Abstract
BACKGROUND:
Pain relief with spinal cord stimulation (SCS) has focused historically on paresthesias overlapping chronically painful areas. A higher level evidence supports the use of SCS in treating leg pain than supports back pain, as it is difficult to achieve adequate paresthesia coverage, and then pain relief, in the low back region. In comparison, 10-kHz high-frequency (HF10 therapy) SCS therapy does not rely on intraoperative paresthesia mapping and remains paresthesia-free during therapy.
OBJECTIVE:
To compare long-term results of HF10 therapy and traditional low-frequency SCS.
METHODS:
A pragmatic randomized, controlled, pivotal trial with 24-month follow-up was conducted across 11 comprehensive pain treatment centers. Subjects had Visual Analog Scale scores of ≥5.0/10.0 cm for both back and leg pain, and were assigned randomly (1:1) to receive HF10 therapy or low-frequency SCS. The primary end point was a responder rate, defined as ≥50% back pain reduction from baseline at 3 months with a secondary end point at 12 months (previously reported). In this article, 24-month secondary results are presented. Non-inferiority was first assessed, and if demonstrated the results were tested for superiority.
RESULTS:
In the study, 198 subjects were randomized (101 HF10 therapy, 97 traditional SCS). One hundred seventy-one subjects (90 HF10 therapy, 81 traditional SCS) successfully completed a short-term trial and were implanted. Subjects averaged 54.9 ± 12.9 years old, 13.6 ± 11.3 years since diagnosis, 86.6% had back surgery, 88.3% were taking opioid analgesics. At 3 months, 84.5% of implanted HF10 therapy subjects were responders for back pain and 83.1% for leg pain, and 43.8% of traditional SCS subjects were responders for back pain and 55.5% for leg pain (P < .001 for both back and leg pain comparisons, non-inferiority and superiority). At 24 months, more subjects were responders to HF10 therapy than traditional SCS (back pain: 76.5% vs 49.3%; 27.2% difference, 95% CI, 10.1%-41.8%; P < .001 for non-inferiority and superiority; leg pain: 72.9% vs 49.3%; 23.6% difference, 95% CI, 5.9%-38.6%; P < .001 for non-inferiority and P = .003 for superiority). Also at 24 months, back pain decreased to a greater degree with HF10 therapy (66.9% ± 31.8%) than traditional SCS (41.1% ± 36.8%, P < .001 for non-inferiority and superiority). Leg pain also decreased to a greater degree with HF10 therapy (65.1% ± 36.0%) than traditional SCS (46.0% ± 40.4%, P < .001 for non-inferiority and P = .002 for superiority).
CONCLUSION:
This study demonstrates long-term superiority of HF10 therapy compared with traditional SCS in treating both back and leg pain. The advantages of HF10 therapy are anticipated to impact the management of chronic pain patients substantially.
ABBREVIATIONS:
IPG, implantable pulse generator
MCID, minimal clinically important difference
PI, permanent implant
ODI, Oswestry Disability Index
SCS, spinal cord stimulation
VAS, Visual Analog Scale
KEY WORDS: Back pain, Chronic pain, Leg pain, Paresthesia, Spinal cord stimulation
Effective pain relief with spinal cord stimulation (SCS) has historically been critically dependent on overlapping stimulation-induced paresthesias with chronically painful areas. Although efficacy of SCS for the treatment of leg pain is well-studied, producing effective, durable paresthesias in the back has been elusive. Thus for >4 decades, the primary focus of innovation has been to improve the reliability of paresthesia coverage.1,2 However, recent technological innovation regarding the delivery of paresthesia-free SCS has shifted focus from optimization of paresthesias to improved patient outcomes, ie, pain relief. With European CE Mark since 2010 and US Food and Drug Administration approval in May 2015, a paresthesia-free therapy provided via high-frequency stimulation at 10-kHz (HF10 therapy) now is available to treat both back and leg pain.3-6
With the objective of providing pragmatic clinical evidence, a pivotal trial was designed to demonstrate safety, effectiveness, and clinical benefit of paresthesia-free HF10 therapy compared with traditional paresthesia-based low-frequency SCS for the treatment of chronic intractable back and leg pain.7 In doing so, this study was the first randomized, controlled, pivotal trial in SCS history to compare device systems directly and to use back pain as the primary end point.
Details of the study design, participants, interventions, and the results through 12-months have been provided previously.3 Described briefly, HF10 therapy involves application of high-frequency (10 kHz), short-duration (30 μsec), low-amplitude (1-5 mA) pulses to the T8-T11 spinal epidural space, which has been shown to produce no paresthesia. Traditional SCS typically involves application of low-frequency (40-60 Hz), longer duration (300-600 μsec), and higher amplitude (4-9 mA) pulses to generate paresthesias as determined by an intraoperative mapping procedure. At the 3-month primary end point, 84.5% of implanted HF10 therapy subjects were responders (≥50% reduction in pain from baseline) for back pain and 83.1% for leg pain. In comparison, 43.8% of traditional SCS subjects were responders for back pain and 55.5% for leg pain (P < .001 for non-inferiority and superiority for both back and leg pain comparisons). The relative ratio for responders was 1.9 (95% confidence interval 1.4-2.5) for back pain and 1.5 (95% confidence interval 1.2-1.9) for leg pain. The superiority of HF10 therapy compared with traditional SCS for leg and back pain was sustained through 12 months (P < .001). None of the HF10 therapy subjects experienced paresthesia.
Building on this evidence base, data collection from this study continued to 24 months, providing comparative long-term evidence important to healthcare providers. The aim of this study was to demonstrate the comparative sustainability of the results, which is particularly notable in that 24-month follow-up is uncommon in chronic pain studies.
METHODS
Study Design
This prospective, randomized, controlled trial was designed to assess primarily non-inferiority and secondarily superiority of HF10 therapy compared with traditional low-frequency SCS in subjects with chronic intractable back and leg pain. The study was conducted across 11 comprehensive pain treatment centers in the United States in compliance with the US Code of Federal Regulations and recommendations guiding physicians in biomedical research by the 18th World Medical Assembly, Helsinki, Finland. The study protocol and informed consent forms were approved by the Institutional Review Board of each study site (Western Institutional Review Board, Puyallup, WA; Forsyth Medical Center IRB, Winston-Salem, NC). The study was designed initially to assess safety and effectiveness during a 12-month follow-up period. Data collection subsequently was extended for an additional year.
Participants
Consenting patients under the care of the study investigators were assessed for eligibility based on inclusion and exclusion criteria. Key inclusion criteria were (1) chronic intractable pain of the trunk and/or limbs, refractory to conservative therapy for a minimum of 3 months (previous conservative treatments included pain medications, physical therapy, spinal injections, pharmacological, and behavioral treatment); (2) average back pain intensity of ≥5.0 out of 10.0 cm on the Visual Analog Scale (VAS); (3) average leg pain intensity of ≥5.0 out of 10.0 cm on the VAS; (4) an Oswestry Disability Index version 2.1a (ODI) score of 41 to 80 out of 1008; and (5) an appropriate candidate for the surgical procedures required in this study. Prior surgical intervention for low back pain was not a study eligibility requirement. Key exclusion criteria were (1) an active disruptive psychological or psychiatric disorder or other known condition significant enough to impact perception of pain, ability to comply with the intervention, or evaluation of treatment outcomes; (2) mechanical spine instability based on flexion/extension films of the lumbar spine; or (3) prior experience with SCS. Near the 12-month follow-up visit, included subjects were asked to re-consent to extended data collection up to 24 months.
Randomization and Masking
Subjects were randomized 1:1 to receive stimulation with either an HF10 therapy system (Senza system; Nevro Corp., Redwood City, California) or a commercially available SCS system (Precision Plus system; Boston Scientific, Natick, Massachusetts). Both SCS systems consisted of two 8-contact leads and a rechargeable implantable pulse generator (IPG). Randomization was stratified by sex and primary area of pain (either back or leg), and administered centrally with each study site assigned randomly chosen alternating blocks of sizes 2, 4, and 6 with frequencies 0.25, 0.50, and 0.25, respectively, as generated by an independent statistician. Consecutive subjects within each site-specific strata block then were assigned sequentially to a treatment group, thus preserving the blinding of the study sites to upcoming treatment group allocations. Study sites were notified by e-mail of each random assignment only after the completion of all baseline assessments. Due to practical considerations (see Discussion section), study subjects and investigators were not masked to the assigned treatment group.
Procedures
Consistent with standard clinical practice, subjects underwent a screening trial of SCS lasting up to 14 days with an external stimulator to determine short-term response. During the trial period, pain was assessed per standard clinical practice. End of trial back and leg pain scores were documented using VAS scales. For traditional SCS subjects, stimulation parameters (pulse frequency, amplitude, and duration; active stimulation contacts) were adjusted optimally to overlap paresthesia with the regions of back and leg pain in the subjects. Paresthesia testing and associated device programming were performed intraoperatively for traditional SCS subjects, then as needed based on subject feedback at standard clinic visits. Subjects with HF10 therapy received 10 000 Hz, 30 μsec stimulation with amplitude and stimulation location adjusted to obtain optimal analgesic response. Because HF10 therapy is paresthesia-free, intraoperative programming was not performed for these subjects. Programming occurred postoperatively and as needed based on subject feedback at standard clinic visits. Programming for each treatment group was provided with the assistance of the respective manufacturer under the guidance of the investigators.
Oral analgesics were stabilized from 28 days prior to enrollment until activation of the implanted SCS system, allowing for perioperative analgesics. Adjustments were allowed subsequently under the guidance of a study investigator as medically necessary, but the study protocol instructed not to exceed baseline levels.
The same type of lead was used for both SCS systems. Two percutaneous leads were placed in the posterior spinal epidural space under radiographic imaging and attached to either an external stimulator (during the short-term screening trial) or a subcutaneously implanted IPG. For HF10 therapy, the distal tip of one lead was placed at T8 while a second lead tip was placed at T9, both near anatomical midline. Lead placement for HF10 therapy did not entail confirmation that they were positioned at physiological midline. Lead position for HF10 therapy was based on extensive empirical observation that most patients respond to stimulation application near T9/10, while allowing for patient variation by covering T8-T11.4-6 For traditional low-frequency SCS, leads were placed at vertebral levels based on intraoperative paresthesia mapping involving patient feedback, typically resulting in parallel lead tip placement at T7-T8.
A subcutaneous pocket was created using standard surgical technique for placement of the IPG. The leads, anchored conventionally with a manufacturer-supplied anchor, were tunneled to the pocket site and connected to the IPG. Intraoperative impedance testing ensured electrical integrity.
Outcomes
The primary outcome of the study was a composite of safety and efficacy: the percentage of subjects who responded to SCS therapy for back pain (≥50% reduction in VAS score) without a stimulation-related neurological deficit. Secondary outcomes included the percentage of subjects who responded for leg pain, the percent pain relief for back and leg pain, and the disability level over the follow-up period.
Standardized outcome measures were assessed at predefined study visits (baseline; 3, 6, 12, 18, and 24 months), including VAS for back and leg pain, ODI, patient and clinician global impression of change, and subject satisfaction. The primary end point was at 3 months, with a secondary end point at 12 months (previously published).3 In this article, 24-month secondary results are presented. In addition to adverse event reporting, a standardized neurological assessment (including motor, sensory, and reflex functions) was performed at each of the scheduled visits. Self-reported outcomes were recorded by subjects on case report forms while isolated from research staff and company representatives.
Statistical Analysis
Primary end point analyses were performed on intention-to-treat (ITT, subjects receiving a randomization assignment), per protocol (PP, subjects completing a primary end point assessment), and permanent implant (PI, subjects passing a short-term screening trial and receiving a permanent SCS system) populations. For subjects who had a successful screening trial and received an IPG implant, the primary efficacy assessment occurred at 3 months post device activation. Subjects who did not have a successful trial phase were considered non-responders for the ITT and PP analyses, and excluded from the PI analysis. As this report focuses on the secondary results at 24 months, the PI analyses are reported.
Sample size for efficacy was based on a non-inferiority comparison of the primary end point between treatment groups. Using an exact binomial test for non-inferiority of HF10 therapy to traditional SCS with a 10% non-inferiority margin, 80% statistical power, 0.05 1-sided significance level, and an estimated success rate of 58% for the test group and 48% for the control group,9 a minimum of 77 randomized subjects per treatment group were required. If non-inferiority was statistically demonstrated, then the results were tested for superiority with 2-sided significance.
Non-inferiority is established if the lower bound of the 95% confidence interval of the difference between the primary outcomes of treatment groups (HF10 therapy—traditional SCS) is greater than −10%. If HF10 therapy is worse (inferior) than traditional SCS by 10%, then there would be <2.5% probability of observing non-inferiority by chance alone. If non-inferiority is established, then superiority may be established if the lower bound of the 95% confidence interval of the difference between the primary outcomes of treatment groups is greater than zero. If HF10 therapy is equal to traditional SCS, then there would be >2.5% probability of observing superiority by chance alone.
In addition to classifying subjects as responders (≥50% decrease in pain from baseline) or non-responders, subjects were post-hoc classified as remitters or non-remitters. By expert consensus prior to the availability of results, we defined a pain remitter as having a VAS pain score of ≤2.5 (irrespective of what treatments or medications are utilized).3
Secondary end points were evaluated successively for tests of non-inferiority (hierarchical closed test approach) with 10% non-inferiority margins and 1-sided .05 significance levels until statistical significance was not achieved. For each end point tested, if non-inferiority was demonstrated then superiority was assessed subsequently post hoc with a 2-sided .05 significance level. Secondary end points included percentage changes from baseline in back pain, leg pain, and ODI. Proportions were compared between treatment groups using Fisher exact test with a 2-sided α level of 5%. Longitudinal results were assessed using repeated measures analysis of variance. Mean results were compared between treatment groups using t tests. Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
The study was registered on ClinicalTrials.gov, identifier NCT01609972. Study execution was overseen by an independent Data and Safety Monitoring Board, comprising an anesthesiologist, a neurologist, a neurosurgeon, and a biostatistician.
RESULTS
From June 7, 2012 to December 28, 2012, 241 patients were enrolled and assessed for eligibility with 198 subjects proceeding through baseline evaluations and randomized to a treatment group (101 HF10 therapy, 97 traditional SCS; Figure 1). Of these, 171 subjects had a successful short-term screening trial and were implanted with an SCS system (90 HF10 therapy, 81 traditional SCS). Data were obtained on a high percentage of subjects through 24 months (85 [94.4%] implanted HF10 therapy subjects, 71 [87.7%] implanted traditional subjects, P = .12).
FIGURE 1.
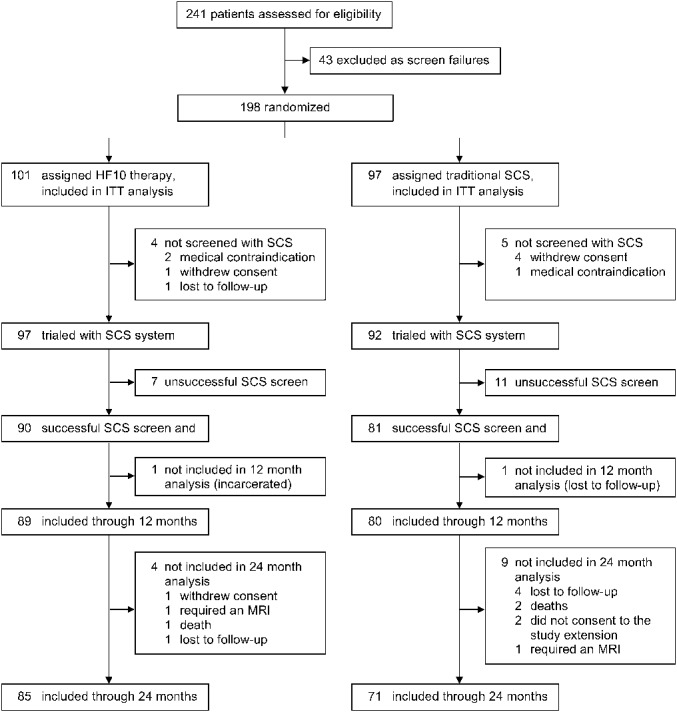
Subject flow diagram. HF10 therapy, 10-kHz high-frequency therapy; ITT, intention-to-treat; MRI, magnetic resonance imaging; SCS, spinal cord stimulation.
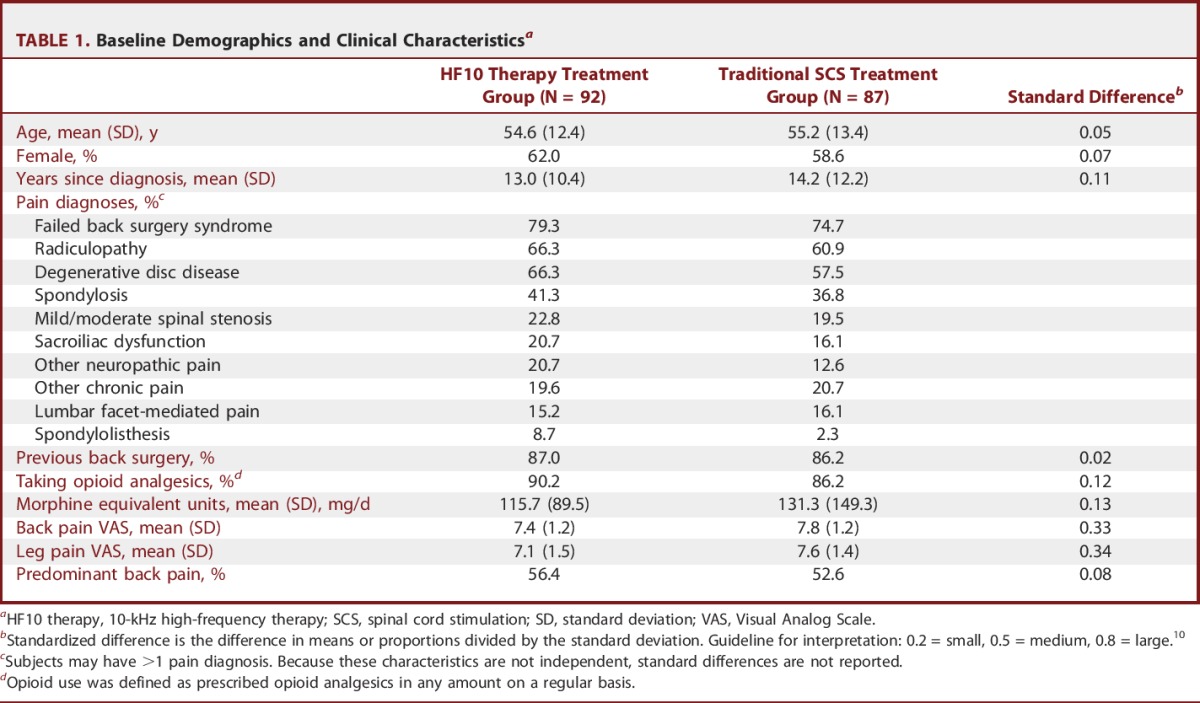
Randomized subjects averaged 54.9 ± 12.9 years of age, 13.6 ± 11.3 years since diagnosis, and 88.3% were taking opioid analgesics with an average morphine-equivalent dose of 118.9 ± 119.3 mg/d (Table 1). At baseline, 86.6% of subjects had previous back surgery, with 77.1% diagnosed by a study investigator as having “failed back surgery syndrome.” Other frequent pain diagnoses were radiculopathy in 63.7% of subjects and degenerative disk disease in 62.2% of subjects. Mean baseline back pain VAS was 7.6 ± 1.2 cm, while mean baseline leg pain was 7.3 ± 1.4 cm. Back pain was the predominant symptom in 54.6% of subjects.
TABLE 1.
Baseline Demographics and Clinical Characteristicsa
For the primary outcome measure, more subjects were responders to HF10 therapy than traditional SCS at 24 months for both back pain (76.5% vs 49.3%; 27.2% difference, 95% CI, 10.1%-41.8%; P < .001 for non-inferiority and superiority) and leg pain (72.9% vs 49.3%; 23.6% difference, 95% CI, 5.9%-38.6%; P < .001 for non-inferiority and P = .003 for superiority; Figure 2). As non-inferiority was established, superiority analyses were justified.
FIGURE 2.
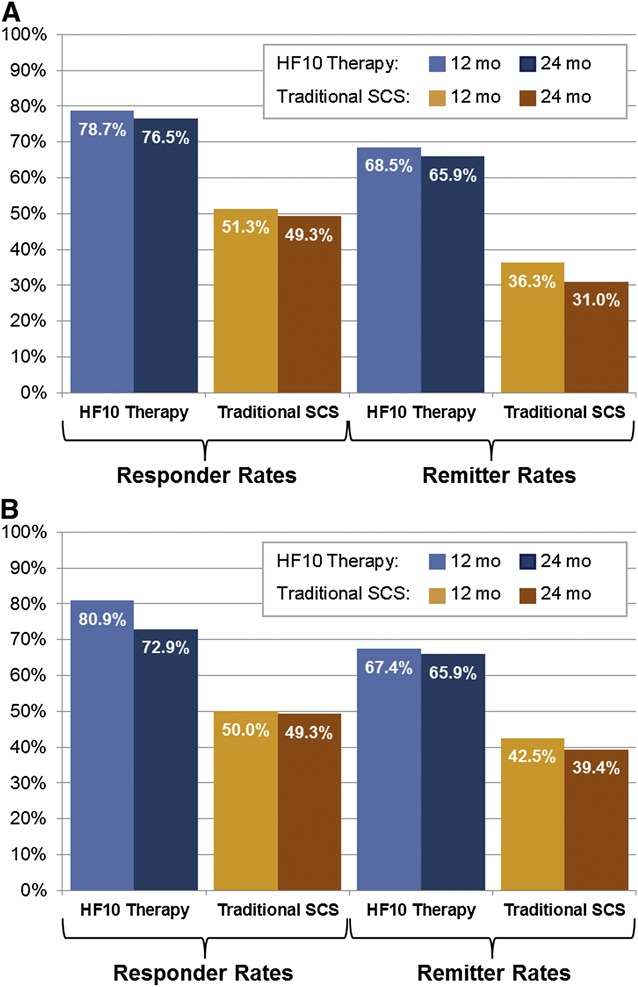
Responder (≥50% decrease in pain score from baseline) and remitter (pain score of ≤2.5) rates at 24 months for (A) back pain and (B) leg pain. HF10 therapy, 10-kHz high-frequency therapy; SCS, spinal cord stimulation.
HF10 therapy showed a similar advantage in terms of remission rates at 24 months (back pain: 65.9% vs 31.0%; 34.9% difference, 95% CI, 18.0%-49.0%, P < .001 for non-inferiority and P = .003 for superiority. Leg pain: 65.9% vs 39.4%; 26.5% difference, 95% CI, 8.0%-41.2%; P < .001 for non-inferiority and P = .001 for superiority). For subjects who achieved remission (including both HF10 therapy and traditional SCS subjects), back pain averaged 1.1 ± 0.7 cm and leg pain averaged 0.9 ± 0.7 cm.
At 24 months, back pain decreased to a greater degree for HF10 therapy subjects (point decrease: 5.0 ± 2.5 cm; percent decrease: 66.9% ± 31.8%) than traditional SCS subjects (3.2 ± 3.0 cm, P < .001 for non-inferiority and superiority; 41.1% ± 36.8%, P < .001 for non-inferiority and superiority; Table 2, Figure 3). Similarly, leg pain decreased to a greater degree for HF10 therapy subjects (4.7 ± 2.8 cm; or pain scores decrease by 65.1% ± 36.0%) than traditional SCS subjects (3.7 ± 3.0 cm, P < .001 for non-inferiority and P = .03 for superiority; 46.0% ± 40.4%, P < .001 for non-inferiority and P = .002 for superiority).
TABLE 2.
Back and Leg Paina,b
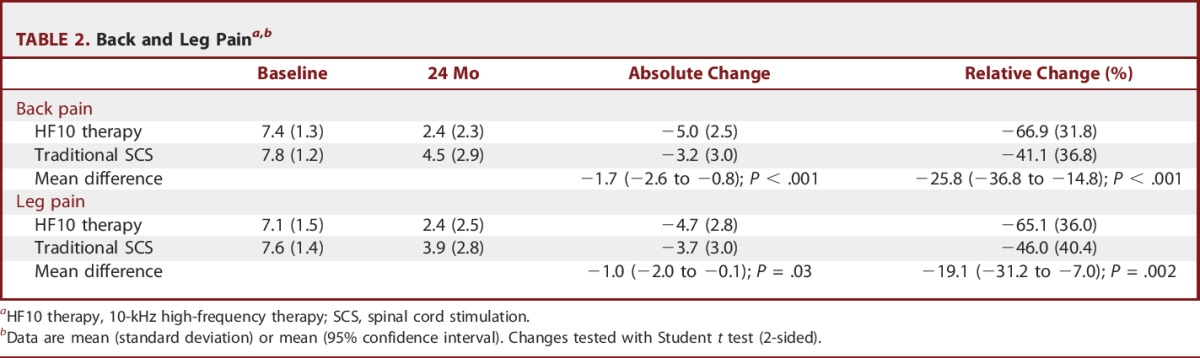
FIGURE 3.
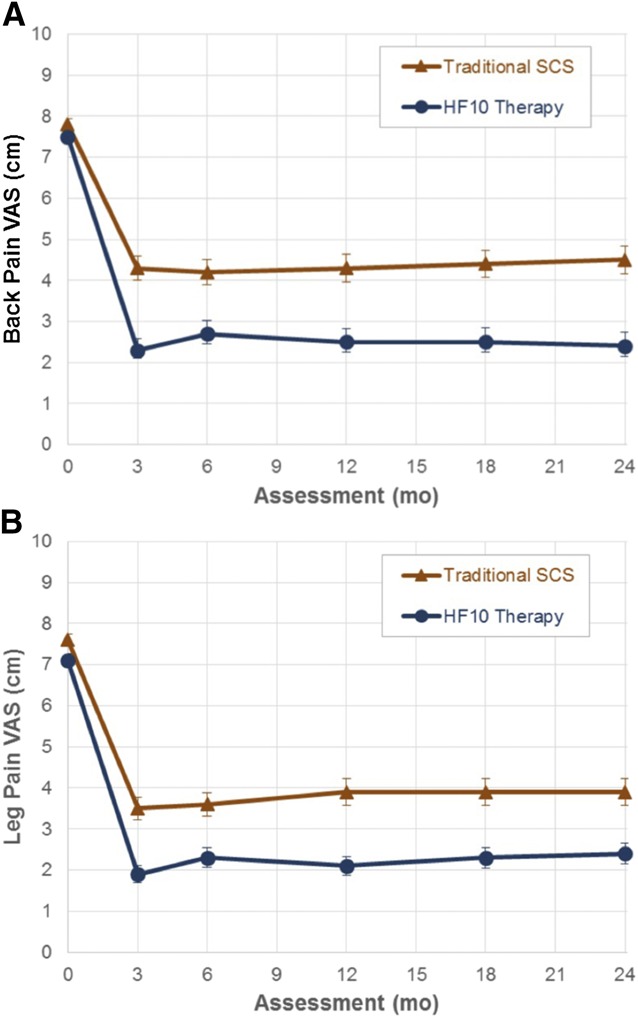
Longitudinal mean pain scores for (A) back pain and (B) leg pain. HF10 therapy, 10-kHz high-frequency; SCS, spinal cord stimulation; VAS, Visual Analog Scale.
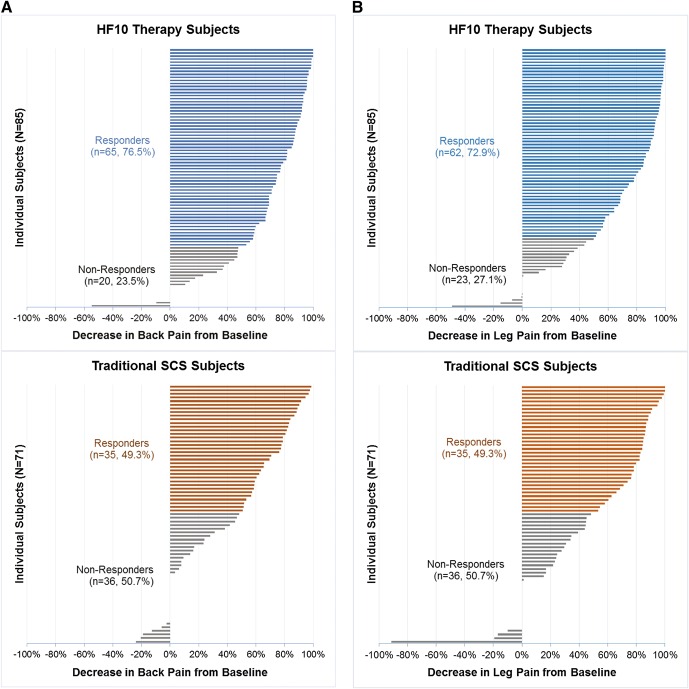
Individual subject responses at 24 months for back and leg pain are shown by treatment group in Figure 4. These charts present the percent decrease in back and leg pain for each study subject, indicating whether each subject was a responder or not.
FIGURE 4.
Individual subject responses at 24 months for (A) back pain and (B) leg pain. Each horizontal line represents the response of a study subject. Responders (colored horizontal lines) are distinguished from non-responders (gray horizontal lines). HF10 therapy, 10-kHz high-frequency therapy; SCS, spinal cord stimulation.
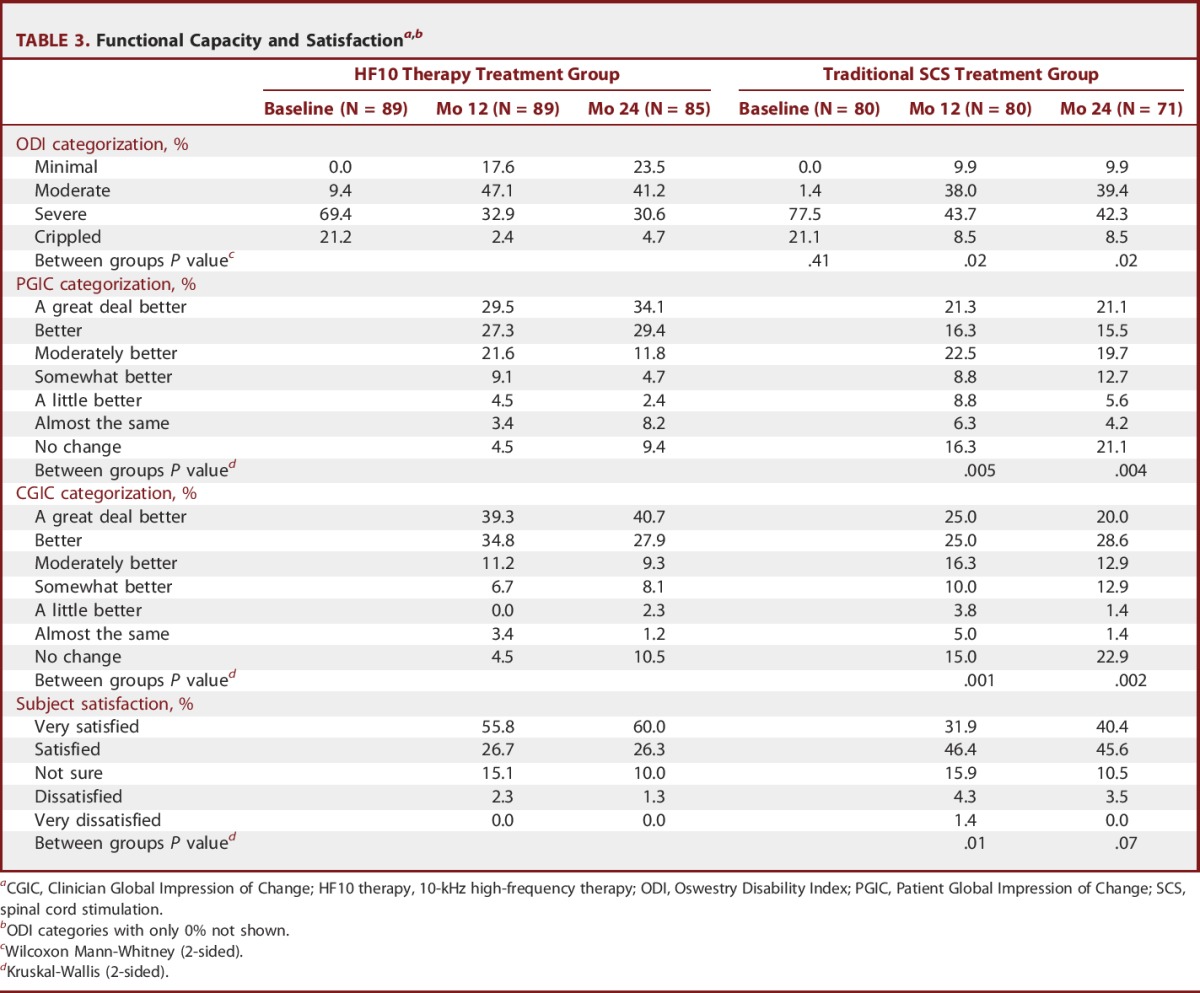
Also at 24 months, HF10 therapy subjects had a favorable distribution of ODI categorizations compared with traditional SCS subjects (P = .02, Table 3), with 23.5% of subjects receiving HF10 therapy having minimal disability compared with 9.9% of subjects receiving traditional SCS. Patient and clinician global impression of change and subject satisfaction distributions also reflect the benefits of HF10 therapy compared with traditional SCS. Particularly notable, 60.0% of subjects were very satisfied with HF10 therapy compared with 40.4% of subjects with traditional SCS.
TABLE 3.
Functional Capacity and Satisfactiona,b
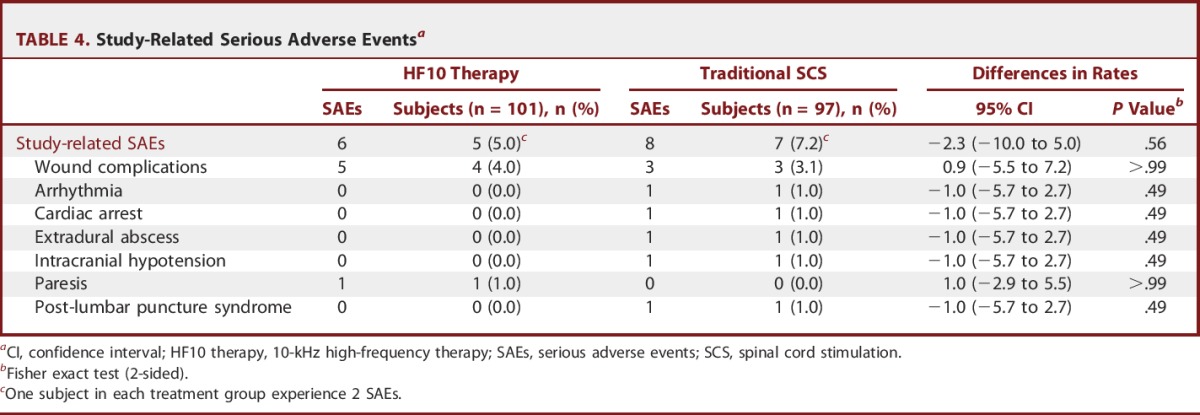
There were few study-related serious adverse events in each treatment group at 24 months, with similar rates of occurrence (5.0% for HF10 therapy, 7.2% for traditional SCS, P = .56, Table 4). Importantly, there were no stimulation-related serious adverse events or neurological deficits in either treatment group. Regarding adverse events that did not reach the level of serious, the most common were implant site pain (in 12.9% of HF10 therapy subjects and 13.4% of traditional SCS subjects, P = .91) and uncomfortable paresthesias (in 0.0% of HF10 therapy subjects and 11.3% of traditional SCS subjects, P < .001). Lead migration resulting in surgical revision occurred in 3.0% of HF10 therapy subjects and 5.2% of traditional SCS subjects (P = .49).
TABLE 4.
Study-Related Serious Adverse Eventsa
DISCUSSION
This study demonstrates not only the non-inferiority, but also the long-term superiority of HF10 therapy compared with traditional low-frequency SCS in treating both back and leg pain at 24 months. Extending comparative safety and efficacy outcomes from 12 to 24 months provides physicians, patients, and payers with rigorous evidence demonstrating the durability of SCS in treating chronic pain. These results are particularly impressive given the degree of pain chronicity and refractoriness of the study subjects.
Randomized controlled trials with substantial follow-up in the SCS field are few. Two previous landmark studies in SCS evaluated subjects with predominant leg pain. In a 2005 study, traditional low-frequency paresthesia-based SCS was compared with reoperation, wherein SCS was found to be more effective in treating persistent radicular pain after lumbosacral spine surgery and often obviated the need for reoperation.11 In a 2008 study, traditional SCS was compared with conventional medical management, wherein more subjects randomized to SCS had significant reduction in leg pain.12 The results of our study confirm the durability of SCS in treating leg pain and also demonstrate long-term effectiveness in treating back pain.
The minimal clinically important difference (MCID) in reducing chronic pain has been shown to be 2 numeric rating scale points (out of a scale maximum of 10 points) or 30%.13 Putting our study results into context, subjects treated with traditional SCS achieved a 3.2 point average decrease in back pain (which is 1.6 times the 2 point MCID) and a 41.4% average decrease in back pain (which is 1.4 times the 30% MCID), a result noteworthy in and of itself. However, subjects treated with HF10 therapy achieved a 5.0 point average decrease in back pain (which is 2.5 times the 2 point MCID) and a 66.9% average decrease in back pain (which is 2.2 times the 30% MCID). Similar ratios were observed for leg pain reduction (traditional SCS: 1.9 and 1.5, respectively; HF10 therapy: 2.2 and 2.2, respectively). Thus, both therapies achieved pain reductions that were beyond the MCID, yet HF10 therapy achieved more than twice the MCID for both back and leg pain reduction.
Lack of paresthesias also might be a factor influencing long-term pain control and improvement in function in subjects who received HF10 therapy. Previous studies have suggested that the presence of mildly to intensely uncomfortable paresthesias, including uncomfortable stimulation surges due to patient movements or repetitive daily activities, influence the compliance with the use of traditional SCS.1,3 Because HF10 therapy is paresthesia-free, such issues completely are absent and therefore do not impact patients' compliance with therapy use.
Summary statistics are important when comparing the results of 2 treatment groups in clinical trials, and are indeed the proper way to convey results in the context of evidence based medicine. However in clinical practice, individual patient results matter. Figure 4 provides the results of each subject implanted with an SCS system in our clinical trial. Doing so allows physicians and their patients to understand more directly the potential outcomes of the selected therapy when treating chronic back and leg pain.
Strengths
Strengths of the study include the study design (sufficiently powered multicenter randomized controlled trial comparing 2 active treatments), degree of oversight (US Food and Drug Administration, independent data, and safety monitoring board), and duration of follow-up (24 months) with high subject retention (94.4% of HF10 therapy subjects and 87.7% of traditional SCS subjects).
Limitations
As with any clinical trial, there are limitations. Study investigators and subjects were not masked to the assigned treatment group. Subject masking was impractical because low-frequency SCS produces paresthesias, whereas high-frequency SCS does not; thus, the therapies themselves become immediately known to the subjects. Due to the differences in stimulator lead placement, intraoperative testing, and device programming between the treatment groups, the study investigators could not be masked. The effect of the lack of masking in this randomized study is not known; nonetheless, the protocol was based on best practices guidance for comparative efficacy trial designs.14-16
Although the study protocol defined specific inclusion and exclusion criteria, heterogeneity in pain diagnoses was observed, with many subjects having multiple diagnoses (Table 1). This etiological heterogeneity reflects the diversity of patients seen when managing chronic back and leg pain, and is therefore a clinically relevant population to evaluate, especially given the pragmatic nature of this study.
Future Directions
Future research regarding HF10 therapy is expected to focus on other chronic pain indications such as arm and neck pain, relative cost-effectiveness,17 as well as a better understanding of the mechanism of action. Clinical application of SCS initially was inspired by the Gate Control Theory of pain, which suggested that increased activity of large innocuous afferents presynaptically inhibit input to pain-transmitting projection neurons via inhibitory interneurons, as well as trigger supraspinal circuits that also modify spinal pain processing.18,19 That core concept remains, but the detailed mechanisms of action of traditional SCS (ie, paresthesia-based) still are not understood completely.20 In recent years, preclinical work investigating traditional SCS have suggested new neural interactions (eg, postsynaptic modulation), as well as key neurotransmitters and neuropeptides that may be involved in pain relief from stimulation.21-25 Indeed, attempts to model and understand these circuits beyond the original Gate Control Theory still are emerging.26,27 Given the gross clinical similarity of application, HF10 therapy also may work with these same neural structures, yet may modulate them in a different manner (eg, desynchronization) in such a way that yields a more robust clinical outcome.28 Additional research is needed to detail the direct spinal effects of HF10 therapy.
CONCLUSION
The advantages of HF10 therapy are anticipated to impact the management of patients with chronic back and leg pain substantially, and possibly other pain conditions. The superior and durable results demonstrated in this study are anticipated to lead to improved long-term cost effectiveness and payer acceptance, making this therapy broadly available to patients suffering from chronic pain.
Disclosures
All authors have completed the International Committee of Medical Journal Editors Form for Disclosure of Potential Conflicts of Interest. Dr Leonardo Kapural reported having received grants from Boston Scientific (Marlborough, Massachusetts) and personal fees from Medtronic (Minneapolis, Minnesota) and St. Jude Medical (St. Paul, Minnesota). Dr Cong Yu reported having received personal fees from Boston Scientific, Medtronic, and St. Jude Medical. Bradford E. Gliner reported having received personal fees from Nevro Corp. (Menlo Park, California). Dr Ricardo Vallejo reported having received grants from Nevro Corp. and Boston Scientific and personal fees from Nevro Corp. and Boston Scientific. Dr Kasra Amirdelfan reported having received personal fees from Nevro Corp., Medtronic, and St. Jude Medical. Dr Richard Bundschu reported having received grants and personal fees from Boston Scientific. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
We thank the SENZA-RCT Investigators and the study Data and Safety Monitoring Board for their valuable contribution.
Footnotes
Funding for this study was provided by Nevro Corp.
COMMENTS
Spinal cord stimulation (SCS) has been used as a modality to treat chronic pain since 1967 when Dr Shealy implanted the first spinal cord stimulator in a terminally ill patient with bronchogenic carcinoma and right lower chest pain.1 Since then there have been advances in lead and battery design, understanding bioelectrical mechanisms, and stimulation methodology. This multicenter, randomized, controlled trial compares the efficacy of high-frequency SCS with traditional SCS for the treatment of intractable leg and back pain. The authors found that both back and leg pain decreased to a greater degree for 10-kHz high-frequency (HF10) therapy patients (66.9% and 65.1%, respectively) at 2 years. Additionally, the streamlined implantation and programming of HF10 systems offers significant benefit to the clinician and is exciting from a workflow perspective. A major limitation to this study is the lack of blinding. Although the improvement in visual analog scale was statistically significant, patients knew which stimulator (newer vs older technology) they received, inherently biasing their perceptions. Furthermore, the traditional stimulation that was used was not the latest generation of SCS offered. That said, the current study makes a meaningful contribution to the pain literature by demonstrating how far SCS technology has come since the PROCESS trial when 48% of patients achieved >50% leg pain, but not back pain relief, at similar follow-up.2 The efficacy of SCS for predominant back pain with latest-generation technology changes our patient selection criteria and expands the pool of patients that may benefit from neuromodulation. Most interesting, scientifically, is that HF10 dispels previous beliefs on SCS mechanism of action, namely that paresthesias are needed. Now that we know this is not the case, we must reframe our understanding of how SCS works. The next step clinically to improve our understanding of HF10 and other new technologies is to determine which patients benefit from which parameters both through subjective and objective outcomes.3
Sebastian Rubino
Julie G. Pilitsis
Albany, New York
- 1.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: a preliminary clinical report. Anesth Analg. 1967;46(4):489-491. [PubMed] [Google Scholar]
- 2.Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicenter randomized controlled trial on patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. [DOI] [PubMed] [Google Scholar]
- 3.Holsheimer J, Buitenweg JR. Review: bioelectrical mechanisms in spinal cord stimulation. Neuromodulation. 2015;18(3):161-170. [DOI] [PubMed] [Google Scholar]
The authors present 24-month data from an industry-sponsored randomized trial, comparing traditional, low-frequency, paresthesia-producing spinal cord stimulation (SCS) to a new non-paresthesia-generating SCS system delivering 10-kHz high-frequency (HF10) stimulation. At 24-month follow-up, more subjects were responsive to HF10 therapy than standard SCS and had a greater degree of pain relief both for back and leg pain. Although traditional SCS has a long track record and a number of well-designed randomized trials to its name, for a variety of reasons it remains stigmatized by many in the medical community. Thus, this study demonstrating superior outcomes to traditional SCS is a welcome addition to the field.
A number of concerns:
The authors introduce the term “remitters,” defined as those with a VAS score of <2.5 after treatment. I would recommend using this term in a prudent and cautious fashion. As the authors note, this does not take into account any changes in pharmacologic therapy. Moreover, the field of chronic pain is littered with the carcasses of interventions initially thought to be near-panaceas that were later found to be, at the very best, effective for a select group of patients, and, at worst, marginally effective or ineffective in the long-term. As traditional SCS has a much longer track record than HF10 therapy, and as the mechanism of action of HF10 therapy is unknown, the longevity of efficacy of this therapy remains unclear.
The authors mention that the paresthesias produced by “traditional” SCS can influence compliance with therapy usage. I have rarely found this to be a significant problem—indeed, for some patient groups (ie, the elderly), absence of paresthesias may be deleterious to ensuring compliance. I would suggest that more emphasis be placed on the superior outcomes of this therapy vs traditional SCS as opposed to the perceived benefits of paresthesia-free stimulation.
Traditionally, when SCS has been effective in the treatment of back pain, it has been for the constant, “neuropathic” component of the pain as opposed to a more mechanical-type, nociceptive pain, frequently experienced even in the absence of instability. Although the authors specifically excluded patients with instability on dynamic spine x-rays, I would warn against using SCS of any type to treat primary axial back as opposed to radicular pain, or mixed pain with a predominant radicular component.
A concern particularly relevant to the neurosurgical community is the lack of availability of a surgical, ie, paddle-type lead. The majority of SCS systems placed by neurosurgeons (as opposed to non-surgical specialists such as anesthesiologists) are paddle leads, as most neurosurgeons are not trained in or comfortable with percutaneous lead placement. Furthermore, in a subset of patients where percutaneous access cannot be obtained (ie, extensive spinal fusions), a paddle electrode is the only option. Given these initial promising results, I would imagine that such a lead will be available in the near future.
It is likely that the future of SCS therapy will not be a “one-size-fits-all” approach—traditional tonic paresthesia-producing stimulation, high-frequency stimulation, “burst” stimulation, and newer targets such as the dorsal root ganglion may, individually or in combination, benefit different subclasses of patient, and even the same patient at different times. At the present time, we have no data to guide our choice of therapy in a particular patient, and I am pessimistic that high-quality data will be available in the near future. Thus, devices that have the ability to provide all of these therapies likely will capture the majority of the market share.
Alon Y. Mogilner
New York, New York
REFERENCES
- 1.Schultz D, Webster L, Kosek P, Dar U, Tan Y, Sun M. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician. 2012;15(1):1-12. [PubMed] [Google Scholar]
- 2.Parker JL, Karantonis DM, Single PS, Obradovic M, Cousins MJ. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain. 2012;153(3):593-601. [DOI] [PubMed] [Google Scholar]
- 3.Kapural L, Doust MW, Gliner BE, et al. Novel 10 kHz high frequency therapy (HF10 therapy) is superior to traditional low frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851-860. [DOI] [PubMed] [Google Scholar]
- 4.Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter european clinical study. Neuromodulation. 2013;16(1):59-66. [DOI] [PubMed] [Google Scholar]
- 6.Tiede J, Brown L, Gekht G, Vallejo R, Yearwood T, Morgan D. Novel spinal cord stimulation parameters in patients with predominant back pain. Neuromodulation. 2013;16(4):370-375. [DOI] [PubMed] [Google Scholar]
- 7.Design Considerations for Pivotal Clinical Investigations for Medical Devices: Guidance for Industry, Clinical Investigators, Institutional Review Boards, and Food and Drug Administration Staff. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration; 2013. UCM373766. [Google Scholar]
- 8.Fairbank JC, Couper J, Davies JB. The Oswestry low back pain questionnaire. Physiotherapy. 1980;66(8):271-273. [PubMed] [Google Scholar]
- 9.Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery. 1995;37(6):1088-1095. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. The t test for means. In: Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 11.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-106. [DOI] [PubMed] [Google Scholar]
- 12.Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness spinal cord stimulation. Neurosurgery. 2008;63(4):762-770. [DOI] [PubMed] [Google Scholar]
- 13.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158. [DOI] [PubMed] [Google Scholar]
- 14.Stanley K. Design of randomized controlled trials. Circulation. 2007;115(9):1164-1169. [DOI] [PubMed] [Google Scholar]
- 15.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010. JAMA. 2012;308(24):2594-2604. [DOI] [PubMed] [Google Scholar]
- 16.Guidance for Industry: Non-inferiority Clinical Trials. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration; 2010. UCM202140. [Google Scholar]
- 17.Annemans L, Van Buyten JP, Smith T, Al-Kaisy A. Cost effectiveness of a novel 10 kHz high-frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). J Long Term Effect Med Implants. 2014;24(2-3):173-183. [DOI] [PubMed] [Google Scholar]
- 18.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971-978. [DOI] [PubMed] [Google Scholar]
- 19.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anes Anal. 1967;46(4):489-491. [PubMed] [Google Scholar]
- 20.Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155(2):210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linderoth B, Bertil G, Johan F, Ernst B. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31(2):289-297. [DOI] [PubMed] [Google Scholar]
- 22.Cui JG, Meyerson BA, Sollevi A, Linderoth B. Effect of spinal cord stimulation on tactile hypersensitivity in mononeuropathic rats is potentiated by simultaneous GABAB and adenosine receptor activation. Neurosci Lett. 1998;247(2-3):183-186. [DOI] [PubMed] [Google Scholar]
- 23.Schechtmann G, Song Z, Meyerson BA, Linderoth B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;139(1):136-145. [DOI] [PubMed] [Google Scholar]
- 24.Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011;152(7):1666-1673. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, Zhang C, Xu Q, et al. Electrical stimulation of dorsal root entry zone attenuates wide-dynamic-range neuronal activity in rats. Neuromodulation. 2015;18(1):33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider SP. Local circuit connections between hamster laminae III and IV dorsal horn neurons. J Neurophysiol. 2008;99(3):1306-1318. [DOI] [PubMed] [Google Scholar]
- 27.Zhang TC, Janik JJ, Grill WM. Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res. 2014;1569:19-31. [DOI] [PubMed] [Google Scholar]
- 28.Litvak LM, Smith ZM, Degutte B, Eddington DK. Desynchronization of electrically evoked auditory-nerve activity by high-frequency pulse trains of long duration. J Acoust Soc Am. 2003;114(4 pt 1):2066-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]