Abstract
Background
Historically, limits to the ability to detect dysplasia in chronic inflammatory bowel disease (IBD)-associated colitis resulted in the recommendation that neoplasia of any grade be treated by proctocolectomy. We hypothesized that with improved optical technologies, most neoplasia in colitis is now detectable and reassessed the prevalence of colitis-associated neoplasia.
Methods
We retrospectively reviewed all our patients with IBD who had pathologist-confirmed neoplasia on surveillance colonoscopy and underwent a subsequent colectomy. We included patients whose index lesions were found between 2005 and 2014 (the dates of our high definition equipment) and recorded the location and grade of these lesions. These findings were compared to the surgical specimens, and in patients with partial colectomies, included follow-up.
Results
Thirty-six patients with IBD (19 [53%] ulcerative colitis, 17 [47%] Crohn’s disease) were found to have neoplastic lesions on surveillance colonoscopy and underwent a subsequent partial colectomy or total proctocolectomy. Fourty-four index lesions were identified by colonoscopy (29 white light and 7 methylene blue chromoscopy): 30 low-grade dysplasia, 6 high-grade dysplasia, and 8 adenocarcinoma. None of the low-grade dysplasia or adenocarcinoma index lesions were associated with synchronous carcinoma at colectomy. One of the patients with high-grade dysplasia had adenocarcinoma of the appendix.
Conclusions
In this experience with high definition colonoscopes in chronic colitis, no synchronous adenocarcinomas were found when colectomy was performed for low grade dysplasia and only 1 adenocarcinoma in the appendix was found in the setting of high-grade dysplasia. These findings suggest that active surveillance or subtotal colectomy, may be safe options for patients with IBD and some grades of neoplasia.
Keywords: Dysplasia, neoplasia, ulcerative colitis, colorectal cancer, inflammatory bowel disease
Introduction
Chronic inflammation in ulcerative colitis (UC) and Crohn’s disease (CD) has long been recognized as a risk factor for colonic neoplasia1, 2. The risk factors for development of neoplasia in inflammatory bowel disease (IBD) include a longer duration of disease,3 greater extent of colonic involvement,3 coexistent primary sclerosing cholangitis,4 and most recently, increasing degree of histologic inflammation.5–8 Because of these acknowledged risks and the younger age of patients with IBD, surveillance colonoscopies have been recommended in order to detect dysplasia and prevent the development of cancer.9–13
Historically, when colonic dysplasia was difficult to visualize (or believed to be “invisible”) it was recommended that random biopsies throughout the colon be obtained in order to systematically sample the at-risk mucosa.9–14 It was also considered that given the diffuse nature of colonic inflammation, there was a field effect of molecular changes predisposing to neoplastic change. Supportive of this pathophysiologic understanding was that identification of dysplasia was predictive of additional, local, or distant, synchronous or metachronous, colonic neoplastic lesions.15 Bernstein analyzed 10 prospective studies on dysplasia surveillance using fiber optic colonoscopies and described that low grade dysplasia (LGD) found during surveillance colonoscopy was associated with a 19% risk of a concurrent adenocarcinoma at the time of immediate colectomy and that high-grade dysplasia (HGD) was associated with a 42% risk of a concurrent adenocarcinoma.14 Therefore, given the risk of a missed synchronous or metachronous invasive cancer, guidelines recommend immediate colectomy when dysplasia of any grade was confirmed.12, 16
More recent advances in technology such as high definition (HD) video colonoscopies and high-resolution monitors have led to the understanding that most neoplasia in IBD is actually visible by white light,17–19 and additional techniques including dye spray chromoendoscopy and the utilization of HD colonoscopes have improved the visibility of neoplasia.20 These newer findings suggest that reflexive colectomy may not be needed for all types of dysplasia. A recent international consensus statement reviewing the status of dysplasia detection in UC recommended chromoscopy for patients with UC undergoing surveillance with standard definition scopes, but acknowledged a limitation in available evidence related to high definition scopes, and therefore concluded that chromoscopy is “suggested” based on low quality limited available evidence.21, 22 The SCENIC consensus did not address patients with CD.
Given the evolving technology, we hypothesized that HD colonoscopic technology offers improved visualization such that when neoplasia was found during colonoscopy, there would not be additional synchronous adenocarcinomas missed.
Materials and Methods
We performed a retrospective review using 2 institutional review board approved registries: the University of Chicago Inflammatory Bowel Disease (IBD) Registry and the University of Chicago IBD Neoplasia Registry. The first is for patients with confirmed IBD at the University of Chicago and is linked to the electronic medical record. All patients seen at the University of Chicago IBD Center are approached to participate in this registry. The second registry is a separate registry database maintained by our IBD center to follow all IBD patients diagnosed with neoplasia. Many, but not all patients in this registry are also in the first registry.
In this study we included all patients with UC and CD with colonic involvement who were found to have pathologist-confirmed neoplasia (LGD, HGD, or adenocarcinoma) on surveillance colonoscopy (defined as the index lesion[s] and index exam, respectively) using an HD colonoscope (models CF-H180AL, CF-HQ190L, and PCF-Q180AL; Olympus America, Melville, NY) between 2005–2014 (the timing of introduction of HD scopes, processors, and monitors at our institution), and who underwent a subsequent colectomy. HD is defined as a resolution of at least 720 active lines of pixels at 24 fps with an aspect ratio of 16:9.23 The three endoscopists (RDC, SBH, DTR) in this study were experts in IBD, each with more than 5 years of IBD endoscopy experience at the time of the earliest patient inclusion.
Using standard database management software, we searched the registries using the terms “grade dysplasia,” “colon,” “surgical pathology report.” We then combined the result set with our IBD registry. A successive search was performed to identify patients who underwent a subsequent partial colectomy or proctocolectomy. The identified patients were extracted into a Microsoft Excel (Redmond, WA) database and combined with identified patients from the Neoplasia Registry using similar search criteria. We excluded patients with missing data. Additional data collection included details regarding diagnosis, demographics, extent of disease, number of colonoscopies before discovery of the index lesion, history of prior colonic neoplasia, quality of bowel preparation, types of biopsies performed (random or targeted), whether dye spray chromoscopy was performed, and the grade and location of the index neoplastic lesions found on colonoscopy, and also how the lesion was identified (visible or by random non-targeted biopsy). We also recorded the indication for surgery, the pathology and colectomy reports, and the location and grade of any macroscopic or microscopic lesion found on the colectomy specimen. Additionally, the grade of the index lesion found on colonoscopy was compared to the lesion found in the same location at colectomy to determine upstaging or downstaging.
Pathology
At the University of Chicago, all colonoscopic biopsy specimens are reviewed and confirmed by at least 2 experienced expert gastrointestinal pathologists. Colectomy specimens are dissected in a standardized fashion, which includes macroscopic inspection as well as both targeted and random histologic sampling every 10 cm throughout the specimen. When surgical resection for neoplasia is performed, the segment of index neoplasia undergoes additional focused scrutiny and dissection.
Based on the colectomy findings, we specified (1) whether the index lesion was found, (2) whether it was upstaged or downstaged, and (3) whether neoplastic lesions elsewhere in the colon but missed on colonoscopy (so-called “synchronous lesions”) were identified.
Statistics
We stratified our data into the different grades of index neoplasia: LGD, HGD, and adenocarcinoma and analyzed the prevalence of synchronous neoplastic lesions for each group.
Statistical analyses using the Kruskal–Wallis test and Fisher’s exact test were performed to evaluate statistically significant variables associated with the presence of synchronous lesions. These variables included the number of scopes before index lesion being found, history of previous neoplasia and less than “adequate” bowel preparation, and yield by white light compared to chromoscopy.
Results
Thirty-six patients with IBD (19 [53%] UC and 17 [47%] CD) were found to have neoplastic lesions in the area of colitis during surveillance colonoscopy and underwent subsequent colectomy (Table 1). These patients had a total of 44 index neoplastic lesions identified on colonoscopy. All patients had surgery for an indication of neoplasia, some of which were due to unresectable neoplasia and others were due to concern for risk of concurrent or subsequent adenocarcinoma and based on the decision and recommendation of the gastroenterologist and patient. Twenty-nine of the colonoscopies used only white light technology and 7 included methylene blue dye spray chromoscopy during scope withdrawal (concentration is approximately 0.1%). Of the patients with UC, 13 (68%) had extensive colitis versus 6 (32%) with limited colitis. Of the patients with CD, 8 (47%) had diffuse pancolitis, 4 had at least 2 segments diffusely involved (but not pancolitis), and 5 had colitis limited to 1 segment. The median age at diagnosis of index lesions was 53.5 (range, 23–82) years and median disease duration was 18.0 (range, 0–40) years.
Table 1.
Demographics of IBD Patients with Neoplasia found on Colonoscopy
| CD | UC | N(%) or Median (range) | |
|---|---|---|---|
| IBD Diagnosis | |||
| – Crohn’s disease | 17 | – | 17 (47%) |
| – Ulcerative colitis | – | 19 | 19 (53%) |
| Median Age at Diagnosis of index lesion | 53.5 (23–82) | ||
| Median Disease Duration at index lesion identification | 18.0 (0–40) | ||
| PSC | 2 | 1 | 8.3% (3) |
| Number of colonoscopies prior to index lesion | 3 (0–13) | ||
|
Surgery types Proctocolectomy Total abdominal colectomy with ileorectal anastomosis Subtotal colectomy |
25 4 7 |
Of the 36 colectomies, all were performed for the primary indication of neoplasia. Twenty-five were proctocolectomies, 4 total abdominal colectomies with ileal-rectal anastomoses, and 7 subtotal colectomies (3 right hemicolectomies with ileo-transverse anastomosis, 2 ileocolectomies with neoterminal ileum-ascending colon anastomosis, 1 left hemicolectomy with distal transverse-sigmoid anastomosis, and 1 sigmoid resection).
Index Neoplasia
Of the 44 index lesions identified, 30 were LGD, 6 were HGD, and 8 were adenocarcinoma. Of the LGD lesions, 19 were in 14 patients with UC and 11 were in 10 patients with CD. Of the HGD lesions, 2 were in 1 patient with UC and 4 were in 4 patients with CD. Of the adenocarcinomas, 4 were in 4 patients with UC and 4 were in 4 patients with CD.
Eleven of the index lesions were associated with 9 additional neoplastic lesions found at colectomy (Fig. 1) versus 33 index lesions that were not associated with any synchronous lesions (Fig. 2). We also reviewed the location of the lesions. Twenty-eight of the 44 (64%) index lesions were in the left colon and were associated with 3 synchronous lesions, versus 4 synchronous lesions associated with the right colon index lesions.
Figure 1.
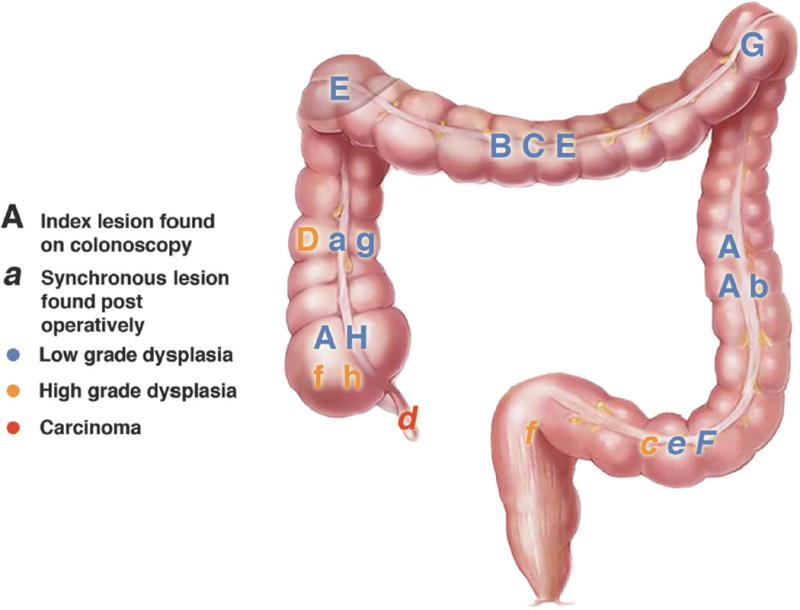
Location and Grade of Index Lesions Found on Colonoscopy that Had Synchronous Lesions Found at Colectomy
Figure 2.
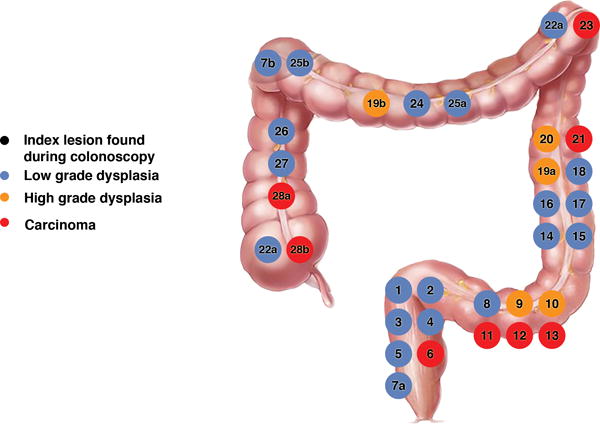
Location and Grade of Index Lesions Found on Colonoscopy that Did Not Have Additional Lesions found at Colectomy
Patients Who Underwent Chromoscopy
Seven of our patients (3 CD and 4 UC) underwent 0.1% methylene blue chromoscopy. Six of these patients had a previous history of dysplasia. All 7 patients had LGD index lesion on chromoscopy and 2 patients (of 7 patients, 28%) were found to have a LGD synchronous lesion on the colectomy specimen. In comparison, 6 patients (of 29 patients, 21%) who had white light examinations had synchronous lesions (chromoscopy versus white light synchronous findings, P = 0.64).
Neoplasia Found at Colectomy
In the group of patients with LGD index lesions at colonoscopy, 11 of 30 (37%) index lesions were also found on the colectomy specimen, and one of which was upstaged to HGD (Table 2). Also in this LGD group, there were 8 associated synchronous lesions found in the colectomy specimens (3 LGD, 4 HGD, and 1 sporadic adenoma proximal to colitis). In the HGD index group, of 6 patients, 5 (83%) of the index lesions were found at colectomy, and one of which was upstaged to adenocarcinoma. One adenocarcinoma synchronous lesion was found on colectomy in the HGD group (a patient with CD), but it was located in the appendix. In the group of patients with adenocarcinoma index lesions, 6 of 8 lesions were found at colectomy (the other 2 lesions were known to have been completely removed during colonoscopy), and this group was not associated with any synchronous lesions (Table 2).
Table 2.
Comparison of colonoscopy and colectomy findings (n=44 Index lesions).
| Grade of Index Neoplasia | Index Lesions found on Colonoscopy | Index Lesion Identified at Colectomy | Upstage or Downstage of Index lesions at Colectomy | Synchronous Lesion Found on Colectomy | Grade of Synchronous Neoplastic Lesions at Colectomy |
|---|---|---|---|---|---|
| LGD | 30/44 (19=UC),(11=CD) |
11/30 | 1/11 Upstaged to HGD |
8 | 3 LGD 4 HGD 1 Sporadic Adenoma |
| HGD | 6/44 (2=UC), (4=CD) |
5/6 | 1/5 Upstaged to Adenocarcinoma |
1 | 1 Adenocarcinoma |
| Adenocarcinoma | 8/44 (4=UC), (4=CD) |
6/8 |
0/6 Downstaged |
0 | – |
The median number of previous negative scopes before the index examination in this study was 1 (range, 0–6) in the group who had synchronous lesions on colectomy (n = 8), versus 3.5 (range, 0–13) in the no synchronous lesions group (n = 28) (P value = 0.06). Fifty percentage of the patients with synchronous lesions had a history of previous neoplasia versus 43% in the group with no synchronous lesions (P value = 1.0). In the synchronous group, there were no patients with less than adequate bowel preparation versus 17.9% in the group with no synchronous lesions (P = 0.56) (Table 3).
Table 3.
Variables predicting the presence of synchronous neoplasia
| Synchronous neoplasia (n=8) | No synchronous lesions (n=28) | P Value | |
|---|---|---|---|
| Median number of scopes prior to index lesion found | 1 (range 0–6) | 3.5 (range 0–13) | 0.06a |
| History of prior neoplasia | 50% (4/8) | 43% (12/28) | 1.0b |
| Less than adequate prepc | 0% (0/7) | 17.9.% (5/28) | 0.56b |
| Methylene blue chromoscopy | 13% (1/8) | 22% (6/28) | 1.00b |
Kruskal-Wallis Test;
Fisher exact test
(n=35)
Follow-Up for Patients with Segmental Colectomies
Of the 7 patients (6 CD and 1 UC) with segmental or subtotal colectomy (5 patients with LGD index lesions and 2 patients with index adenocarcinoma), there was a median of 6 months of follow-up (range, 3–81 mo) with median of 2 endoscopic examinations (range, 1–5). No colitis-associated neoplasia was found on any of these follow-up examinations.
Discussion
This study examined neoplastic findings in colectomy specimens from patients with colitis-associated neoplasia diagnosed using HD colonoscopic equipment. With HD colonoscopes and monitors, we found that most neoplasia was visible. Importantly, when colectomy was performed for LGD, no LGD lesions were upstaged, and no synchronous adenocarcinomas were found. This challenges previous recommendations that colectomy should be performed immediately when dysplasia of any grade was confirmed.2, 12, 15, 24, 25 Importantly, and also unique to our study, we also demonstrate similar findings for neoplasia found in UC and in CD of the colon.
The association of neoplasia and long-standing colitis has been well described and was the impetus for the development of prevention strategies. Based on the recognition that early technologies such as barium enema and first-generation endoscopy equipment could not adequately visualize precancerous (or cancerous) findings, gastroenterologists relied on the premise that once neoplasia of any grade is found, the prudent recommendation was surgical removal of the at-risk bowel.
These recommendations were based on studies that demonstrated a high rate of synchronous adenocarcinoma in patients with known neoplasia on colonoscopy.15 Gorfine et al15 in 2000 looked at 590 pathology reports of patients who underwent total proctocolectomy or restorative proctocolectomy for chronic UC and found that patients with dysplasia of any grade were 36 times more likely to harbor a synchronous invasive carcinoma.15 In Bernstein’s review, LGD was associated with a 19% risk of concurrent adenocarcinoma, and HGD was associated with a 42% risk of concurrent adenocarcinoma. Both Gorfine et al and Bernstein et al concluded that neoplasia of any grade had an unacceptably high concurrent cancer rate and therefore should prompt immediate colectomy.
In recent years, the detection of neoplasia has improved, and we have begun to adjust our expectations for management. Two prior retrospective studies described a high rate of visibility of UC-associated neoplasia using white light standard definition scopes. Rutter et al found that of 525 UC patients who underwent 2204 surveillance colonoscopies, 77.3% of lesions were macroscopically visible at colonoscopy.16 At our center, we reviewed 1339 surveillance examinations in 622 patients with UC and found that 58% of dysplastic lesions and 80% of cancers were visible to the endoscopist.18
More recently, Murphy et al examined the extent to which the preoperative colonoscopic detection of dysplasia is associated with synchronous cancer in UC patients. In this retrospective review, the presence of LGD was associated with 2% to 3% risk of undetected cancer, and similarly, HGD was associated with a 3% risk of undetected cancer. The study did not specify the type of colonoscopic equipment used.26
Contributing further to this discussion are recent studies that suggest that some patients with UC and confirmed dysplasia may be followed rather than having immediate surgery.20 Despite the acknowledged improvements in our “active surveillance” approach, there remain important gaps in the scientific literature and a lag time in adoption of such advances into clinical guidelines and clinical practice. Such gaps also include appropriate studies of HD colonoscopic equipment.
In this study of HD scope and colectomy findings, there were no synchronous adenocarcinomas in the patients with LGD. In addition, we found no synchronous or secondary adenocarcinomas in our patients who had colectomy for an index adenocarcinoma.
Of our 6 patients with index HGD, we only found 1 adenocarcinoma. Importantly, this was in a patient with CD and located in the appendix, an area that is not visible by colonoscopy, so technically was not “missed,” but obviously is an association that warrants caution in such patients. Involvement of the appendix in CD is not uncommon,27, 28 however, few appendiceal adenocarcinoma in CD have been previously described.29, 30 While this finding is undoubtedly important, it is unclear whether the colonic dysplasia was at all related to the appendiceal cancer or whether this was a coincidence. However, given this patient’s history of pancolitis involving the cecum, it is reasonable to believe that the adenocarcinoma was preceded by long standing CD involving the appendix.
In addition, it was of great interest to us that there were few synchronous LGD or HGD lesions found at colectomy, and in fact, 22 of the 43 index lesions were not found at colectomy. In careful review of the colonoscopy reports and pathology, we found that this was due to the fact that the index lesion was removed endoscopically in entirety prior to the colectomy. Further confirmation of this explanation is that our pathologists routinely focus extra attention on the segments that were described to have pre-operative neoplasia.
Of note, although not the primary focus of this retrospective study, the outcomes of patients in this series who had chromoscopy with methylene blue dye spray were not different from the outcomes of those with white light examinations, both in the number of synchronous lesions and, perhaps most clinically relevant, in findings of adencarcinoma. This is quite important to acknowledge given ongoing international discussions and emphasis on chromoendoscopy.21 Given the limited available evidence for the value of chromoscopy in the setting of HD scopes, we believe that our findings are important and reassuring to those who are not yet performing chromoscopy, but do have HD scopes. In fact, although the SCENIC consensus paper suggests chromoscopy for patients undergoing surveillance with HD scopes, it also specifies that the quality of evidence to support a need for chromoscopy with high definition scopes is low because it is based on only one small study.21, 22
We had specific interest in patients with CD in this study, and note that their outcomes were similar to the patients with UC, despite the acknowledged difference in morphology of inflammation in patients with CD. We believe that these findings contribute significantly to the current approach to cancer prevention in CD, which has mostly been adopted from previous studies of UC.31
An additional outcome of this study was the follow-up of patients with subtotal colectomies. Given that most neoplasia is visible in this series and with these techniques, it is reasonable to consider subtotal colectomy and ongoing endoscopic surveillance for some patients. The reassuring follow-up in our 7 patients with subtotal colectomy is a further support for this approach.
The major limitation of this study was that it was a retrospective analysis. Errors in data collection may have affected our results. However, the use of overlapping data sources (electronic records, colonoscopy, and also biopsy and colectomy reports) makes this limitation less likely. Given the strictness of our inclusion criteria, there were small numbers of patients included in the analysis. Additionally, despite our experienced GI pathologists’ techniques, it is possible that some synchronous lesions were missed. In addition, we acknowledge the existence of a rare type of adenocarcinoma found beneath surface of mucosal LGD. Although none of these were found in this cohort, clinicians should be aware of this possibility.32 Finally, this study was performed at a tertiary center, and colonoscopies and the decision to recommend surgery occurred with our experienced expert IBD endoscopists and the bias of a largely referral population. Therefore, our findings may not easily translate to the broader community-based patient population.
In conclusion, we have shown that HD colonoscopes and an experienced endoscopist identify most dysplastic lesions in both UC and CD of the colon, and most importantly, that colonic adenocarcinomas are not missed when LGD is found in the colon. These findings support the evolving practice of active surveillance in such patients and suggest that some patients may benefit from subtotal colectomies and ongoing surveillance rather than total proctocolectomies.
Acknowledgments
Funding: Partial funding for this study came from the Scholtz Family Foundation and the Digestive Disease Research Core Center of the University of Chicago (DK42086).
Footnotes
Conflicts of Interest: The authors have no relevant conflicts of interest.
References
- 1.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441–52. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 2.Ullman T, C V, Harpaz N, et al. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311–9. doi: 10.1016/j.gastro.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soetikno RM, Lin OS, Heidenreich PA, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 5.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol. 2013;11:1601–8. e1–4. doi: 10.1016/j.cgh.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 9.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 10.Farraye FA, Odze RD, Eaden J, et al. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–74. 774.e1–4. doi: 10.1053/j.gastro.2009.12.035. quiz e12–3. [DOI] [PubMed] [Google Scholar]
- 11.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–45. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 13.Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558–65. doi: 10.1016/j.gie.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71–4. doi: 10.1016/s0140-6736(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 15.Gorfine SR, Bauer JJ, Harris MT, et al. Dysplasia complicating chronic ulcerative colitis: is immediate colectomy warranted? Dis Colon Rectum. 2000;43:1575–81. doi: 10.1007/BF02236742. [DOI] [PubMed] [Google Scholar]
- 16.Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–21. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 17.Blonski W, Kundu R, Lewis J, et al. Is dysplasia visible during surveillance colonoscopy in patients with ulcerative colitis? Scandinavian Journal of Gastroenterology. 2008;43:698–703. doi: 10.1080/00365520701866150. [DOI] [PubMed] [Google Scholar]
- 18.Rubin DT, Rothe JA, Hetzel JT, et al. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65:998–1004. doi: 10.1016/j.gie.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Rutter MD, Saunders BP, Wilkinson KH, et al. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334–9. doi: 10.1016/s0016-5107(04)01710-9. [DOI] [PubMed] [Google Scholar]
- 20.Kiesslich R, Neurath MF. Surveillance colonoscopy in ulcerative colitis: magnifying chromoendoscopy in the spotlight. Gut. 2004;53:165–7. doi: 10.1136/gut.2003.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639–651.e28. doi: 10.1053/j.gastro.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Picco MF, Pasha S, Leighton JA, et al. Procedure time and the determination of polypoid abnormalities with experience: implementation of a chromoendoscopy program for surveillance colonoscopy for ulcerative colitis. Inflamm Bowel Dis. 2013;19:1913–20. doi: 10.1097/MIB.0b013e3182902aba. [DOI] [PubMed] [Google Scholar]
- 23.Hurbis-Cherrier M. The Digital Video System. In: Francis T, editor. Voice and Vision: A Creative Approach to Narrative Film and DV Production. Oxford: 2007. [Google Scholar]
- 24.Ullman TA. Patients with low-grade dysplasia should be advised to undergo colectomy. Inflamm Bowel Dis. 2003;9:267–9. doi: 10.1097/00054725-200307000-00007. discussion 273–5. [DOI] [PubMed] [Google Scholar]
- 25.Blackstone MO, Riddell RH, Rogers BH, et al. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366–374. [PubMed] [Google Scholar]
- 26.Murphy J, Kalkbrenner KA, Pemberton JH, et al. Dysplasia in ulcerative colitis as a predictor of unsuspected synchronous colorectal cancer. Dis Colon Rectum. 2014;57:993–8. doi: 10.1097/DCR.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 27.Ripolles T, Martinez MJ, Morote V, et al. Appendiceal involvement in Crohn’s disease: gray-scale sonography and color Doppler flow features. AJR Am J Roentgenol. 2006;186:1071–8. doi: 10.2214/AJR.04.1839. [DOI] [PubMed] [Google Scholar]
- 28.Yang SS, Gibson P, McCaughey RS, et al. Primary Crohn’s disease of the appendix: report of 14 cases and review of the literature. Ann Surg. 1979;189:334–9. doi: 10.1097/00000658-197903000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonwalkar SA, Denyer ME, Verbeke CS, et al. Cancer of appendix as a presenting feature of Crohn’s disease. Eur J Gastroenterol Hepatol. 2002;14:1029–32. doi: 10.1097/00042737-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Almogy G, Fellig Y, Paz K, et al. Adenocarcinoma of the appendix associated with long-standing Crohn’s disease. Int J Colorectal Dis. 2001;16:408–9. doi: 10.1007/s003840100328. [DOI] [PubMed] [Google Scholar]
- 31.Friedman S, Rubin PH, Bodian C, et al. Screening and surveillance colonoscopy in chronic Crohn’s colitis: results of a surveillance program spanning 25 years. Clin Gastroenterol Hepatol. 2008;6:993–8. doi: 10.1016/j.cgh.2008.03.019. quiz 953–4. [DOI] [PubMed] [Google Scholar]
- 32.Levi GS, H N. Intestinal low-grade tubuloglandular adenocarcinoma in inflammatory bowel disease. Am J Surg Pathol. 2006;30:1022–1029. doi: 10.1097/00000478-200608000-00014. [DOI] [PubMed] [Google Scholar]


