Abstract
Objective
Electroconvulsive therapy (ECT) is the most rapid and effective antidepressant treatment but with concerns about cognitive adverse effects. A new form of ECT, focal electrically administered seizure therapy (FEAST), was designed to increase the focality of stimulation and better match stimulus parameters with neurophysiology. We recently reported on the safety and feasibility of FEAST in a cohort (n = 17) of depressed patients. We now report on the safety, feasibility, preliminary efficacy, and cognitive effects of FEAST in a new cohort.
Methods
Open-label FEAST was administered to 20 depressed adults (6 men; 3 with bipolar disorder; age 49.1 ± 10.6 years). Clinical and cognitive assessments were obtained at baseline and end of course. Time to orientation recovery was assessed at each treatment. Nonresponders switched to conventional ECT.
Results
Participants tolerated the treatment well with no dropouts. Five patients (25%) transitioned from FEAST to conventional ECT due to inadequate response. After FEAST (mean, 9.3 ± 3.5 sessions; range, 4–14), there was a 58.1% ± 36.0% improvement in Hamilton Rating Scale for Depression scores compared with that in the baseline (P < 0.0001); 13 (65%) of 20 patients met response criteria, and 11 (55%) of 20 met remission criteria. Patients achieved reorientation (4 of 5 items) in 4.4 ± 3.0 minutes (median, 4.5 minutes), timed from eyes opening. There was no deterioration in neuropsychological measures.
Conclusions
These findings provide further support for the safety and efficacy of FEAST. The remission and response rates were in the range found using conventional ECT, and the time to reorientation may be quicker. However, without a randomized comparison group, conclusions are tentative.
Key Words: electroconvulsive therapy, ECT, depression, FEAST, efficacy, cognitive adverse effects
Electroconvulsive therapy (ECT) is the gold standard intervention for treatment-resistant depression. Historically, cognitive adverse effects have been a barrier to its use in circumstances other than high levels of treatment resistance, or where there is an urgent need for rapid clinical improvement (eg, acute suicidality, poor per os intake). Technical modifications of ECT have substantially reduced cognitive adverse effects. Refinements in electrode positioning,1–3 improvements in electrical stimulation parameters,4,5 and individualized dosing strategies6 have resulted in an improved adverse effect profile without compromising efficacy. Generally, more focal stimulation and more efficient delivery of the electrical stimulus have resulted in reduced cognitive adverse effects. For example, ultrabrief pulse right unilateral (UBP-RUL) ECT has a similar rate of response and remission as traditional brief pulse right unilateral (BP-RUL) or bitemporal ECT, but with a much improved cognitive adverse effect profile.4,7 Ultrabrief pulse right unilateral ECT has become the standard method for delivering ECT for depression at many facilities.
Building on this line of research, focal electrically administered seizure therapy (FEAST) was designed to further increase stimulus focality and the efficiency of the electrical stimulus.8,9 Focal electrically administered seizure therapy differs from UBP-RUL ECT in 3 key respects: (1) FEAST uses unidirectional current, whereas UBP-RUL and all other forms of modern ECT rely on bidirectional current. Thus, in FEAST the electrodes consistently are either an anode or cathode. In conventional ECT, the electrodes flip in their roles as anode or cathode with each change in current direction. (2) FEAST uses a large oblong, posterior electrode, and a considerably smaller circular, anterior electrode. Until now, and throughout ECT's history, the 2 electrodes have been symmetric in size and shape. (3) FEAST involves a novel electrode placement. The posterior electrode is placed anterior to the right motor cortex, over the supplementary motor area, and the anterior electrode, targeting the right orbitofrontal cortex, is placed just above the center of the right eyebrow. In contrast, UBP-RUL uses the traditional d'Elia placement.10 In an empirical primate model, the alterations in stimulus delivery embodied in FEAST resulted in increased focality and efficiency of seizure induction.11 Studies modeling electrical paths have also found FEAST to concentrate stimulation in prefrontal regions.12–14
The rationale for FEAST rests on the notion that distinct neural systems subserve the antidepressant and amnestic properties of ECT. That there is localization in the neural underpinnings of the efficacy and adverse effects of ECT was amply demonstrated in a series of studies examining the impact of electrode positioning and electrical dosage.4,5,15,16 This work established that the current path of the electrical stimulus and the dosage within that path determine both efficacy and amnestic effects. Imaging research strongly supported this view by tying antidepressant effects to prefrontal reductions in blood flow17 and glucose metabolism,18 and to increased prefrontal slow wave activity in the electroencephalogram (EEG).19 In contrast, amnestic effects have been linked to the extent of these physiological alterations in medial temporal lobe regions.18,20 Thus, a strategy to restrict seizure initiation to prefrontal regions might maintain the efficacy of ECT while further limiting its amnestic effects. This view considers the sites of seizure initiation as more critical to the biological and behavioral consequences of ECT-induced seizures than are the sites of seizure propagation.8 As with secondarily generalized, focal seizures in epilepsy, surround inhibition is greatest at the site(s) of seizure initiation rather than at the sites of propagation.21–24 In essence, this theory suggests that evoking a robust inhibitory process in prefrontal cortical regions is key to achieving antidepressant effects.
Two strategies have emerged to induce focal frontal seizures, and both are under active investigation. Focal electrically administered seizure therapy attempts to accomplish this goal by maximally focusing electrical stimulation. Magnetic seizure therapy, or MST, alternately uses supraconvulsive doses of magnetic stimulation.25–27 The energy transfer from current in a coil to electricity in brain is highly inefficient, and MST has often had difficulty triggering seizures, despite the use of nonfocal, round coils often centered over the vertex, away from frontal tissue. While MST seems to have a mild cognitive profile, comparability of efficacy to traditional ECT is uncertain.12,28–30
The particular innovations exemplified by FEAST were inspired by the work of Amassian and colleagues, maximizing the focality of transcranial stimulation—both magnetic and electrical. For electrical stimulation, they found that use of a small anode and large cathode produced highly focal stimulation and with low thresholds for depolarization. Reversing the direction of current resulted in more variable thresholds and loss of focality.31–38 The use of unidirectional stimulation was common in the 1940s, soon after the introduction of ECT, and appears to further improve the efficiency of stimulation and may be associated with lower seizure threshold.11,39–45
We recently reported on the safety and feasibility of this new form of ECT (FEAST) in a cohort of depressed patients (n = 17) who were free of antidepressant medication.9 During the conduct of that study, the FEAST methods were refined. Changes were made to the size of the electrodes, with the initial electrodes consisting of a circular anterior electrode with a diameter of 0.75 inch and an oblong posterior electrode with the dimensions of 1 × 2.5 inches, and the final electrodes consisting of a circular anterior electrode with a diameter of 1.25 inches and an oblong posterior electrode with the dimensions of 2 × 3 inches. In addition, in the initial report, seizure threshold was determined by titrating in the current domain, using escalating current (pulse amplitude) to identify seizure threshold (ie, 200 mA, 400 mA, 600 mA, and 800 mA), and subsequent treatments were delivered using that current. The size of the electrodes was increased because of elevated dynamic impedance with the smaller electrodes. The current dose was restricted to the conventionally used 800 mA to exclude differences in dosing strategies as a potential confound in evaluating FEAST. We now report on the safety, feasibility, preliminary efficacy, and preliminary cognitive effects of FEAST in an expanded cohort of depressed patients, using a revised and consistent treatment technique.
MATERIALS AND METHODS
General Design and Patient Characteristics
This was an open-label trial designed to provide safety, tolerability, and efficacy data on FEAST after the earlier refinement of the technique. Participants were recruited from the Brain Stimulation Service of the Medical University of South Carolina (MUSC). They were referred for evaluation and treatment between April 2013 and April 2015. The protocol was approved by the MUSC institutional review board and was conducted under an investigational device exemption from the US Food and Drug Administration with Dr George as the principle investigator. All participants provided written informed consent before participating in any research-related activities, and all activities were performed in accordance with the Declaration of Helsinki (Clintrials.gov registration number NCT01589315).
Patients were considered for inclusion if they currently were in a major depressive episode (unipolar or bipolar), had a score of 21 or greater on the Hamilton Rating Scale for Depression (HRSD, 24 item),46 and ECT was indicated. Electroconvulsive therapy was indicated independent of referral for the study, and subsequently, patients not interested in participating in our study went on to receive traditional ECT. We excluded potential participants if they had a history of any nonmood disorder psychosis (eg, schizophrenia or schizoaffective disorder), history of substance abuse/dependence within the past 6 months, neurological illness, or unstable medical condition.
For the first 16 participants, psychotropic medications were discontinued a minimum of 4 days before starting FEAST (other than lorazepam given as needed [up to 3 mg/d]). The final 4 participants received either concomitant nortriptyline (n = 2) or venlafaxine (n = 2) while participating in the study. This change was introduced to enhance recruitment and to mirror current clinical ECT practice at MUSC.
Treatments were given 3 times per week (Mondays, Wednesdays, Fridays) until maximum therapeutic benefit was achieved. After 6 treatments, participants who showed less than 40% improvement in HRSD24 scores were offered the option of either continuing with FEAST with a dosage increase from 6 times seizure threshold (6×ST) to 9 times seizure threshold (9×ST) or withdrawing from the study and receiving traditional ECT (UBP-RUL ECT).
Participants were oxygenated by mask (100% O2) before anesthetic administration and until resumption of spontaneous respiration. Methohexital (0.75–1.0 mg/kg) was the anesthetic agent, and succinylcholine (0.75–1.0 mg/kg) was the muscle relaxant. Glycopyrrolate (0.2–0.4 mg) was administered at each titration session before anesthesia induction. We administered FEAST with a custom-modified MECTA spECTrum 5000Q device (MECTA Corp, Tualatin, Oregon). The modified device had a unidirectional stimulation waveform (with an anode and cathode electrode), compared with the standard 5000Q device, which uses a bidirectional stimulation waveform. The modified device had a maximal output voltage of 400 V, like the standard device. Focal electrically administered seizure therapy used a small (1.25 inches diameter) circular frontal anode electrode and a large oblong (2 × 3 inches curved) posterior cathode electrode. The smaller anterior anode was placed with the lower boundary just above the center of the right eyebrow, and the larger posterior cathode with the medial aspect tangential to the nasion-to-inion line, and the posterior boundary 1 inch anterior to the vertex, with the lateral portion extending over the right hemisphere (see Fig. 1).
FIGURE 1.
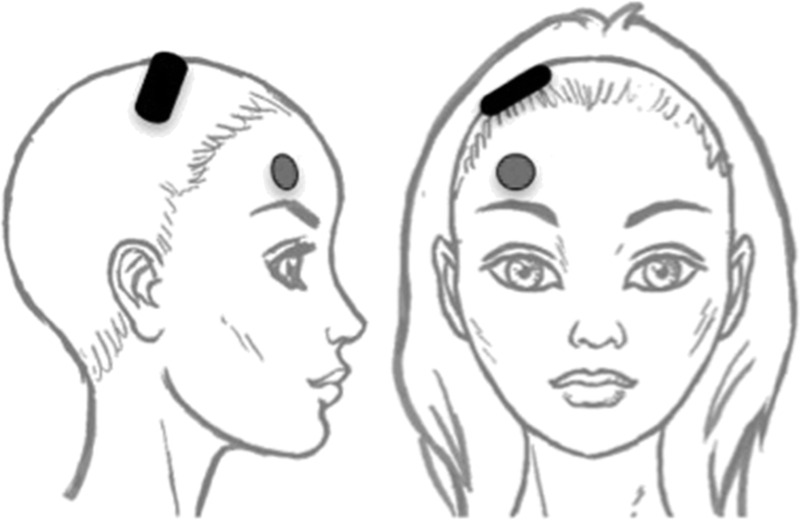
FEAST electrode placement representation. The anterior anode is placed over the middle of the right eyebrow. The posterior cathode electrode is tangential to the nasion-inion line, and the posterior boundary is 1 inch anterior to the vertex. The electrode extends over the right supplementary motor area. This figure is taken from Nahas et al.9
Each patient's seizure threshold was determined during the first treatment session using the empirical titration procedure.6 In the first cohort of FEAST patients, we titrated the seizure threshold by increasing the current amplitude. However, in this new cohort, we titrated the seizure threshold by using a fixed current (800 mA) as well as adjusting frequency and the duration of the stimulation train. We considered a motor or EEG seizure that was 20 seconds or longer to be of adequate duration. Seizures failing to meet the criteria for minimum duration were followed 30 seconds later by restimulation at the next step in the titration table. Dosing at subsequent treatments was substantially above seizure threshold (6×ST) as commonly adopted for UBP-RUL ECT.4,7 After 6 treatments, this dosage could be increased up to approximately 9×ST before terminating use of FEAST in those participants not achieving a satisfactory response.
Assessments
Diagnosis was established using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition47 and applying Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria. The Antidepressant Treatment History Form48 documented treatment history in the current episode. Our primary efficacy outcome measure was based on the 24-item HRSD.46 Clinical antidepressant response was defined a priori as a 50% reduction in the mean HRSD24 scores at the final assessment after the last FEAST session relative to baseline. Remission was defined a priori as an HRSD24 score of 10 or less after treatment. For a secondary analysis of depressive symptoms, we included the Inventory of Depressive Symptoms–Self-Report (IDS-SR). We defined response on the IDS-SR as a 50% reduction in mean score after the final FEAST session relative to baseline and remission as a score of 13 or less on the IDS-SR after treatment.49 Other secondary efficacy measures included the Clinical Global Impression (CGI) scales (CGI-S indicates severity; CGI-I, improvement),50 the Global Assessment of Function scale,51 and the Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ).52 We collected these measures before the start of FEAST and after treatment termination.
As our primary outcome measure of acute cognitive adverse effects, after each treatment, we measured the time from eyes open to correctly answering 4 of 5 orientation questions (name, place, date of birth, day of week, and age). We chose this as our primary outcome measure as time to recover full orientation is highly sensitive to variations in ECT technique, may be acquired at each treatment to enhance reliability of measurement, and is a predictor of long-term amnestic effects.5,53–55 To determine the time to reorientation, every 30 seconds after seizure termination, we gently asked participants to open their eyes. Once participants opened their eyes, we progressively asked each orientation question above every 30 seconds until all were correctly answered. In addition, we administered a neuropsychological battery before the first FEAST treatment and the day after the final FEAST treatment. This battery included the following measures: total score on the modified Mini-Mental State Examination (MMSE)56; total recall of unrelated words across 6 trials of the Buschke Selective Reminding Test (BSRT)57; score on the Autobiographical Memory Interview, Short Form (AMI-SF)58; and the Repeatable Battery for Assessment of Neurocognitive Status (RBANS).59 The MMSE assessed global cognitive status, the BSRT assessed anterograde amnesia for verbal information, the AMI-SF assessed retrograde amnesia for autobiographical information, and the RBANS is a brief neurocognitive battery with 4 alternate forms, measuring immediate and delayed memory, attention, language, and visuospatial skills. Retrograde amnesia for autobiographical information is the most well-established persistent deficit after ECT.60
Statistical Analyses
All data were quality checked, and queries clarified before final analyses were conducted. For clinical and neuropsychological data, paired sample t tests were used to assess for change over time. All statistical tests were 2-tailed with an α level of 0.05.
RESULTS
Patient Characteristics
A total of 20 participants signed informed consent, all underwent a titration session, and all completed the trial. Of this cohort, 16 were free of antidepressant medications throughout, and 4 received antidepressant medication concurrent with FEAST (2 nortriptyline, 2 venlafaxine). The average age of participants was 49.1 ± 10.6 years; 14 were women, and 3 had bipolar depression (Tables 1, 2). The average length of the current depressive episode was 77.4 ± 92 weeks (median, 46 weeks). The average total antidepressant treatment history form score was 7.9 ± 4.6, and the average number of failed adequate trials was 1.4 ± 1.3 trials. Those participants failing to show at least a 40% improvement in HRSD score after the 6th treatment were offered the option of switching to 9×ST FEAST or conventional ECT (UBP-RUL). A total of 6 participants transitioned from FEAST 6×ST to FEAST 9×ST, and 4 transitioned directly to UBP-RUL ECT. Of those transitioning from 6×ST to 9×ST FEAST, 1 participant further switched to UBP-RUL ECT. Of those switching from 6×ST FEAST to UBP-RUL ECT, 2 later received brief pulse, bitemporal ECT due to lack of response to UBP-RUL ECT.
TABLE 1.
Demographic and Clinical Characteristics of the Sample
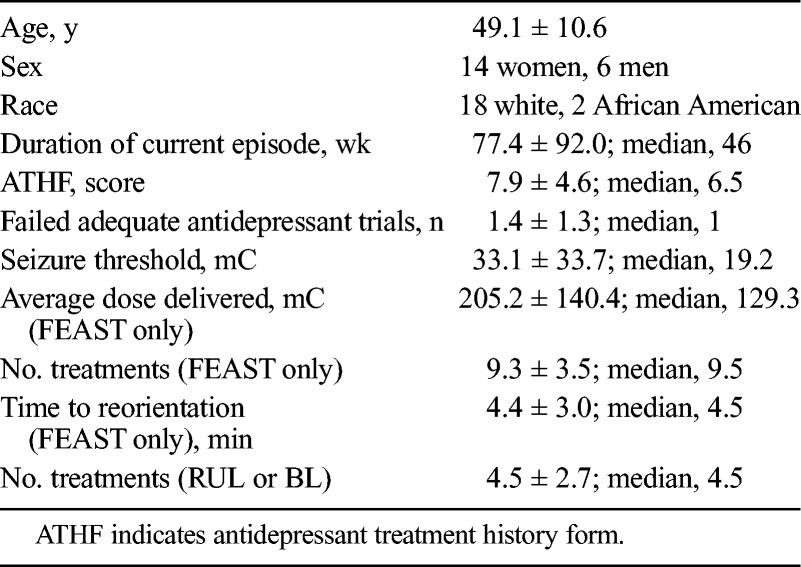
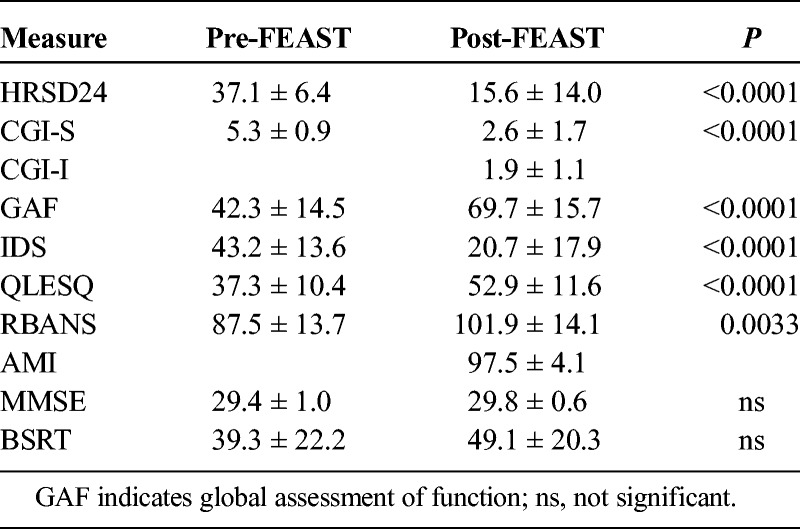
TABLE 2.
Scores on Clinical and Neuropsychological Measures Before and After the FEAST Course
Titration and Seizure Duration
The average charge needed to elicit a seizure during titration of FEAST was 33.1 ± 33.7 mC (median, 19.2 mC). Across all FEAST treatments, the average duration of motor convulsions was 32.4 ± 17.7 seconds and the EEG seizure duration was 57.8 ± 26.9 seconds. The average charge delivered during FEAST treatments was 205.2 ± 140.4 mC (median, 129.3 mC).
Clinical Outcomes
The average number of FEAST treatment sessions per patient was 9.3 ± 3.5, and the median was 10.5 treatments. Patients averaged a 58.1% ± 36.0% improvement after FEAST compared with baseline on the HRSD24 (37.1 ± 6.4, 15.6 ± 14.0; P < 0.0001) (see Figs. 2A, B) and a 54.7% ± 32.3% improvement on the IDS-SR (43.2 ± 13.6, 20.7 ± 17.9; P < 0.0001). The sample also showed a robust increase in the quality of life measure, QLESQ (37.3 ± 10.4, 52.9 ± 11.6; P < 0.0001).
FIGURE 2.
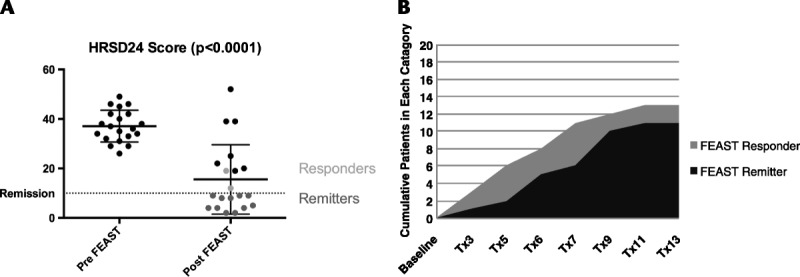
A, Pre-FEAST and post-FEAST HRSD scores. B, Cumulative number of participants meeting response and remission criteria by treatment number.
At the end of the FEAST course, 13 (65%) of 20 patients met response criteria and 11 (55%) of 20 met remission criteria on the HRSD. For the IDS-SR, the response rate was 65% and the remission rate was 45%. Both of the participants on concomitant venlafaxine achieved remission, and one of the participants on concomitant nortriptyline achieved remission based on HRSD24 scores.
Of the 6 participants who transitioned from 6×ST FEAST to 9×ST FEAST, one eventually achieved remission, and one other achieved response. Of the 5 nonresponders to FEAST (HRSD24 criteria) who transitioned to UBP-RUL ECT, 1 later achieved response, and none achieved remission. Of the 2 participants who transitioned to brief pulse bitemporal ECT, none went on to achieve response or remission (see Fig. 3 for a charting of clinical improvement based upon type of ECT).
FIGURE 3.
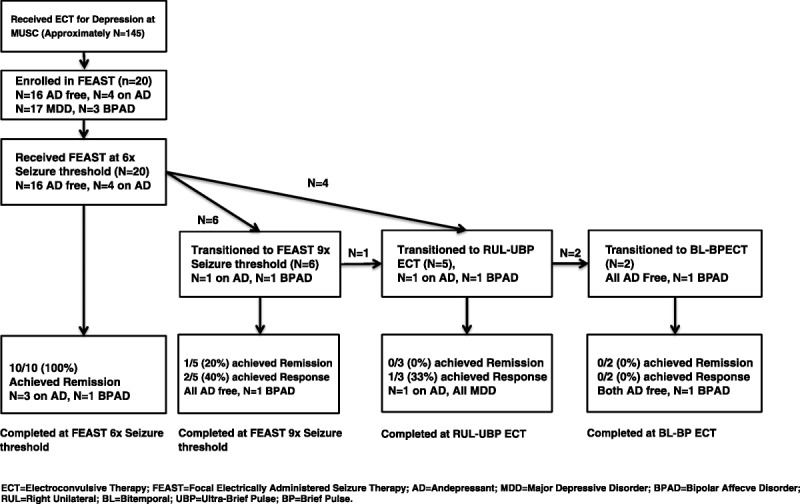
Consort style flow chart of clinical outcomes as a function of type of ECT.
Cognitive Measures
Patients achieved full reorientation (defined as the time from eyes opening on command to correctly answering 4 of 5 questions) in 4.4 ± 3.0 minutes (median, 4.5 minutes). Two participants experienced postictal agitation with each treatment, requiring midazolam, were not able to cooperate, and were excluded from this analysis. Neither of these participants had amnestic adverse effects as measured by the AMI-SF (98% and 97%) or RBANS (both substantially improved). The MMSE scores at baseline and end of treatment scores were (29.4 ± 1.0, 29.8 ± 0.6; P = ns).
Across the sample, the post-FEAST AMI-SF consistency score was (97.5% ± 4.1%), and the RBANS were (87.5 ± 13.7, 101.9 ± 14.1; P = 0.003). Thus, there was no evidence that FEAST resulted in deterioration in global cognitive status (MMSE) or across RBANS measures of attention and anterograde learning and memory. There was no evidence that FEAST produced retrograde amnesia for autobiographical information (AMI-SF). Scores on the BSRT nonsignificantly improved from 39.3 ± 22.2 to 49.1 ± 20.3. Thus, there was also no indication that FEAST resulted in anterograde amnesia for verbal material.
Adverse Events
There were no unanticipated adverse events. There was 1 case of induced hypomania. This state resolved spontaneously, without intervention, and the patient met remission criteria.
DISCUSSION
The findings further indicate that FEAST is feasible, tolerable, and safe. Furthermore, the findings demonstrate that, in this cohort of patients, the therapeutic efficacy of FEAST is in the range of that seen in other prospective treatment studies using conventional UBP-RUL and BP-RUL ECT or other techniques. The FEAST adverse effects were minimal, and in cognitive effects, FEAST may have had an advantage compared with other recent trials using UBP-RUL and BP-RUL.
In a recent meta-analysis of BP-RUL and UBP-RUL trials, 2 of the most commonly used forms of conventional ECT for depression, the authors found an aggregate response rate of 58.1% for BP-RUL and 55.3% for UBP-RUL as well as and an aggregate remission rate of 44.9% for BP-RUL and 33.8% for UBP-RUL.7 Our cohort's response (65%) and remission (55%) rates are within the range or higher than of the published average response/remission rates. In 2 other recent multisite studies where UBP-RUL was compared with BP-bilateral (BL) ECT4 or BP-bifrontal (BF) ECT,61 the remission rates for UBP-RUL were 61.3% and 43.8%, respectively, again in the range of our findings. In a recent multisite study randomizing patients to BP-RUL or BP-BL ECT (and randomized as well to placebo or nortriptyline or venlafaxine), the remission rates for BP-RUL ECT was 61.3%, and it was 51.8% for BP-BL ECT.1 In another recent multisite trial, patients were randomized to RUL ECT, BL ECT, or BF ECT, all using a brief pulse.2 The remission rates were 54.6%, 63.9%, and 60.5% for BP-RUL, BP-BL, and BP-BF ECT, respectively. Thus, the remission rate in this new cohort of FEAST patients compares favorably with the remission rates across much of the recent literature comparing different forms of ECT.
It is noteworthy that 16 of the 20 patients received FEAST without any concomitant psychotropic medications other than lorazepam (up to 3 mg/d). Recent evidence suggests that concomitant antidepressant medication use increases ECT remission rates by approximately 15%.1 Thus, the overall remission rate we observed with FEAST might have been higher had antidepressants also been prescribed. Indeed, 3 (75%) of the 4 patients who received FEAST while also being treated with venlafaxine or nortriptyline were remitters.
Interestingly, in this cohort, those who elected to transition to conventional ECT after slow or nonresponse to FEAST were unlikely to respond to additional treatments with conventional ECT (only 1 of 5 eventually responded and none remitted). This finding might also suggest that FEAST is as robust in efficacy as conventional forms of ECT and that failure to benefit from FEAST predicts poor outcome with conventional treatment. Alternate explanations include the possibilities that an additional number of treatments of conventional ECT beyond what was given were needed for response or, less likely, that FEAST given before conventional ECT somehow reduced the efficacy of subsequent conventional ECT treatments. Another notable finding from our cohort was that those who showed at least a 40% improvement after 6 treatments using 6×ST threshold FEAST all went on to achieve remission. This finding may be valuable prognostically if it holds up with further study.
Our primary outcome measure for adverse cognitive adverse effects was time to reorientation after treatment. Our mean time to reorientation was 4.4 minutes. This compares favorably to published means of reorientation time in other investigations using UBP RUL (10–15 minutes) and BP RUL (21–22 minutes).4,62 Our main secondary outcome measure of amnestic effects was the AMI-SF. Patients averaged 97.5% in the consistency score from pre-FEAST baseline to post-FEAST completion. This remarkable level of consistency in memories across these 2 time points also compares favorably to reports of UBP RUL (consistency scores of 71%–93%) and BP RUL (consistency scores of 53%–80%).62,63 We had 2 participants who experienced postictal confusion after treatment, and their time to reorientation was excluded from analysis. Both of these participants still had excellent AMI-SF consistency scores and showed no other impairment on any other cognitive measure.
In the early primate studies comparing the seizure threshold of FEAST to other forms of ECT, it was found that FEAST resulted in a lower seizure threshold with directionality of stimulus, and electrode geometries both playing a role.11 In contrast, our average seizure threshold of 33.1 mC was not lower than other reported mean seizure thresholds using UBP-RUL, which ranged from 22 to 38 mC.1,4,61–65 Our participants were allowed to be on 3 mg per day, or less of lorazepam. In addition, they were potentially on other anticonvulsant medications up until 4 days before their first treatment. It is possible that the average seizure threshold of our cohort was elevated by either the concomitant use of lorazepam, or the residual effect of a long-acting anticonvulsant medication. Although this is possible, we do not believe it would have elevated seizure threshold above that found in the other investigations we cited. In the trials, we cited for comparison that low-dose lorazepam was also commonly allowed, and there was not a reported period of medication washout that was longer.
Although we have similar rates of response and remission as well as reduced markers of amnestic adverse effects as compared with the recently published literature using UBP and BP RUL ECT, it is important to remember that a comparison of our data with these other published studies is only suggestive of similarities and differences. Randomized comparative trials are needed to test these possibilities. Although the investigators offered this treatment to all patients who were about to undergo ECT, it is possible that the cohort studied was higher functioning (specifically desiring reduced cognitive adverse effects) and less severe (very severely depressed patients may have opted for the more established form of ECT). However, baseline HRSD scores were in a similar range to other ECT investigations, but the number of failed adequate medication trials was lower than in some4,63 but not all1,62 published comparison trials. This potential selection bias can be avoided in the future by performing controlled trials, ideally with a randomized, masked design. In addition to the potential selection bias, the open-label design may have led to an expectancy bias in both the participants and the raters. The expectation of improvement without amnestic effects may have resulted in unintentional extra effort during cognitive tests by the participants or a tendency to optimistically rate cognitive measures. The least susceptible outcome for this sort of bias was the time to reorientation, which would be difficult for a participant to alter and difficult for a rater to influence. The fact that the short time to reorientation was consistent with minimal amnestic effects on other measures of cognition lends credence to the overall pattern of findings.
Limitations
This study was designed to test the safety, feasibility, and preliminary efficacy and cognitive adverse effects of FEAST. There were a number of methodological limitations that warrant mention. This was an open-label trial where participants self-selected for enrollment. There was also no randomized or otherwise concurrent comparison group. These limitations may have led to both selection and expectancy biases as discussed previously.
CONCLUSIONS
In aggregate, the findings from our present study suggest that FEAST may have similar efficacy, with reduced amnestic affects, as compared with other forms of conventional ECT. While further study is needed to reach firm conclusions, these promising data support continued study into this novel method of delivering ECT, and the possible neurophysiological bases of these differences. Future studies directly comparing FEAST to conventional ECT are needed to determine if FEAST in fact has comparable efficacy and reduced adverse effects.
Footnotes
This study was supported in part by an unrestricted educational grant from the MECTA Corporation. The MECTA Corporation also provided a custom-modified MECTA spECTrum 5000Q device. Dr Sackeim is the inventor on a patent for FEAST (US8712532 B2) and serves as a consultant to the MECTA Corporation and Neuronetics, Inc. All other authors have no conflicts of interest or financial disclosures to report.
REFERENCES
- 1.Sackeim HA, Dillingham EM, Prudic J, et al. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Arch Gen Psychiatry. 2009;66:729–737. [DOI] [PubMed] [Google Scholar]
- 2.Kellner CH, Knapp R, Husain MM, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. 2010;196:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackeim HA, Prudic J, Fuller R, et al. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32:244–254. [DOI] [PubMed] [Google Scholar]
- 4.Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328:839–846. [DOI] [PubMed] [Google Scholar]
- 6.Sackeim H, Decina P, Prohovnik I, et al. Seizure threshold in electroconvulsive therapy. Effects of sex, age, electrode placement, and number of treatments. Arch Gen Psychiatry. 1987;44:355–360. [DOI] [PubMed] [Google Scholar]
- 7.Tor PC, Bautovich A, Wang MJ, et al. A systematic review and meta-analysis of brief versus ultrabrief right unilateral electroconvulsive therapy for depression. J Clin Psychiatry. 2015;76:e1092–e1098. [DOI] [PubMed] [Google Scholar]
- 8.Sackeim HA. The convulsant and anticonvulsant properties of electroconvulsive therapy: towards a focal form of brain stimulation. Clin Neurosci Rev. 2004;4:39–57. [Google Scholar]
- 9.Nahas Z, Short B, Burns C, et al. A feasibility study of a new method for electrically producing seizures in man: focal electrically administered seizure therapy [FEAST]. Brain Stimul. 2013;6:403–408. [DOI] [PubMed] [Google Scholar]
- 10.d'Elia G. Unilateral electroconvulsive therapy. Acta Psychiatr Scand Suppl. 1970;215:1–98. [PubMed] [Google Scholar]
- 11.Spellman T, Peterchev AV, Lisanby SH. Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology. 2009;34:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng ZD, Lisanby SH, Peterchev AV. Effect of anatomical variability on neural stimulation strength and focality in electroconvulsive therapy (ECT) and magnetic seizure therapy (MST). Conf Proc IEEE Eng Med Biol Soc. 2009;2009:682–688. [DOI] [PubMed] [Google Scholar]
- 13.Deng ZD, Lisanby SH, Peterchev AV. Effect of anatomical variability on electric field characteristics of electroconvulsive therapy and magnetic seizure therapy: a parametric modeling study. IEEE Trans Neural Syst Rehabil Eng. 2015;23:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee WH, Deng ZD, Kim TS, et al. Regional electric field induced by electroconvulsive therapy: a finite element simulation study. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2045–2048. [DOI] [PubMed] [Google Scholar]
- 15.Sackeim HA, Decina P, Kanzler M, et al. Effects of electrode placement on the efficacy of titrated, low-dose ECT. Am J Psychiatry. 1987;144:1449–1455. [DOI] [PubMed] [Google Scholar]
- 16.Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–434. [DOI] [PubMed] [Google Scholar]
- 17.Nobler MS, Sackeim HA, Prohovnik I, et al. Regional cerebral blood flow in mood disorders, III. Treatment and clinical response. Arch Gen Psychiatry. 1994;51:884–897. [DOI] [PubMed] [Google Scholar]
- 18.Nobler MS, Oquendo MA, Kegeles LS, et al. Decreased regional brain metabolism after ECT. Am J Psychiatry. 2001;158:305–308. [DOI] [PubMed] [Google Scholar]
- 19.Sackeim HA, Luber B, Katzman GP, et al. The effects of electroconvulsive therapy on quantitative electroencephalograms. Relationship to clinical outcome. Arch Gen Psychiatry. 1996;53:814–824. [DOI] [PubMed] [Google Scholar]
- 20.Sackeim HA, Luber B, Moeller JR, et al. Electrophysiological correlates of the adverse cognitive effects of electroconvulsive therapy. J ECT. 2000;16:110–120. [DOI] [PubMed] [Google Scholar]
- 21.Ackermann RF, Engel J, Jr, Baxter L. Positron emission tomography and autoradiographic studies of glucose utilization following electroconvulsive seizures in humans and rats. Ann N Y Acad Sci. 1986;462:263–269. [DOI] [PubMed] [Google Scholar]
- 22.Engel JJ. Seizures and Epilepsy. Philadelphia, PA: F.A. Davis; 1989. [Google Scholar]
- 23.Engel JJ. New concepts of the epileptic focus. In: Wieser HG, Speckmann EJ, Engel JJ, eds. The Epileptic Focus. London, United Kingdom: John Libbey; 1987:83–94. [Google Scholar]
- 24.Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Arch Neurol. 1967;16:194–202. [DOI] [PubMed] [Google Scholar]
- 25.Lisanby SH, Luber B, Finck AD, et al. Deliberate seizure induction with repetitive transcranial magnetic stimulation in nonhuman primates. Arch Gen Psychiatry. 2001;58:199–200. [DOI] [PubMed] [Google Scholar]
- 26.Lisanby SH, Luber B, Schlaepfer TE, et al. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28:1852–1865. [DOI] [PubMed] [Google Scholar]
- 27.Lisanby SH, Schlaepfer TE, Fisch HU, et al. Magnetic seizure therapy of major depression. Arch Gen Psychiatry. 2001;58:303–305. [DOI] [PubMed] [Google Scholar]
- 28.Kosel M, Frick C, Lisanby SH, et al. Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology. 2003;28:2045–2048. [DOI] [PubMed] [Google Scholar]
- 29.Moscrip TD, Terrace HS, Sackeim HA, et al. Randomized controlled trial of the cognitive side-effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS). Int J Neuropsychopharmacol. 2006;9:1–11. [DOI] [PubMed] [Google Scholar]
- 30.Lee WH, Lisanby SH, Laine AF. Stimulation strength and focality of electroconvulsive therapy and magnetic seizure therapy in a realistic head model. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:410–413. [DOI] [PubMed] [Google Scholar]
- 31.Maccabee PJ, Nagarajan SS, Amassian VE, et al. Influence of pulse sequence, polarity and amplitude on magnetic stimulation of human and porcine peripheral nerve. J Physiol. 1998;513(Pt 2):571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maccabee PJ, Amassian VE, Eberle LP, et al. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J Physiol. 1993;460:201–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amassian VE, Eberle L, Maccabee PJ, et al. Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol. 1992;85:291–301. [DOI] [PubMed] [Google Scholar]
- 34.Maccabee PJ, Amassian VE, Cracco RQ, et al. Mechanisms of peripheral nervous system stimulation using the magnetic coil. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:344–361. [PubMed] [Google Scholar]
- 35.Amassian VE, Cracco RQ, Maccabee PJ, et al. Matching focal and non-focal magnetic coil stimulation to properties of human nervous system: mapping motor unit fields in motor cortex contrasted with altering sequential digit movements by premotor-SMA stimulation. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:3–28. [PubMed] [Google Scholar]
- 36.Amassian VE, Quirk GJ, Stewart M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalogr Clin Neurophysiol. 1990;77:390–401. [DOI] [PubMed] [Google Scholar]
- 37.Cracco RQ, Amassian VE, Maccabee PJ, et al. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:417–424. [DOI] [PubMed] [Google Scholar]
- 38.Amassian VE, Maccabee PJ, Cracco RQ. Focal stimulation of human peripheral nerve with the magnetic coil: a comparison with electrical stimulation. Exp Neurol. 1989;103:282–289. [DOI] [PubMed] [Google Scholar]
- 39.Alexander SP. Modified electro-convulsive therapy with unidirectional current. Dis Nerv Syst. 1955;16:72–76. [PubMed] [Google Scholar]
- 40.Epstein J, Wender L. Alternating current vs. unidirectional current for electroconvulsive therapy—comparative studies. Confin Neurol. 1956;16:137–146. [DOI] [PubMed] [Google Scholar]
- 41.Friedman E. Unidirectional electrostimulated convulsive therapy: I. The effect of wave form and stimulus characteristics on the convulsive dose. Am J Psychiatry. 1942;99:218–223. [Google Scholar]
- 42.Friedman E. Unidirectional electro-stimulated convulsive therapy. II Therapeutic results in 536 patients. J Nerv Ment Dis. 1949;109:540–549. [DOI] [PubMed] [Google Scholar]
- 43.Friedman E, Wilcox PH. Electro-stimulated convulsive doses in intact humans by means of unidirectional currents. J Nerv Ment Dis. 1942;96:56–63. [Google Scholar]
- 44.Pacella BL. Comparative studies of convulsive therapy administered by unidirectional current (Reiter type) and by the usual alternating current apparatus. Confin Neurol. 1952;12:330–336. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox PH. Electroshock therapy. A review of over 23,000 treatments using unidirectional currents. Am J Psychiatry. 1947;104:100–112. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.First MW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5® Disorders—Clinician Version (SCID-5-CV). New York, NY: 2016. [Google Scholar]
- 48.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 49.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. [DOI] [PubMed] [Google Scholar]
- 50.Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: Superintendent of Documents, US Government Printing Office, US Department of Health, Education, and Welfare Publication; 1976:76–338. [Google Scholar]
- 51.American Psychiatric Association. Electronic DSM-IV-TR plus. 2000; 1 CD-ROM. [Google Scholar]
- 52.Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 53.Sobin C, Sackeim HA, Prudic J, et al. Predictors of retrograde amnesia following ECT. Am J Psychiatry. 1995;152:995–1001. [DOI] [PubMed] [Google Scholar]
- 54.Martin DM, Gálvez V, Loo CK. Predicting retrograde autobiographical memory changes following electroconvulsive therapy: relationships between individual, treatment, and early clinical factors. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sackeim HA. The cognitive effects of electroconvulsive therapy. In: Moos WH, Gamzu ER, Thal LJ, eds. Cognitive Disorders: Pathophysiology and Treatment. New York, NY: Marcel Dekker; 1992:183–228. [Google Scholar]
- 56.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 57.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. [DOI] [PubMed] [Google Scholar]
- 58.McElhiney M, Moody B, Sackeim H. The Autobiographical Memory Interview - Short Form. New York, NY: New York State Psychiatric Institute; 1997. [Google Scholar]
- 59.Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 60.Sackeim HA. Autobiographical memory and electroconvulsive therapy: do not throw out the baby. J ECT. 2014;30:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sienaert P, Vansteelandt K, Demyttenaere K, et al. Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: clinical efficacy. J Affect Disord. 2009;116:106–112. [DOI] [PubMed] [Google Scholar]
- 62.Loo CK, Katalinic N, Smith DJ, et al. A randomized controlled trial of brief and ultrabrief pulse right unilateral electroconvulsive therapy. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loo CK, Sainsbury K, Sheehan P, et al. A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. Int J Neuropsychopharmacol. 2008;11:883–890. [DOI] [PubMed] [Google Scholar]
- 64.Mayur P, Byth K, Harris A. Acute antidepressant effects of right unilateral ultra-brief ECT: a double-blind randomised controlled trial. J Affect Disord. 2013;149:426–429. [DOI] [PubMed] [Google Scholar]
- 65.Spaans HP, Verwijk E, Comijs HC, et al. Efficacy and cognitive side effects after brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy for major depression: a randomized, double-blind, controlled study. J Clin Psychiatry. 2013;74:e1029–e1036. [DOI] [PubMed] [Google Scholar]


