 5% CO2, for 10 minutes. Fluorescently labeled antibodies were added at 10 µg/ml final concentration; CD16 was labeled with Alexa Fluor 647 conjugated anti-CD16 antibody (clone 3G8, BD PharMingen, 302023), HER2 was labeled with an Alexa Fluor 555 conjugated Fab, clone 76.5.
Tumor xenograft sections
At termination, mice were dissected and fresh tumors were embedded in cryomatrix (Thermo Fischer Scientific, 6769006) and snap frozen in liquid nitrogen. Serial 14 µm thick cryosections were made with a Shandon Cryotome (Thermo Fischer Scientific) at −24°C and air-dried. Labeling was carried out at room temperature and all antibodies were diluted in HEPES buffer supplemented with 1% BSA (A7906). After 5 min of rehydration in HEPES buffer containing 1% BSA and 0.01% TritonX-100 (Thermo Fischer Scientific, 28314) HER2 was labeled with Alexa Fluor 488 conjugated 76.5 antibody, NK cells were labeled with Alexa Fluor 647 conjugated anti-CD45 (clone CD45.1). Both antibodies were used at 2 µg/ml concentration at 4°C for 10 hours. Nuclei were stained with DAPI (D9564) at 10 µg/ml for 2 hours. Sections were washed for 5, 20, and 60 minutes, fixed in formaldehyde, and mounted in Mowiol (Sigma, 81381) antifade.
Confocal laser scanning microscopy
Fluorescence-labeled cells and tissue sections were analyzed with confocal laser scanning microscope (LSM 510, Carl Zeiss GmbH) using a 40× C-Apochromat water immersion objective (NA = 1.2). DAPI was excited at 351 nm, Alexa Fluor 488 and eGFP were excited at 488 nm, Alexa Fluor 546 and Alexa Fluor 555 at 543 nm, and Alexa Fluor 647 at 633 nm. Corresponding fluorescence emission was separated with an appropriate quad-band dichroic mirror, and detected through 385 to 470 nm, 505 to 550 nm, 560 to 615 nm bandpass and 650 nm longpass filters, respectively. For tissue samples, 3 consecutive, 4 µm thick optical sections were taken at 3 µm intervals, covering the central 10 µm part of the sections. For detecting immune synapses between NK cells and target cells, 1.5 µm optical sections were used.
Statistical evaluation of data
To analyze dose effect curves, data were fitted with the Hill equation. For comparison of the efficacy of ADCC evoked by trastuzumab, pertuzumab and their combination, data points in each experiment were normalized to the maximum effect reached with saturating concentrations, and the mean for each treatment and concentration was normalized to the overall maximum effect. In vivo tumor sizes at each sampling time point were compared with one-way ANOVA followed by Tukey's multiple comparison test, at α = 0.05. SigmaPlot 12 and GraphPad Prism 6 were used for analysis.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge the financial support from the Hungarian Scientific Research Fund (OTKA NK 101337), and the New Hungary Development Plan co-financed by the European Social Fund and the European Regional Development Fund (TÁMOP-4.2.2.A-11/1/KONV-2012-0025). G.T. was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4.A/2-11-1-2012-0001 and by the Gedeon Richter Plc. CD16.NK-92 cells were obtained from Dr. Kerry S. Campbell (Fox Chase Cancer Center, Philadelphia, PA, USA), are protected by patents or pending patents worldwide, and were licensed by Nantkwest, Inc. (www.nantkwest.com). We are indebted to Dr. Kerry S Campbell for his help in establishing the optimal culture conditions for the CD16.NK-92 cells, the useful discussions on ADCC assays, and for critical reading of the manuscript.
Authors' contributions
GT carried out in vitro, in vivo and ex vivo experiments, performed data analysis and wrote the manuscript, ÁS participated in study design, performed data analysis, and wrote the manuscript, LS carried out and evaluated in vivo experiments and wrote the manuscript, YY conceived the project, participated in study design and wrote the manuscript, JS participated in study design, analyzed results and wrote the manuscript, GV conceived and coordinated the study, designed experiments, carried out in vitro experiments, analyzed data, and wrote the manuscript. All authors have read and approved the final manuscript.
References
- 1.Paik S, Liu ET. HER2 as a predictor of therapeutic response in breast cancer. Breast Dis 2000; 11:91-102; PMID:15687595; http://dx.doi.org/ 10.3233/BD-1999-11108 [DOI] [PubMed] [Google Scholar]
- 2.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clinical Breast Cancer 2004; 5:63-9; PMID:15140287; http://dx.doi.org/ 10.3816/CBC.2004.n.011 [DOI] [PubMed] [Google Scholar]
- 3.Tanner M, Hollmen M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al.. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 2005; 16:273-8; PMID:15668283; http://dx.doi.org/ 10.1093/annonc/mdi064 [DOI] [PubMed] [Google Scholar]
- 4.Friedlander E, Barok M, Szollosi J, Vereb G. ErbB-directed immunotherapy: antibodies in current practice and promising new agents. Immunol Lett 2008; 116:126-40; PMID:18201769; http://dx.doi.org/ 10.1016/j.imlet.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Monoclonal antibody approved for metastatic breast cancer. Oncology (Williston Park, NY) 1998; 12:1727. [PubMed] [Google Scholar]
- 6.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, Cristofanilli M, Arun B, Esmaeli B, Fritsche HA, et al.. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20:1800-8; PMID:11919237; http://dx.doi.org/ 10.1200/JCO.2002.07.058 [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al.. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783-92; PMID:11248153; http://dx.doi.org/ 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 8.Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, Dennis PA, Lipkowitz S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res 2001; 61:4892-900; PMID:11406568 [PubMed] [Google Scholar]
- 9.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al.. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004; 6:117-27; PMID:15324695; http://dx.doi.org/ 10.1016/j.ccr.2004.06.022 [DOI] [PubMed] [Google Scholar]
- 10.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 2002; 416:279-80; PMID:11907566; http://dx.doi.org/ 10.1038/416279b [DOI] [PubMed] [Google Scholar]
- 11.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443-6; PMID:10742152; http://dx.doi.org/ 10.1038/74704 [DOI] [PubMed] [Google Scholar]
- 12.Barok M, Isola J, Palyi-Krekk Z, Nagy P, Juhasz I, Vereb G, Kauraniemi P, Kapanen A, Tanner M, Vereb G. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther 2007; 6:2065-72; PMID:17620435; http://dx.doi.org/ 10.1158/1535-7163.MCT-06-0766 [DOI] [PubMed] [Google Scholar]
- 13.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res 2005; 65:473-82; PMID:15695389 [PubMed] [Google Scholar]
- 14.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 2001; 93:1852-7; PMID:11752009; http://dx.doi.org/ 10.1093/jnci/93.24.1852 [DOI] [PubMed] [Google Scholar]
- 15.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 2002; 62:4132-41; PMID:12124352 [PubMed] [Google Scholar]
- 16.Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res 2002; 62:5813-7; PMID:12384543 [PubMed] [Google Scholar]
- 17.Kute T, Stehle JR Jr, Ornelles D, Walker N, Delbono O, Vaughn JP. Understanding key assay parameters that affect measurements of trastuzumab-mediated ADCC against Her2 positive breast cancer cells. Oncoimmunology 2012; 1:810-21; PMID:23162748; http://dx.doi.org/ 10.4161/onci.20447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palyi-Krekk Z, Barok M, Kovacs T, Saya H, Nagano O, Szollosi J, Nagy P. EGFR and ErbB2 are functionally coupled to CD44 and regulate shedding, internalization and motogenic effect of CD44. Cancer Lett 2008; 263:231-42; PMID:18276068; http://dx.doi.org/ 10.1016/j.canlet.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 19.Barok M, Balazs M, Nagy P, Rakosy Z, Treszl A, Toth E, Juhász I, Park JW, Isola J, Vereb G, et al.. Trastuzumab decreases the number of circulating and disseminated tumor cells despite trastuzumab resistance of the primary tumor. Cancer Lett 2008; 260:198-208; PMID:18096313; http://dx.doi.org/ 10.1016/j.canlet.2007.10.043 [DOI] [PubMed] [Google Scholar]
- 20.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004; 5:317-28; PMID:15093539; http://dx.doi.org/ 10.1016/S1535-6108(04)00083-2 [DOI] [PubMed] [Google Scholar]
- 21.Ben-Kasus T, Schechter B, Lavi S, Yarden Y, Sela M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: relevance of receptor endocytosis. Proc Natl Acad Sci U S Am 2009; 106:3294-9; PMID:19218427; http://dx.doi.org/ 10.1073/pnas.0812059106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagossi P, Horvath G, Vereb G, Szollosi J, Tozser J. Molecular modeling of nearly full-length ErbB2 receptor. Biophys J 2005; 88:1354-63; PMID:15596490; http://dx.doi.org/ 10.1529/biophysj.104.046003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard SR. EGF receptor inhibition: attacks on multiple fronts. Cancer Cell 2005; 7:287-8; PMID:15837615; http://dx.doi.org/ 10.1016/j.ccr.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 24.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009; 69:9330-6; PMID:19934333; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4597 [DOI] [PubMed] [Google Scholar]
- 25.Amiri-Kordestani L, Wedam S, Zhang L, Tang S, Tilley A, Ibrahim A, Justice R, Pazdur R, Cortazar P. First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res 2014; 20:5359-64; PMID:25204553; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-1268 [DOI] [PubMed] [Google Scholar]
- 26.Boix-Perales H, Borregaard J, Jensen KB, Ersboll J, Galluzzo S, Giuliani R, Ciceroni C, Melchiorri D, Salmonson T, Bergh J, et al.. The European Medicines Agency Review of Pertuzumab for the treatment of adult patients with HER2-positive metastatic or locally recurrent unresectable breast cancer: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist 2014; 19:766-73; PMID:24928613; http://dx.doi.org/ 10.1634/theoncologist.2013-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, et al.. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015; 372:724-34; PMID:25693012; http://dx.doi.org/ 10.1056/NEJMoa1413513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavaud P, Andre F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med 2014; 12:132; PMID:25285786; http://dx.doi.org/ 10.1186/s12916-014-0132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roszik J, Toth G, Szollosi J, Vereb G. Validating pharmacological disruption of protein-protein interactions by acceptor photobleaching FRET imaging. Methods Mol Biol 2013; 986:165-78; PMID:23436412; http://dx.doi.org/19924542 10.1007/978-1-62703-311-4_11 [DOI] [PubMed] [Google Scholar]
- 30.Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J 2010; 12:33-43; PMID:19924542; http://dx.doi.org/ 10.1208/s12248-009-9157-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boswell CA, Bumbaca D, Fielder PJ, Khawli LA. Compartmental tissue distribution of antibody therapeutics: experimental approaches and interpretations. AAPS J 2012; 14:612-8; PMID:22648903; http://dx.doi.org/ 10.1208/s12248-012-9374-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baselga J, Cortes J, Im SA, Clark E, Ross G, Kiermaier A, Swain SM. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol 2014; 32:3753-61; PMID:25332247; http://dx.doi.org/ 10.1200/JCO.2013.54.5384 [DOI] [PubMed] [Google Scholar]
- 33.Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko CW, Sridhara R, Justice R, Pazdur R. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. The oncologist 2008; 13:1114-9; PMID:18849320; http://dx.doi.org/ 10.1634/theoncologist.2008-0816 [DOI] [PubMed] [Google Scholar]
- 34.Bundred N, Cameron D, Armstrong A, Brunt A, Cramer A, Dodwell D, et al.. Effects of perioperative lapatinib and trastuzumab, alone and in combination, in early HER2+ breast cancer - the UK EPHOS-B trial (CRUK/08/002). Proceedings of the Official 10th European Breast Cancer Conference Amsterdam, The Netherlands, 2016. [Google Scholar]
- 35.Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, et al.. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012; 30:2585-92; PMID:22689807; http://dx.doi.org/ 10.1200/JCO.2011.35.6725 [DOI] [PubMed] [Google Scholar]
- 36.Ahn ER, Wang E, Gluck S. Is the Improved Efficacy of Trastuzumab and Lapatinib Combination Worth the Added Toxicity? A Discussion of Current Evidence, Recommendations, and Ethical Issues Regarding Dual HER2-Targeted Therapy. Breast Cancer 2012; 6:191-207; PMID:23226023; http://dx.doi.org/ 10.4137/BCBCR.S9301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanner M, Kapanen AI, Junttila T, Raheem O, Grenman S, Elo J, Elenius K, Isola J. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther 2004; 3:1585-92; PMID:15634652 [PubMed] [Google Scholar]
- 38.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994; 8:652-8; PMID:8152260 [PubMed] [Google Scholar]
- 39.Anikeeva N, Steblyanko M, Fayngerts S, Kopylova N, Marshall DJ, Powers GD, Sato T, Campbell KS, Sykulev Y. Integrin receptors on tumor cells facilitate NK cell-mediated antibody-dependent cytotoxicity. Eur J Immunol 2014; 44:2331-9; PMID:24810893; http://dx.doi.org/ 10.1002/eji.201344179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol 2008; 180:6392-401; PMID:18424763; http://dx.doi.org/ 10.4049/jimmunol.180.9.6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


