Abstract
Purpose:
The objective of this study is to investigate changes in corneal densitometry after accelerated transepithelial corneal collagen cross-linking (ATE-CXL) for patients with progressive keratoconus (KC).
Methods:
Seventeen progressive KC patients who underwent ATE-CXL (KC group) were examined and compared against 17 non-KC myopes (control group). For the KC group, corneal topography and densitometry were evaluated preoperatively and at 1, 6, and 12 months postoperatively. Manifest refraction spherical equivalent and best spectacle-corrected distant visual acuity were assessed preoperatively and at 12 months postoperatively. These parameters were also evaluated in the control group.
Results:
Preoperatively, in the KC group, the densitometry values of the total layer over the annular diameters (Φ) 0 to 2 and Φ 2 to 6 mm were 18.47 ± 1.81 and 16.62 ± 1.60, respectively. In the control group, the values were 14.98 ± 1.18 and 13.39 ± 1.33, respectively, significantly lower than those of the KC group (both post hoc P values < 0.001). At postoperative month 12, the densitometry values of Φ 0 to 2 and Φ 2 to 6 mm of the total layer in the KC group were 16.88 ± 1.57 and 15.28 ± 1.40, which were significantly lower than the preoperative values (post hoc P = 0.012 and 0.030, respectively). However, they were still higher than those of the myopes (post hoc P = 0.002 and 0.001, respectively).
Conclusions:
KC patients have much higher corneal densitometry values than myopes without KC. The KC patients' corneal densitometry values decreased significantly when measured at 12 months after ATE-CXL. However, they remain higher than those of the myopes.
Key Words: corneal densitometry, accelerated transepithelial corneal collagen cross-linking, keratoconus
The conventional corneal collagen cross-linking (C-CXL) procedure involves removing the corneal epithelium and applying riboflavin (vitamin B2) for 5 to 15 minutes before a 30-minute UV-A irradiation (power: 3 mW/cm2 and total energy: 5.4 J/cm2).1–5 Large quantities of literature have reported its safety and efficacy.2–4 However, more and more studies have shown that in the early stages after epithelium-off CXL, corneal densitometry is significantly increased, in addition to possible development of a temporary or permanent corneal haze.2,6–8 Moreover, the epithelial removal and prolonged exposure to UV-A irradiation during the C-CXL results in a time-consuming procedure, which may lead to discomfort, temporary vision loss or increased risk of infection.9 Accelerated transepithelial corneal collagen cross-linking (ATE-CXL) is a newly developed CXL technique that maintains the integrity of the corneal epithelium layer, thus avoiding stromal exposure. With a higher irradiation intensity of UV-A light, the duration of the irradiation is dramatically reduced. So far, the effects of ATE-CXL on corneal densitometry have not been fully elucidated. In this study, we used a 3-dimensional anterior segment analyzer and an automated Scheimpflug densitometry program to assess the changes in corneal densitometry values.
METHODS
Patients
This was a retrospective cohort study. A total of 34 patients were involved in this study at the Eye and ENT Hospital of Fudan University from December 2013 to July 2015. Seventeen eyes of 17 patients (10 male and 7 female, with ages between 22.4 and 31.4 years) diagnosed with progressive keratoconus (KC) (KC group) and 17 eyes of 17 myopic patients (control group) with matching age and gender were examined. This study was approved by the Ethics Committee of the Eye and ENT Hospital of Fudan University (ethical approval number: ky2012-017) and was carried out following the tenets of the Declaration of Helsinki. Written informed consent for the treatment was obtained from each patient before the procedures. Progressive KC was defined as ≥1 diopter (D) increase in the steepest keratometry (k) value, ≥1 D increase in the manifest cylinder, or ≥0.5 D increase in the manifest refraction spherical equivalent (MRSE) over the past 2 years.10 Exclusion criteria of the procedure were corneal thickness of less than 340 μm at the thinnest point (evaluated by a 3-dimensional anterior segment analyzer, Pentacam HR, Type 70900; Pentacam, Oculus, Germany), endothelial cell density of less than 2000 cells per square millimeter (Specular microscope, SP-2000P; Topcon Corporation, Japan), corneal scarring, severe dry eye, autoimmune diseases, or pregnancy.9,10
Preoperative Ophthalmologic Examinations
Before their operations, the eyes of the KC patients underwent MRSE, best spectacle-corrected distant visual acuity (BSCDVA), and slit-lamp anterior segment and fundus examinations. Corneal topography, including central corneal thickness (CCT), thinnest point pachymetry (TPP), flat k value (k1), steep k value (k2), mean k value (km), and corneal densitometry were obtained by using a Pentacam HR in a sitting position. Images with acceptable quality11 were analyzed.
Follow-up Examinations
For the KC group, MRSE and BSCDVA were assessed again 12 months after the procedure. In addition, their CCT, TPP, k1, k2, km, and corneal densitometry values were postoperatively remeasured at 1, 6, and 12 months. Those parameters were only assessed once in the control group.
Corneal Densitometry Analysis
Densitometry is expressed in gray scale units and ranges from 0 (100% transparent) to 100 (completely opaque, 0% transparent).11 In this study, corneal densitometry values were analyzed using the Cornea Densitometry Average Table (version 1.20r29), which refers to the average densitometry values in different annuli around the apex and in 4 different layers of the cornea. The “anterior layer” and the “posterior layer” represent the first 120 μm and the last 60 μm of the complete corneal thickness. The “central layer,” which has no fixed thickness value, refers to the volume between the 2 boundary layers. The “total layer” refers to the volume between the epithelium and endothelium of a cornea. For each eye, the measurements on the central annuli of diameters from 0 to 2 mm (Φ 0–2 mm) and from 2 to 6 mm (Φ 2–6 mm) of the corneas' anterior layer, central layer, posterior layer, and the total layer were analyzed. This is because densitometry value measurements were much more accurate and repeatable in the central areas compared with the peripheral.11,12
Surgical Techniques
All procedures were performed by 1 surgeon (X.Z.). Before each procedure, 3 drops of 0.4% oxybuprocaine hydrochloride (Santen Pharmaceutical Co, Ltd, Osaka, Japan) were applied on the cornea for topical anesthesia. After a lid speculum was applied to keep the whole cornea fully exposed, a trephine with an inner diameter of 8.5 mm (Model 52503B; 66 vision Tech Co, Ltd, Suzhou, China) was placed in the center of the cornea. ParaCel Solution (riboflavin 0.25%, hydroxyl-propyl-methyl-cellulose, NaCl, ethylene-diamine-tetra-acetic acid, Tris, and benzalkonium chloride; Medio-Haus-Medizinprodukte GmbH, Kiel, Germany) was then instilled into the trephine to increase the permeability of the corneal epithelium for 240 seconds and removed immediately with a cellulose sponge. Subsequently, Vibex-Xtra Solution (riboflavin phosphate 2.80 mg/mL and NaCl, Avedro, Inc.) was continually dripped into the trephine for 6 minutes to saturate the total layer of the corneal tissue. Then, the trephine was removed and ATE-CXL was performed using a 365-nm UV-A light (Avedro's KXL System; Avedro, Inc) with 45-mW/cm2 irradiation in pulsed mode (0.5-hertz flash rate, ie, one second on, next second off) for a total of 320 seconds (total energy: 7.2 J/cm2). Balanced salt solution was administered every 40 seconds to protect ocular surface from dehydration during irradiation. Finally, a bandage contact lens (Acuvue Oasys, Inc, Jacksonville, FL) was placed on the cornea for 3 days postoperation. Topical antibiotics (ofloxacin ophthalmic solution 0.5%; Santen Pharmaceutical Co, Ltd) were used 4 times per day for 7 days. Artificial tears (Tears Naturale; Alcon Laboratories, Inc, Fort Worth, TX) were applied 4 times per day for 20 days. Topical steroids (fluorometholone 0.1%; Santen Pharmaceutical Co, Ltd) were applied 8 times per day initially, with gradually reduced frequencies for a period of 20 days.
Data Analysis and Statistical Evaluation
Statistical analysis was performed using SPSS 19 (SPSS Inc, IBM). Normality check was conducted using the Kolmogorov–Smirnov Z test. Paired t test was performed to assess the differences in the baseline of refraction and topographic parameters between the KC group and the control group. Repeated measures analyses of variance (RM-ANOVA) with Bonferroni-adjusted post hoc comparisons were performed to evaluate the changes in corneal densitometry values and topographic parameters obtained preoperation, and at 1, 6, and 12 months postoperations. Multifactor ANOVAs with Bonferroni-adjusted post hoc comparisons were performed to assess the differences between the densitometry values of the control group and those obtained before and at 12 months after ATE-CXL of the KC group. The cut-off P value was 0.05.
RESULTS
Baseline Data of Demographics and Topographic Parameters
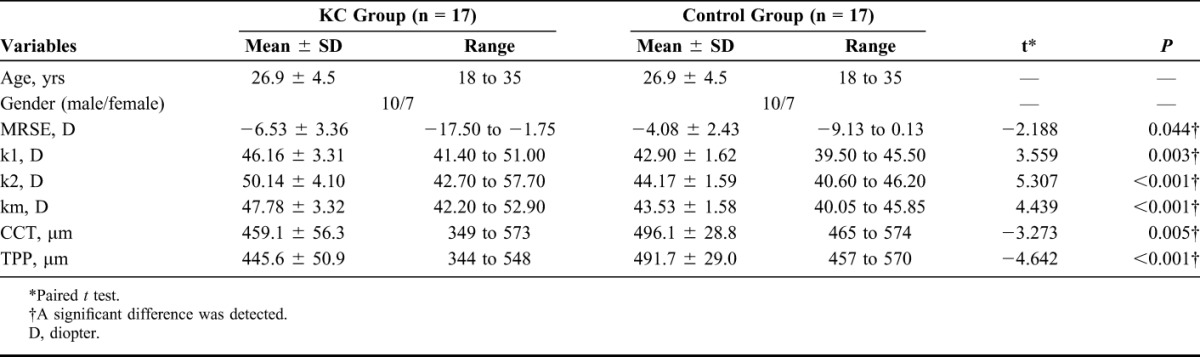
Baseline data of patient demographics are shown in Table 1. Paired t test revealed that the values of MRSE, k1, k2, and km in the KC group were significantly higher than those in the control group (t = −2.188, P = 0.044; t = 3.559, P = 0.003; t = 5.307, P < 0.001; t = 4.439, P < 0.001, respectively), whereas the mean values of CCT and TPP were lower (t = −3.273, P = 0.005 and t = −4.642, P < 0.001). All surgical procedures were uneventful. No postoperative complications (eg, severe dry eye, corneal haze, edema, or infection) were detected at the 1-, 6-, and 12-month marks.
TABLE 1.
Baseline Data of Main Topographic Parameters and Patient Demographics (n = 34)
Visual Acuity
Before the procedure, 6 KC patients' eyes (35.3%) had a BSCDVA of equal to or better than 20/25; 8 (47.1%) had a BDCVA between 20/25 and 6/20. The remaining 3 (17.6%) were less than 6/20. Twelve-month postoperative results showed that the postoperative BSCDVA improved significantly (Z = −2.634, P = 0.008) as 9 KC patients' eyes (52.9%) had a BSCDVA of equal to or better than 20/25; 5 (29.4%) had a BDCVA between 20/25 and 6/20; and the remaining 3 (17.6%) were less than 6/20.
Changes in Main Topographic Parameters and Corneal Densitometry After ATE-CXL
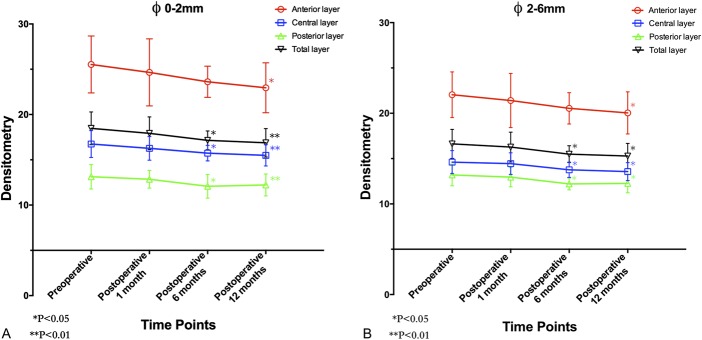
The mean values of topographic parameters before and after ATE-CXL were listed in Table 2. RM-ANOVA revealed that the mean values of k1, k2, km, CCT, and TPP remained stable (F = 2.352, P = 0.084; F = 0.848, P = 0.474; F = 0.938, P = 0.430; F = 0.694, P = 0.560; F = 0.801, P = 0.471, respectively) after the procedure. However, as demonstrated in Table 3, the densitometry values of the anterior layer (F = 4.186, P = 0.010), the central layer (F = 6.438, P = 0.001), the posterior layer (F = 6.109, P = 0.001), and the total layer (F = 5.590, P = 0.002) over Φ 0 to 2 mm, and the values obtained at Φ 2 to 6 mm of the anterior layer (F = 3.738, P = 0.017), the central layer (F = 7.611, P < 0.001), the posterior layer (F = 6.293, P = 0.001), and the total layer (F = 5.570, P = 0.002) changed significantly after the ATE-CXL procedure. As illustrated in Figures 1A, B, a Bonferroni post hoc comparison test detected that the mean densitometry values of the anterior layer over Φ 0 to 2 mm and Φ 2 to 6 mm areas remained unchanged at the 1-month mark (Φ 0–2 mm post hoc P = 1.000; Φ 2–6 mm post hoc P = 1.000) and the 6-month mark (Φ 0–2 mm post hoc P = 0.093; Φ 2–6 mm post hoc P = 0.089), but decreased significantly after 12 months (Φ 0–2 mm post hoc P = 0.011; Φ 2–6 mm post hoc P = 0.012) when compared with the preoperative values. The mean densitometry values decreased significantly after 6 months in the central layer (Φ 0–2 mm post hoc P = 0.024; Φ 2–6 mm post hoc P = 0.020), the posterior layer (Φ 0–2 mm post hoc P = 0.025; Φ 2–6 mm post hoc P = 0.027), and the total layer (Φ 0–2 mm post hoc P = 0.035; Φ 2–6 mm post hoc P = 0.033). Those parameters, obtained 12 months postoperations, were still significantly lower than preoperative ones (central layer Φ 0–2 mm: post hoc P = 0.006; central layer Φ 2–6 mm: post hoc P = 0.016; posterior layer Φ 0–2 mm: post hoc P = 0.010; posterior layer Φ 2–6 mm: post hoc P = 0.013; the total layer Φ 0–2 mm: post hoc P = 0.007 and the total layer Φ 2–6 mm: post hoc P = 0.011).
TABLE 2.
Changes in Topographic Parameters After ATE-CXL (n = 17)
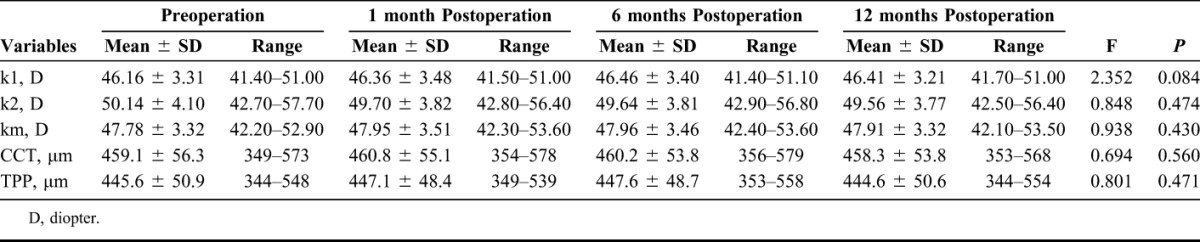
TABLE 3.
Changes in Densitometry Values of Each Position of a Cornea After ATE-CXL (n = 17)
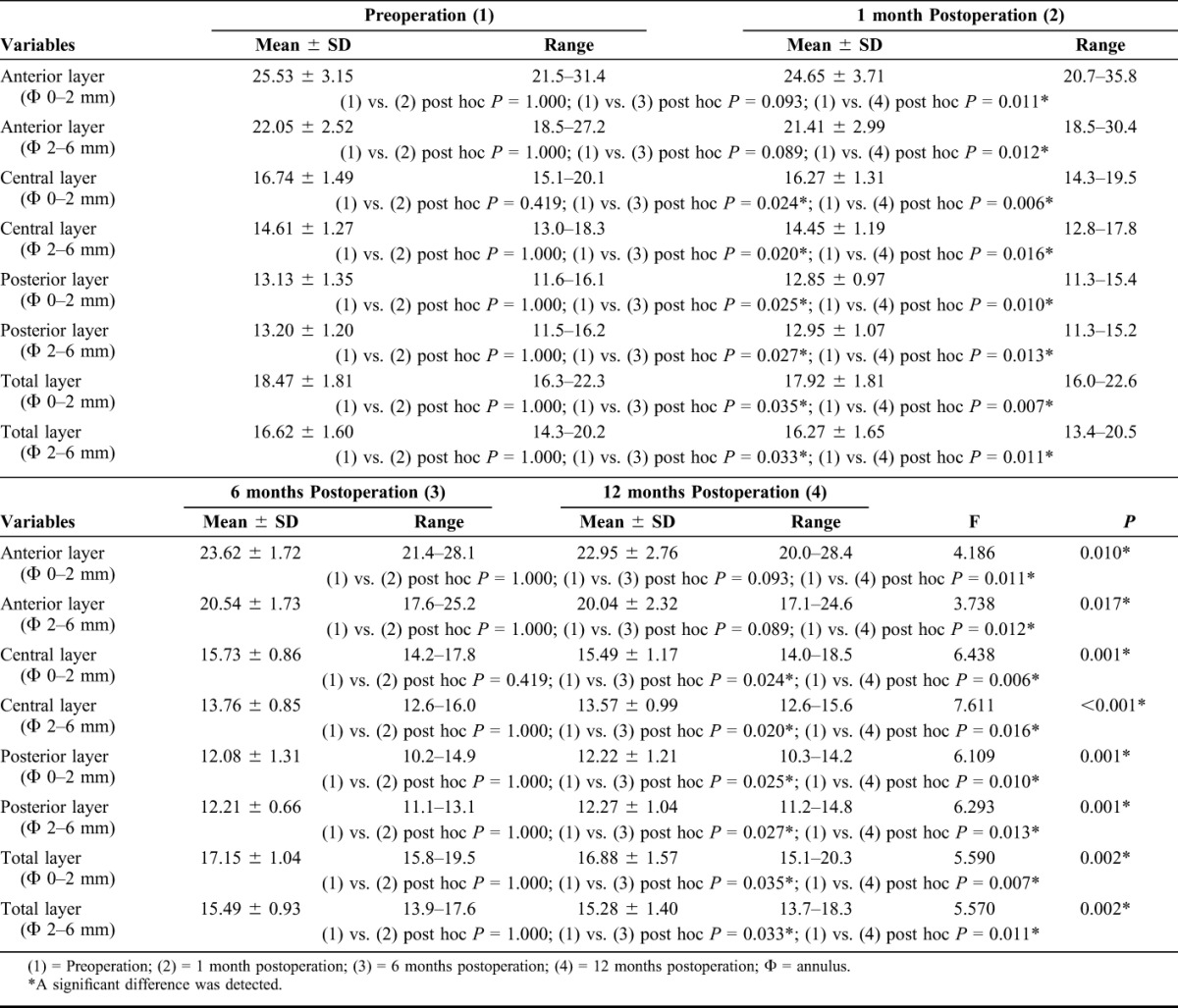
FIGURE 1.
A, The densitometry values over Φ 0 to 2 mm of the anterior layer, central layer, posterior layer, and total layer of the corneas measured before and at 1, 6, and 12 months after ATE-CXL. (The asterisk refers to a significant difference that was detected when compared with the preoperative value.) B, The densitometry values over Φ 2 to 6 mm of the anterior layer, central layer, posterior layer, and total layer of the corneas measured before and at 1, 6, and 12 months after ATE-CXL. (The asterisk refers to a significant difference that was detected when compared with the preoperative value.)
Differences in Corneal Densitometry Between Groups
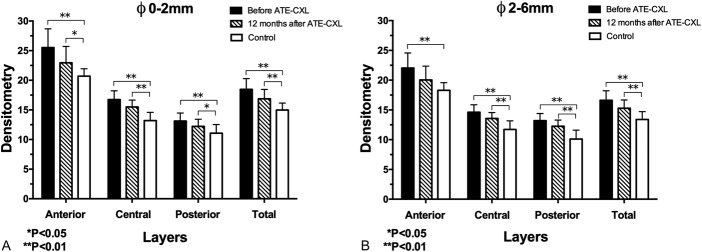
The mean densitometry values of each layer in the control group were listed in Table 4. Multifactor ANOVA and Bonferroni post hoc comparisons tests revealed that the densitometry values of the myopes were significantly lower than those of the KC patients in each location of the cornea over Φ 0 to 2 mm and Φ 2 to 6 mm (all post hoc P values were less than 0.001). Moreover, except for the densitometry value of anterior layer over Φ 2 to 6 mm (post hoc P = 0.062), the densitometry values of the anterior layer over Φ 0 to 2 mm (post hoc P = 0.036), the central layer over Φ 0 to 2 mm (post hoc P < 0.001), the central layer over Φ 2 to 6 mm (post hoc P < 0.001), the posterior layer over Φ 0 to 2 mm (post hoc P = 0.049), the posterior layer over Φ 2 to 6 mm (post hoc P < 0.001), the total layer over Φ 0 to 2 mm (post hoc P = 0.002), and the total layer over Φ 2 to 6 mm (post hoc P = 0.001) of the KC group were still significantly higher than those of the control group 12 months after the ATE-CXL procedure (Figs. 2A, B).
TABLE 4.
Corneal Densitometry Average Table (n = 34)
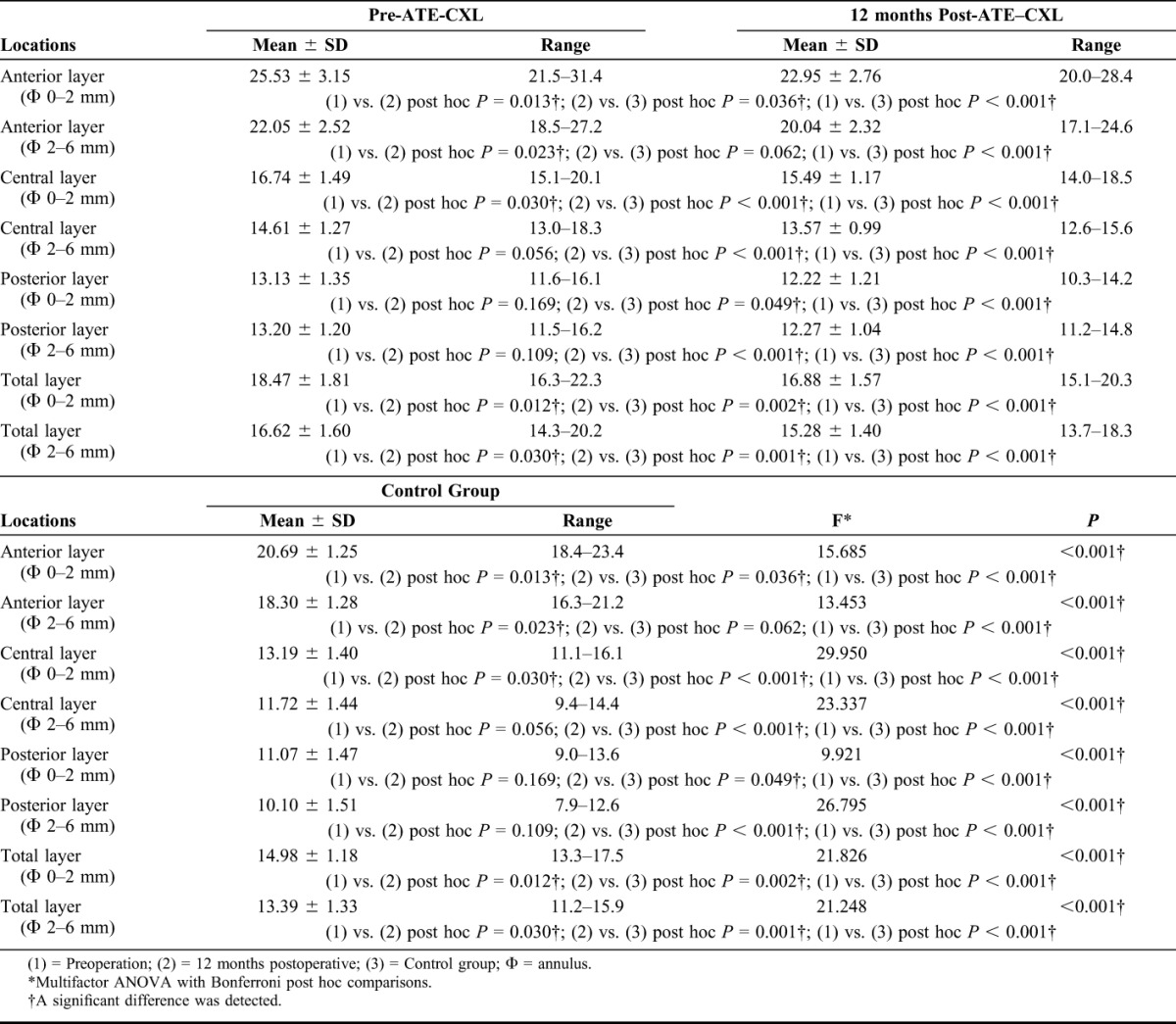
FIGURE 2.
A, The mean densitometry values over Φ 0 to 2 mm of preoperation, 12-month postoperation, and control group. B, The mean densitometry values over Φ 2 to 6 mm of preoperation, 12-month postoperation, and control group.
DISCUSSION
Disarrangement in corneal histology (such as changes in the corneal lamellar array and spacing, inflammation, haze clouding, and scarring formation), which may compromise corneal transparency, is considered as the most probable cause of corneal densitometry elevation.6,11–15 Several in vivo studies16,17 have also reported a significant negative correlation between the severity of KC and the mean epithelial cell density, and the mean anterior and posterior stromal keratocyte densities. In the present study, we found that the mean corneal densitometry values in the KC group were significantly higher than those in the control group, which is similar to the findings of Lopes et al.11 We hypothesize that the decrease in the density and quantity of epithelial cells and keratocytes in the KC corneas compromised the regularity in corneal histology, thus increasing the densitometry values of these “sick corneas.” Furthermore, we found that the mean values of MRSE, k1, k2, km, and CCT remain stable after ATE-CXL, which is consistent with the reports of Elbaz et al18 and Waszczykowska and Jurowski.19 However, the densitometry values dramatically decreased postoperation, indicating that the change in densitometry measurements may be independent of those topographic parameters. Greenstein et al's study6 has shown that densitometry values peak 1 month after the C-CXL procedure, decrease between 3 and 12 months, but do not completely return to the baseline 12 months postoperation. Alnawaiseh et al20,21 found that densitometry measurements were stable at 12 months after epithelium-off accelerated CXL, whereas decreasing below the preoperative baseline after another 12 months. We originally studied the changes in corneal densitometry after ATE-CXL and found that the mean densitometry measurements of these KC corneas (over Φ 0–6 mm areas) steadily decreased within the first year after ATE-CXL, in contrast with the results of Greenstein et al and Alnawaiseh et al. A possible explanation is that the epithelial removal and the long duration of UV-A exposure of the epithelium-off CXL technique may trigger more severe inflammatory responses and activate more keratocytes.22 Instead of debriding the epithelium, ATE-CXL uses infiltration agents to facilitate the photosensitizer saturating the entire cornea. As a result, the corneal epithelial barrier is maximally maintained and the anterior stromal tissue is minimally invaded. Moreover, MRSE and topographic parameters kept stable after ATE-CXL, whereas BSCDVA improved significantly 12 months postoperation. We believe the improvement in BSCDVA was partly attributed to the decreased corneal densitometry. Although densitometry significantly decreased after ATE-CXL, the mean values were still statistically higher than those of the myopes. This implies that although the ATE-CXL procedure may significantly ameliorate the arrangement and transparency of the corneal histology in progressive KC patients, they are still compromised when compared with the myopes without KC.
One limitation of our study is the potential bias because of the relatively small sample size in each group. However, we mainly investigated the changes in densitometry values before and after the ATE-CXL procedure. In addition, age and gender of the 2 groups were strictly paired, thus minimizing the bias. Another limitation is that we did not evaluate in vivo corneal morphology and biomechanical properties in this study. The potential relations between the altered densitometry and the pathophysiologic process or biomechanical change after ATE-CXL should be clarified in further studies.
In conclusion, the densitometry values of progressive KC patients are higher than those of the myopes. Although the densitometry values decrease significantly at 12 months postoperation, they still remain higher than those of the myopes.
Footnotes
The authors have no conflicts of interest to disclose.
Y. Shen and W. Jian contributed equally to this work.
Supported by National Natural Science Foundation of China (Grant 81570879; http://www.nsfc.gov.cn) and Outstanding Academic Leaders Program of Shanghai Health System (Grant XBR2013098; http://www.wsjsw.gov.cn).
REFERENCES
- 1.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;35:620–627. [DOI] [PubMed] [Google Scholar]
- 2.Caporossi A, Mazzotta C, Baiocchi S, et al. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–593. [DOI] [PubMed] [Google Scholar]
- 3.Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. [DOI] [PubMed] [Google Scholar]
- 4.Wollensak G, Spoerl E, Wilsch M, et al. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg. 2003;29:1786–1790. [DOI] [PubMed] [Google Scholar]
- 5.Caporossi A, Mazzotta C, Paradiso AL, et al. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 2013;39:1157–1163. [DOI] [PubMed] [Google Scholar]
- 6.Greenstein SA, Fry KL, Bhatt J, et al. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010;36:2105–2114. [DOI] [PubMed] [Google Scholar]
- 7.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35:1358–1362. [DOI] [PubMed] [Google Scholar]
- 8.Connon CJ, Marshall J, Patmore AL, et al. Persistent haze and disorganization of anterior stromal collagen appear unrelated following phototherapeutic keratectomy. J Refract Surg. 2003;19:323–332. [DOI] [PubMed] [Google Scholar]
- 9.Vinciguerra P, Randleman JB, Romano V, et al. Transepithelial iontophoresis corneal collagen cross-linking for progressive keratoconus: initial clinical outcomes. J Refract Surg. 2014;30:746–753. [DOI] [PubMed] [Google Scholar]
- 10.Chan TC, Chow VW, Jhanji V, et al. Different topographic response between mild to moderate and advanced keratoconus after accelerated collagen cross-linking. Cornea. 2015;34:922–927. [DOI] [PubMed] [Google Scholar]
- 11.Lopes B, Ramos I, Ambrósio R., Jr Corneal densitometry in keratoconus. Cornea. 2014;33:1282–1286. [DOI] [PubMed] [Google Scholar]
- 12.Ni Dhubhghaill S, Rozema JJ, Jongenelen S, et al. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Invest Ophthalmol Vis Sci. 2014;55:162–168. [DOI] [PubMed] [Google Scholar]
- 13.Elflein HM, Hofherr T, Berisha-Ramadani F, et al. Measuring corneal clouding in patients suffering from mucopolysaccharidosis with the pentacam densitometry programme. Br J Ophthalmol. 2013;97:829–833. [DOI] [PubMed] [Google Scholar]
- 14.Otri AM, Fares U, Al-Aqaba MA, et al. Corneal densitometry as an indicator of corneal health. Ophthalmology. 2012;119:501–508. [DOI] [PubMed] [Google Scholar]
- 15.Cennamo G, Forte R, Aufiero B, et al. Computerized scheimpflug densitometry as a measure of corneal optical density after excimer laser refractive surgery in myopic eyes. J Cataract Refract Surg. 2011;37:1502–1506. [DOI] [PubMed] [Google Scholar]
- 16.Bitirgen G, Ozkagnici A, Bozkurt B, et al. In vivo corneal confocal microscopic analysis in patients with keratoconus. Int J Ophthalmol. 2015;8:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozgurhan EB, Kara N, Yildirim A, et al. Evaluation of corneal microstructure in keratoconus: a confocal microscopy study. Am J Ophthalmol. 2013;156:885–893. [DOI] [PubMed] [Google Scholar]
- 18.Elbaz U, Shen C, Lichtinger A, et al. Accelerated (9-mW/cm2) corneal collagen crosslinking for keratoconus-A 1-year follow-up. Cornea. 2014;33:769–773. [DOI] [PubMed] [Google Scholar]
- 19.Waszczykowska A, Jurowski P. Two-year accelerated corneal cross-linking outcome in patients with progressive keratoconus. Biomed Res Int. 2015;2015:325157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alnawaiseh M, Rosentreter A, Böhm MR, et al. Accelerated (18 mW/cm2) corneal collagen cross-linking for progressive keratoconus. Cornea. 2015;34:1427–1431. [DOI] [PubMed] [Google Scholar]
- 21.Alnawaiseh M, Rosentreter A, Eveslage M, et al. Changes in corneal transparency after cross-linking for progressive keratoconus: long-term follow-up. J Refract Surg. 2015;31:614–618. [DOI] [PubMed] [Google Scholar]
- 22.Wollensak G, Spoerl E, Wilsch M, et al. Keratocyteapoptosis after corneal collagen cross-linking using riboflavin/UVA treatment. Cornea. 2004;23:43–49. [DOI] [PubMed] [Google Scholar]