Supplemental Digital Content is available in the text.
Keywords: antihypertensive therapy, hypertension, labetalol, methyldopa, pregnancy
Abstract
To determine whether clinical outcomes differed by occurrence of severe hypertension in the international CHIPS trial (Control of Hypertension in Pregnancy Study), adjusting for the interventions of “less tight” (target diastolic blood pressure [dBP] 100 mm Hg) versus “tight” control (target dBP 85 mm Hg). In this post-hoc analysis of CHIPS data from 987 women with nonsevere nonproteinuric preexisting or gestational hypertension, mixed effects logistic regression was used to compare the following outcomes according to occurrence of severe hypertension, adjusting for allocated group and the influence of baseline factors: CHIPS primary (perinatal loss or high-level neonatal care for >48 hours) and secondary outcomes (serious maternal complications), birth weight <10th percentile, preeclampsia, delivery at <34 or <37 weeks, platelets <100×109/L, elevated liver enzymes with symptoms, maternal length of stay ≥10 days, and maternal readmission before 6 weeks postpartum. Three hundred and thirty-four (34.1%) women in CHIPS developed severe hypertension that was associated with all outcomes examined except for maternal readmission (P=0.20): CHIPS primary outcome, birth weight <10th percentile, preeclampsia, preterm delivery, elevated liver enzymes (all P<0.001), platelets <100×109/L (P=0.006), and prolonged hospital stay (P=0.03). The association between severe hypertension and serious maternal complications was seen only in less tight control (P=0.02). Adjustment for preeclampsia (464, 47.3%) did not negate the relationship between severe hypertension and the CHIPS primary outcome (P<0.001), birth weight <10th percentile (P=0.005), delivery at <37 (P<0.001) or <34 weeks (P<0.001), or elevated liver enzymes with symptoms (P=0.02). Severe hypertension is a risk marker for adverse maternal and perinatal outcomes, independent of BP control or preeclampsia co-occurrence.
Clinical Trial Registration—
URL: http://pre-empt.cfri.ca/. Unique identifier: ISRCTN 71416914. URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01192412.
The treatment of nonsevere pregnancy hypertension has been controversial. Although prior randomized controlled trials identified that antihypertensive therapy (to normalize blood pressure [BP]) was associated with a lower incidence of severe hypertension, it was not clear that this was harmful in and of itself, particularly when coupled with concerns that such therapy could impair fetal growth and increase perinatal mortality and morbidity.1
The CHIPS trial (Control of Hypertension in Pregnancy Study) has provided evidence that antihypertensive treatment of nonsevere hypertension in pregnancy is of benefit to the mother, without associated perinatal risk. The CHIPS trial randomized women with nonsevere pregnancy hypertension to a diastolic blood pressure (dBP) target of 100 mm Hg (“less tight” control) versus 85 mm Hg (“tight” control) for a planned between-group difference in dBP of 5 mm Hg.2 The BP achieved was higher in less tight (versus tight) control by a mean of 5.8 mm Hg systolic (95% confidence interval, 4.5–7.0; 138.8±0.5 mm Hg versus 133.1±0.5 mm Hg; P<0.001) and 4.6 mm Hg diastolic (95% confidence interval, 3.7–5.4; 89.9±0.3 mm Hg versus 85.3±0.3 mm Hg; P<0.001). There was no impact of less tight (versus tight) control on perinatal death or high-level neonatal care for >48 hours (155, 31.4% in less tight versus 150, 30.7% in tight) or serious maternal complications (including death; 18, 3.7% versus 10, 2.0%, respectively). However, there was more severe maternal hypertension (200, 40.6% versus 134, 27.5%, respectively), despite almost half the women using home BP monitoring (231, 46.5% versus 225, 46.0%, respectively) and attending frequent antenatal visits (ie, a median [interquartile range] of 7.0 [4.0, 11.0] clinic visits from a mean gestational age at enrollment of 24 weeks [23.7±6.3 versus 24.2±6.3 weeks, respectively]). The distribution of observed systolic BP and dBP values was similar between allocated groups, and the excess of severe BP values in less tight control was not restricted to values just above the 160/110 mm Hg diagnostic threshold for severe hypertension. In addition, predictive modeling did not demonstrate that women destined to develop subsequent severe hypertension could be identified by clinical characteristics at randomization, when a BP management strategy was instituted.3
The CHIPS trial has generated controversy over whether the increase in severe hypertension associated with less tight control merits a recommendation to use tight control because there is disagreement about whether the increased frequency of severe hypertension with less tight control: (1) is important to prevent because it would otherwise translate into an excess of adverse maternal outcomes for which CHIPS was underpowered to detect; (2) represents any risk to the fetus; or (3) can be identified easily and treated promptly in the course of antenatal care.3 In this secondary analysis of CHIPS data, we sought to examine whether the occurrence of severe hypertension was associated with adverse perinatal and maternal outcomes, independent of allocated group and the occurrence of preeclampsia, one of the recognized pathways to adverse outcomes for hypertensive mothers and their babies.
Methods
In brief, CHIPS was an open pragmatic international multicenter trial (ISRCTN 71416914, NCT01192412, http://pre-empt.cfri.ca/CHIPS) that was approved by the Research Ethics Board at the University of British Columbia as the Coordinating Center (H08-00882) and at all study sites. Women at 14+0- to 33+6-week gestation with nonproteinuric preexisting or gestational hypertension, office dBP 90 to 105 mm Hg (or 85–105 mm Hg if on antihypertensives), and a live fetus were randomized (centrally and stratified by center and type of hypertension) to less tight (target dBP 100 mm Hg) or tight control (target dBP 85 mm Hg). For additional details, see Appendix in the online-only Data Supplement.
Women could be recruited on an antihypertensive agent other than atenolol from ≥14 weeks’ gestation. Post randomization, labetalol was the recommended antihypertensive of first choice, but women could stay on their existing antihypertensive agent if they wished or if labetalol were contraindicated or unavailable. (The only exceptions were atenolol, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or direct renin inhibitors.)
The composite primary outcome was pregnancy loss or high-level neonatal care for >48 hours in the first 28 days of life, and the secondary outcome was maternal death or serious maternal complications before 6 weeks postpartum. Severe hypertension was defined as a systolic BP ≥160 mm Hg or a dBP ≥110 mm Hg, measured twice, 15 minutes apart. Preeclampsia was defined according to Canadian clinical practice guidelines4 broadly as the development of new proteinuria or one/more suggestive maternal symptoms (ie, headache, visual disturbances, persistent right upper quadrant or epigastric pain, severe nausea or vomiting, chest pain, or dyspnea), signs (ie, severe hypertension, eclampsia, placental abruption, or pulmonary edema), or abnormal laboratory results (ie, elevated aspartate or alanine aminotransferase or lactate dehydrogenase [according to local laboratory criteria] with symptoms, platelet count <100×109/L, or serum creatinine >2.26 mg/dL [>200 μmol/L]). Further details can be found in Table S2 in the online-only Data Supplement and in the CHIPS protocol (www.pre-empt.cfri.ca/CHIPS) and in the main CHIPS publication.2
Statistical Analysis
Descriptive analyses were undertaken to describe the relationship between the occurrence of severe hypertension and the occurrence of preeclampsia.
To compare the effect of less tight (versus tight) control among women who developed severe hypertension, as opposed to those who did not, and the effect of severe hypertension among women in less tight, as opposed to those in tight control, a mixed-effects logistic regression model was used with an interaction term between severe hypertension and allocation group. An additional model without the interaction term was also considered to assess the overall effect of severe hypertension. We adjusted for the influence of baseline factors as in the main CHIPS analysis (ie, stratification factors [hypertension type and center] and key prognostics factors [antihypertensive therapy at randomization, prior BP ≥160/110 mm Hg in this pregnancy, gestational age at randomization, region, in-hospital status at enrollment, and systolic BP at randomization]) and any others that may have differed between those with and those without severe hypertension. The same process was undertaken for preeclampsia, given the acceptance that it increases maternal and perinatal risk. A P value <0.05 was considered statistically significant for the homogeneity of the odds ratios (ORs) between women with and without each of severe hypertension or preeclampsia, for each of the following adverse outcomes: primary perinatal outcome, secondary maternal outcome, severe hypertension, preeclampsia, delivery at <37 or 34 weeks, platelet count <100×109/L, elevated aspartate aminotransferase or alanine aminotransferase with symptoms, maternal length of stay ≥10 days, or maternal readmission before 6 weeks postpartum, as applicable.
We further considered the co-occurrence of severe hypertension and preeclampsia. These analyses examined the association with adverse outcomes for (1) severe hypertension after adjustment for preeclampsia, and (2) preeclampsia after adjustment for severe hypertension. A P value <0.05 was considered statistically significant.
All analyses were repeated using a restrictive definition of preeclampsia (ie, the development of new proteinuria, defined as ≥2+ by urinary dipstick, ≥0.3 g/d by 24 hours urine collection, or ≥30 mg/mmol [0.26 mg/mL] urinary creatinine).
Results
For the 981 women randomized and included in the primary CHIPS analyses, severe hypertension (N=334, 33.9%) and preeclampsia (N=464, 47.3%) were common (Table S3). Most women with severe hypertension had preeclampsia (248/334, 74.5%). Just over half of women with preeclampsia (248/464, 53.4%) had severe hypertension. No woman had preeclampsia defined only by severe hypertension; one woman had preeclampsia defined only by signs, but she had both severe hypertension and placental abruption, another diagnostic criterion for preeclampsia.4 Four hundred and thirty women (43.8%) had neither severe hypertension nor preeclampsia.
Because women with severe hypertension or preeclampsia were different from those without each of these outcomes according to baseline characteristics (Table S4), additional adjustments were required to the mixed logistic regression. In brief, women who developed severe hypertension were more likely to have been randomized to less tight control (as previously reported), be non-Caucasian, have had prior severe hypertension in the index pregnancy, be taking aspirin at enrollment, and be from regions outside of North and South America that had low perinatal mortality ratios. Women who developed preeclampsia were randomized at a later gestational age, and were more likely to: have gestational (versus preexisting) hypertension, have experienced prior severe hypertension, be in hospital at enrollment, taking folic acid or aprenatal vitamin at enrollment, and have been recruited from South America or UK/Europe.
The adjusted OR (aOR) for adverse outcomes in less tight versus tight control was similar among women with and those without each of severe hypertension and preeclampsia, as reflected by the P value for the homogeneity of the OR (Table S5). As in the main results overall, women in less tight control more frequently developed severe hypertension.
Table 1 shows raw adverse outcome event rates, presented according to the occurrence of severe hypertension or preeclampsia. The raw outcome rates are representative as the ORs changed little after adjustment. Adverse outcomes appeared to be more frequent among women who developed (as opposed to those who did not) severe hypertension or preeclampsia. Not shown in Table 1 is the apparently lower frequency of adverse outcomes among the 85 women with severe hypertension without preeclampsia: primary perinatal outcome (23, 27.1%), birth weight <10th percentile (14, 16.9%), secondary maternal outcome (1, 1.2%), delivery at <37 (26, 31.0%) or <34 weeks (9, 10.7%), platelets <100×109/L (0), elevated liver enzymes with symptoms (0), and maternal length of stay ≥10 days (0); readmission before 6 weeks was similar (5, 6.0%). Adverse outcomes appeared to be lower still among the 430 women with neither severe hypertension nor preeclampsia: primary perinatal outcome (76, 17.7%); birth weight <10th percentile (55, 12.9%); secondary maternal outcome (6, 1.4%); delivery at <37 weeks (67, 15.6%) or <34 weeks (25, 5.8%); low platelets (0); elevated liver enzymes with symptoms (0); and maternal length of stay ≥10 days (2, 0.5%).
Table 1.
Outcome Rates According to the Occurrence of Postrandomization Severe Hypertension or Preeclampsia (N Women, %)
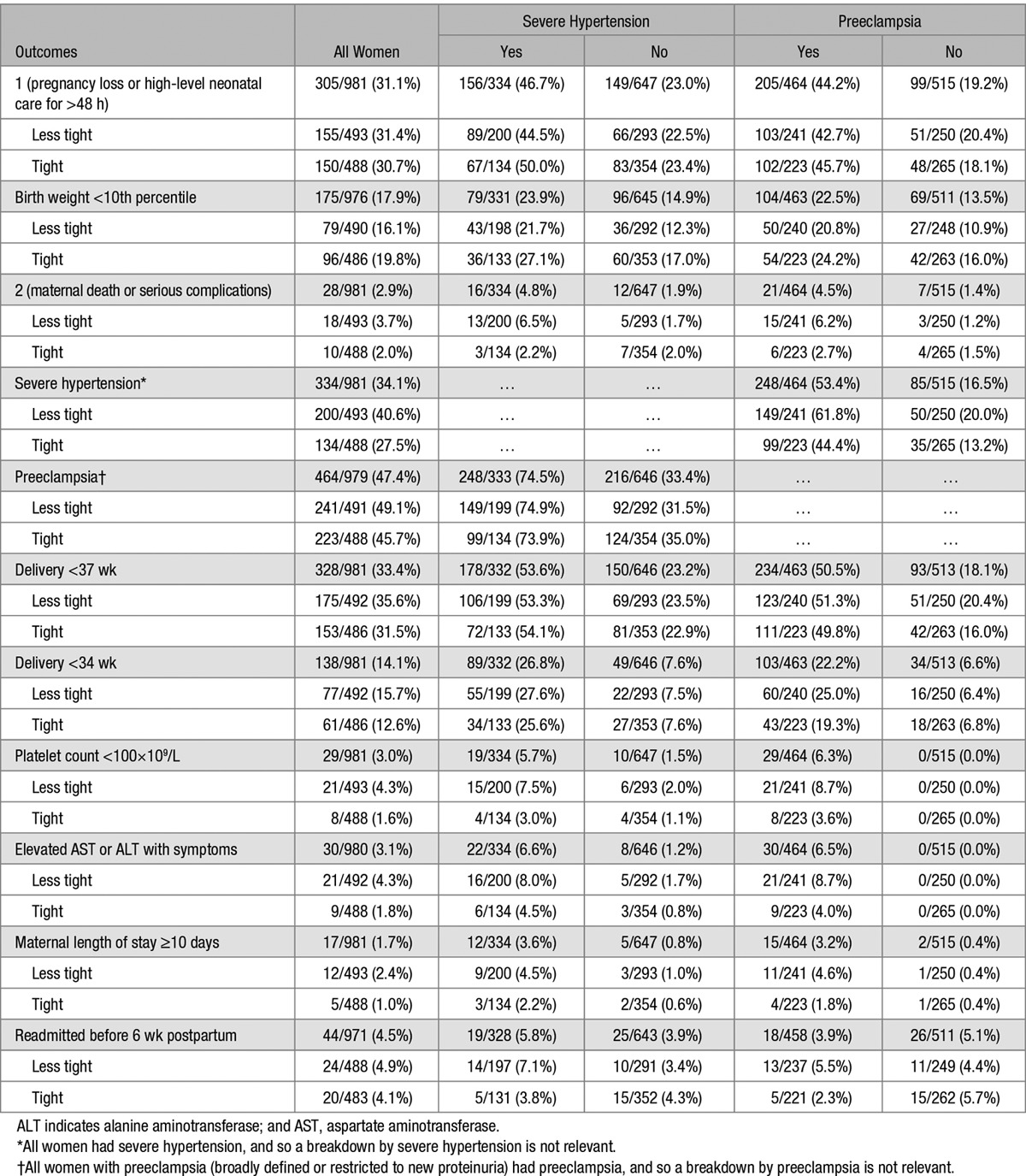
Table 2 presents aORs for adverse outcomes according to the occurrence of severe hypertension or preeclampsia.
Table 2.
Odds Ratios for Adverse Perinatal and Maternal Outcomes, According to Severe Hypertension, Preeclampsia, and Allocated Group
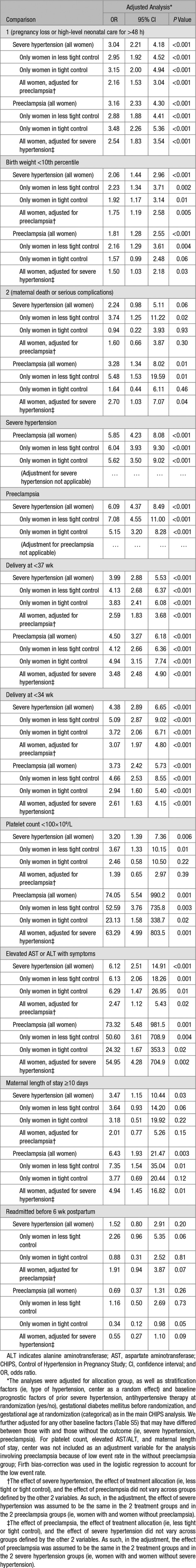
Development of severe hypertension was associated with more adverse perinatal and maternal outcomes. With few exceptions, this was true for all women, those within either less tight or tight control, and all women following adjustment for the co-occurrence of preeclampsia. Severe hypertension was associated with the secondary maternal outcome only in less tight (aOR 3.74; P=0.02) but not tight control (aOR 0.94; P=0.93), although the interaction was not statistically significant (P=0.13). A similar pattern was seen for maternal readmission before 6 weeks postpartum in less tight (aOR 2.26; P=0.06) but not in tight control (aOR 0.88; P=0.81), although power was limited with only 44 women readmitted, and there was no significant interaction (P=0.17).
Similarly, development of preeclampsia was associated with more adverse outcomes. With few exceptions, this was true overall, within each of less tight and tight control, and overall following additional adjustment for the co-occurrence of postrandomization severe hypertension. Preeclampsia was associated with the secondary maternal outcome in less tight (aOR 5.48; P=0.01) but not tight control (aOR 1.64; P=0.46), although there was no significant interaction (P=0.19). A pattern of reduced risk was seen in tight (aOR 0.34; P=0.05) but not less tight control (aOR 1.16; P=0.73) for maternal readmission before 6 weeks postpartum, although the results did not reach statistical significance and there was no significant interaction (P=0.07).
New proteinuria was common (N=280, 28.5%; Table S3) and present in almost half of women with severe hypertension (162/334, 48.5%). All results were similar to those obtained using a broad definition of preeclampsia. In brief, after adjustment for baseline differences between women with and those without new proteinuria (Table S4), adverse outcomes in less tight versus tight control were similar among women with and those without new proteinuria (Table S5). Adverse outcome rates appeared to be more frequent among women who developed (as opposed to those who did not) new proteinuria (Table S6), and development of new proteinuria was associated with more adverse perinatal and maternal outcomes (Table S7).
Discussion
Main Findings
In the CHIPS trial, women randomized to less tight control more frequently developed severe hypertension, and predictive modeling was unable to identify which women were destined to do so.2,3 Both severe hypertension and preeclampsia, a recognized risk marker for adverse outcome, were common (33.9% and 47.3%, respectively) and often developed in the same woman (25.3%), but neither severe hypertension nor preeclampsia fully accounted for all women with one or more of the adverse outcomes considered. However, severe hypertension was associated with higher rates of each of the CHIPS primary perinatal outcome, birth weight <10th percentile, preeclampsia, delivery at <34 or 37 weeks, platelets <100×109/L, elevated liver enzymes with symptoms, and maternal length of hospital stay ≥10 days. Only among women in less tight control was severe hypertension, which also developed more frequently in these women, associated with serious maternal complications (the CHIPS secondary outcome) and, possibly, maternal readmission within 6 weeks postpartum. The negative impact of severe hypertension on outcomes was evident even after adjusting for the negative effect of preeclampsia.
Strengths and Limitations
Strengths of our study include the multicenter, international nature of the CHIPS trial that speaks to the generalizability of the results. Our outcome definitions were rigorous, and we were able to define and adjust for preeclampsia defined both broadly and narrowly, increasing the relevance of the results to settings where preeclampsia is variably defined in guidelines or by practitioners.
A limitation of our study is that these analyses were post hoc, albeit in response to views that severe hypertension was a benign development and not an outcome worthy of avoidance. Also, comparisons of women with/without severe hypertension or preeclampsia, as with all nonrandomized comparisons, may reflect the impact of unknown sources of bias related to ways that clinicians manage women. Finally, CHIPS as with all randomized controlled trials was powered to detect a major effect of less tight (versus tight) control of nonsevere hypertension and underpowered to look at interactions.
Interpretation
The CHIPS cohort had a high incidence of adverse outcomes, consistent with literature showing a dBP of 90 mm Hg in pregnancy identifies a level above which perinatal morbidity and other adverse maternal outcomes are increased in nonproteinuric hypertension.2 Our results are consistent with existing randomized controlled trial data that show that less tight control is associated with more severe hypertension (N=3293 women).5–7 To date, this has been recognized as a risk marker for maternal stroke, a rare but devastating (and increasing) complication in pregnancy.8,9 Also, our results are consistent with a limited literature on the association between severe hypertension and adverse outcomes among women with chronic hypertension that is severe early in pregnancy5,6 or after 20 weeks7–9 and among women with severe gestational hypertension.10,11 However, our study adds to this literature by demonstrating within the same population that less tight control is associated with more severe hypertension, that severe hypertension is a risk marker for adverse maternal and perinatal outcomes, and that the risks associated with severe hypertension are over and above those associated with the co-occurrence of preeclampsia.
Conclusions
The development of severe hypertension raises concern about elevated stroke risk, but the CHIPS data demonstrate that the risk of other adverse perinatal and maternal outcomes, including serious maternal complications, is also increased, independently of the co-occurrence of preeclampsia.
Perspectives
There is more than one pathway that may lead to adverse outcomes in hypertensive pregnancy, and women with those outcomes may have neither severe hypertension nor preeclampsia, a fact that justifies close antenatal surveillance. However, CHIPS data indicate that severe hypertension is an outcome worthy of avoidance to minimize maternal and perinatal risk. As such, we should move from detection and prompt treatment of severe hypertension to prevention. This can be achieved with antihypertensive therapy to normalize maternal BP, as practiced in the tight BP control arm of the CHIPS trial, aiming for a modest dBP of 85 mm Hg. Future work should focus on whether one antihypertensive agent offers advantages over another.
Acknowledgments
We sincerely thank all of the women who gave of themselves by participating in CHIPS. We continue to dedicate our analyses to Dr Andrée Gruslin, our friend, and colleague who is so dearly missed.
Sources of Funding
CHIPS was funded by the Canadian Institutes of Health Research (MCT 87522).
Disclosures
All named authors contributed to the design of the project, its execution and analysis, and the writing and revision of the article. The CHIPS trial was approved by the Research Ethics Board at the University of British Columbia as the Co-ordinating Centre (H08-00882) and at all study sites. Dr von Dadelszen has received consultancy fees and placental growth factor (PLGF) cartridges for research from Alere International. The other authors have no relevant conflicts of interest to disclose.
Supplementary Material
Footnotes
A list of all CHIPS Study Group participants is given in Table S1 in the online-only Data Supplement.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.116.07862/-/DC1.
Novelty and Significance
What Is New?
Severe hypertension increases the risk of adverse perinatal and maternal outcomes beyond stroke.
These risks are independent of the risks of preeclampsia.
What Is Relevant?
Severe hypertension is not just a bigger blood pressure. Rather, severe hypertension is an important clinical outcome worthy of avoidance.
Summary
Women randomized to less tight control in the CHIPS trial (Control of Hypertension in Pregnancy Study) more often developed severe hypertension, which could not be predicted from clinical characteristics when hypertension developed. Severe hypertension was associated with higher rates of the primary perinatal outcome (pregnancy loss or high-level neonatal care for >48 hours), birth weight <10th percentile, preeclampsia, preterm delivery, platelets <100×109/L, elevated liver enzymes with symptoms, and maternal length of hospital stay for ≥10 days. Severe hypertension was associated with the secondary maternal outcomes (maternal death or serious maternal complications) only among women in less tight control. Severe hypertension remained a significant risk factor for adverse maternal and perinatal outcomes even after adjustment for preeclampsia.
References
- 1.Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014;2:CD002252. doi: 10.1002/14651858.CD002252.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 3.Magee LA, von Dadelszen P, Singer J, et al. Can adverse maternal and perinatal outcomes be predicted when blood pressure becomes elevated? Secondary analyses from the CHIPS (Control of Hypertension In Pregnancy Study) randomized controlled trial. Acta Obstet Gynecol Scand. 2016;95:763–776. doi: 10.1111/aogs.12877. doi: 10.1111/aogs.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P Canadian Hypertensive Disorders of Pregnancy (HDP) Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–145. doi: 10.1016/j.preghy.2014.01.003. doi: 10.1016/j.preghy.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Bagga R, Aggarwal N, Chopra V, Saha SC, Prasad GR, Dhaliwal LK. Pregnancy complicated by severe chronic hypertension: a 10-year analysis from a developing country. Hypertens Pregnancy. 2007;26:139–149. doi: 10.1080/10641950701204588. doi: 10.1080/10641950701204588. [DOI] [PubMed] [Google Scholar]
- 6.Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M, MacPherson C, Landon M, Miodovnik M, Paul R, Meis P, Dombrowski M. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;339:667–671. doi: 10.1056/NEJM199809033391004. doi: 10.1056/NEJM199809033391004. [DOI] [PubMed] [Google Scholar]
- 7.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigil-De Gracia P, Lasso M, Montufar-Rueda C. Perinatal outcome in women with severe chronic hypertension during the second half of pregnancy. Int J Gynaecol Obstet. 2004;85:139–144. doi: 10.1016/j.ijgo.2003.12.002. doi: 10.1016/j.ijgo.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Vigil-De Gracia P, Montufar-Rueda C, Smith A. Pregnancy and severe chronic hypertension: maternal outcome. Hypertens Pregnancy. 2004;23:285–293. doi: 10.1081/PRG-200030315. doi: 10.1081/PRG-200030315. [DOI] [PubMed] [Google Scholar]
- 10.Buchbinder A, Sibai BM, Caritis S, Macpherson C, Hauth J, Lindheimer MD, Klebanoff M, Vandorsten P, Landon M, Paul R, Miodovnik M, Meis P, Thurnau G National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66–71. doi: 10.1067/mob.2002.120080. [DOI] [PubMed] [Google Scholar]
- 11.Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, Catalano PM, Morris CD. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–28. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]


