Abstract
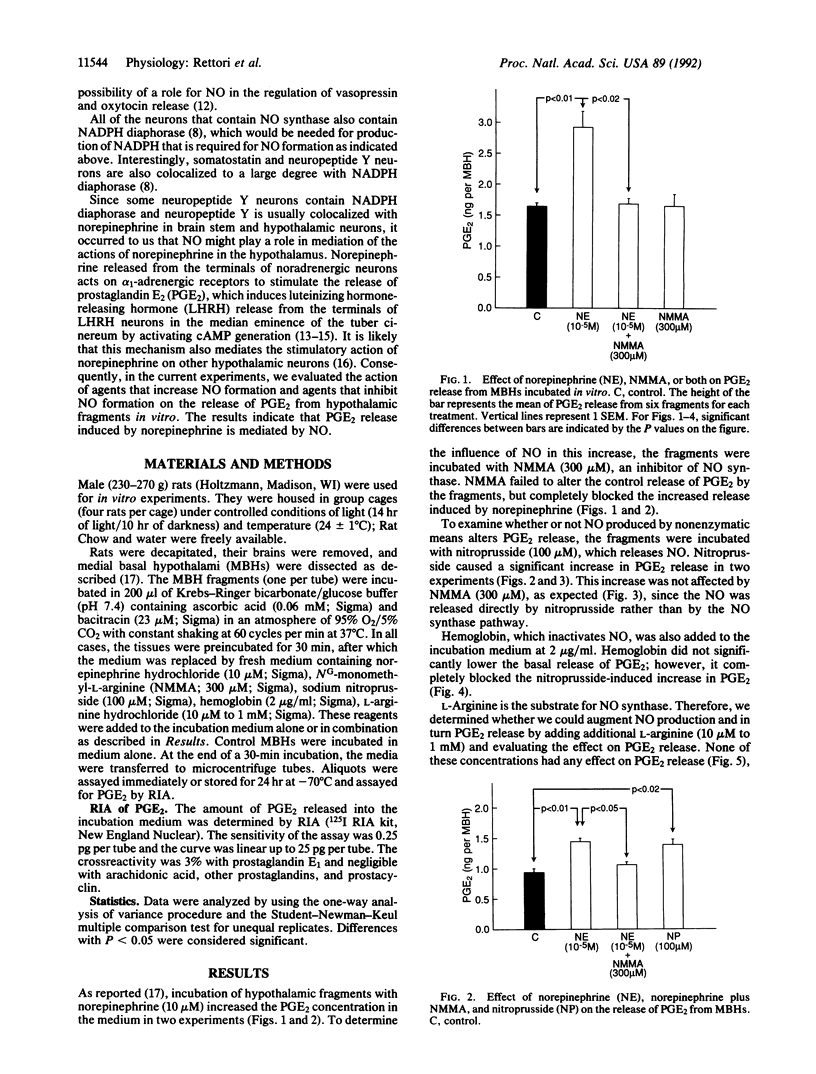
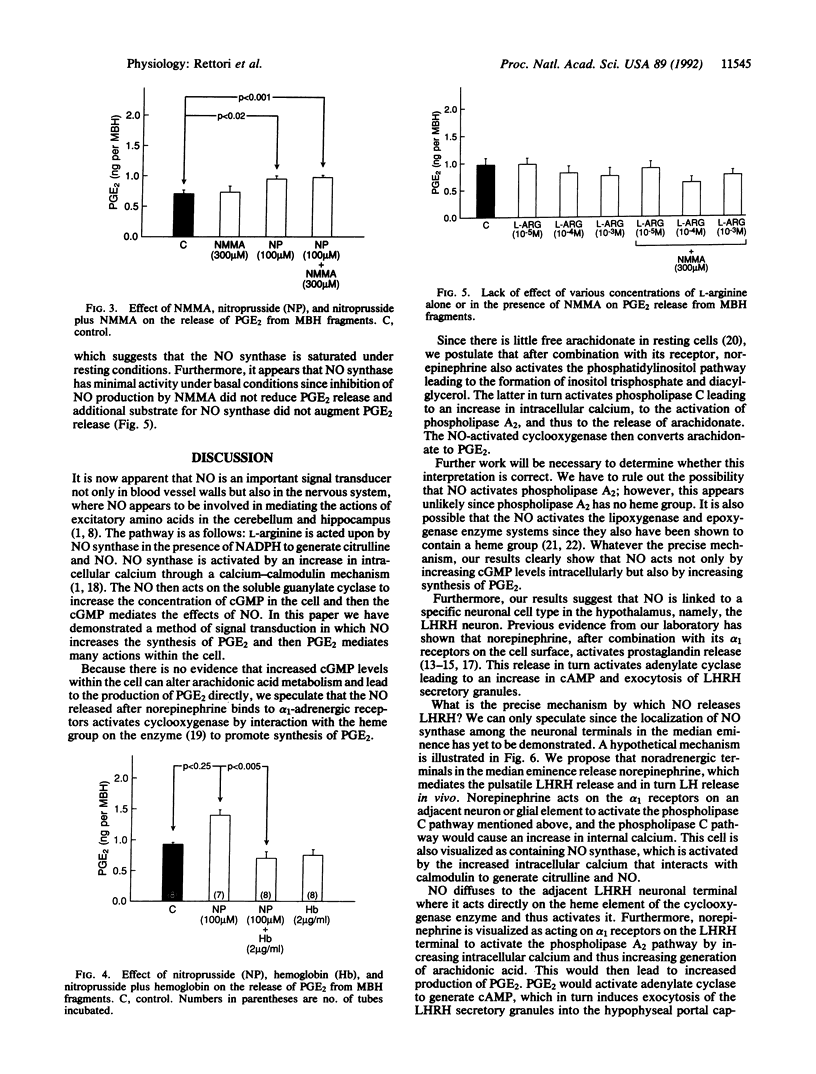
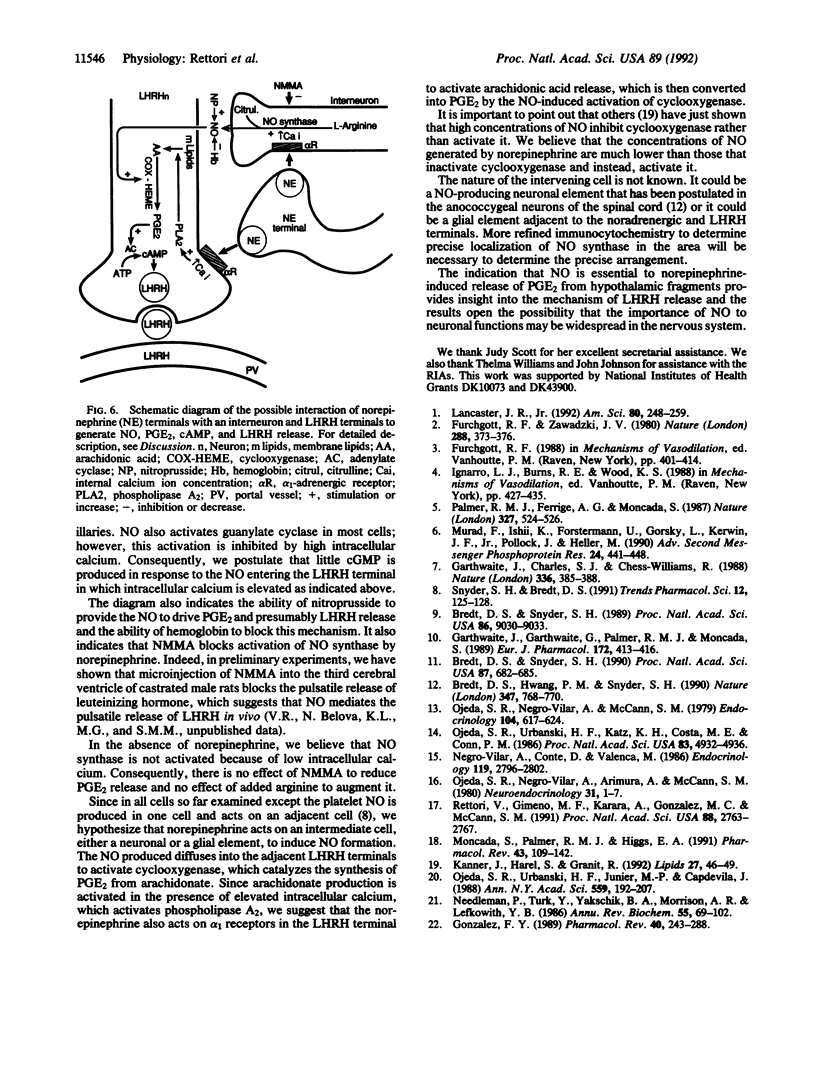
Nitric oxide (NO), formed by conversion of arginine to citrulline and NO by NO synthase, mediates relaxation of vascular smooth muscle. NO synthase has been demonstrated by immunocytochemical methods in neurons in various parts of the central nervous system including the hypothalamus. The latter finding suggested to us that NO might play a role in controlling the release of hypothalamic peptides. We have previously shown that norepinephrine mediates the release of luteinizing hormone-releasing hormone (LHRH) from LHRH terminals in the median eminence into the hypophyseal portal veins, which transport LHRH to the anterior pituitary gland to trigger release of luteinizing hormone from gonadotrophs. LHRH release from these terminals requires increased release of prostaglandin E2 (PGE2). PGE2 activates adenylate cyclase to produce cAMP, and then cAMP induces the exocytosis of LHRH secretory granules. In view of the evidence above and because of the developing evidence for the importance of NO in the central nervous system, it occurred to us that NO might be involved in this process. Consequently, we evaluated the role of NO in the release of PGE2 from medial basal hypothalamic fragments. As previously reported, norepinephrine (10 microM) increased PGE2 release from the hypothalamic fragments. The inhibitor of NO synthase NG-monomethyl-L-arginine (NMMA, 300 microM) blocked the stimulation of PGE2 release induced by norepinephrine but had no effect on the basal release of PGE2. Sodium nitroprusside (100 microM), which liberates NO, also elevated PGE2 release from the hypothalamic fragments. This elevation was not affected by NMMA, presumably because NMMA blocks enzymatic generation of NO but does not alter NO liberated by nitroprusside. When the NO liberated by nitroprusside was inactivated by hemoglobin (2 micrograms/ml), the effect of nitroprusside on PGE2 release was completely inhibited. Neither NMMA nor hemoglobin altered the basal release of PGE2, which indicates that NO is not responsible for basal PGE2 release. Addition of L-arginine (10 microM to 1 mM), the substrate for NO synthase, had no effect on basal PGE2 production. These results indicate that NO synthase is not activated in unstimulated hypothalamic fragments in vitro. The results suggest that norepinephrine activates NO synthase leading to the production of NO, which subsequently activates cyclooxygenase and results in the production of PGE2. PGE2 then activates adenylate cyclase leading to generation of increased cAMP, which induces exocytosis of secretory granules of LHRH and other neuropeptides released by PGE2. The indication that NO is essential to norepinephrine-induced release of PGE2 from hypothalamic fragments provides insight into the mechanism of LHRH release and the results open the possibility that the importance of NO to neuronal functions may be widespread in the nervous system.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Palmer R. M., Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989 Oct 17;172(4-5):413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids. 1992 Jan;27(1):46–49. doi: 10.1007/BF02537058. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Murad F., Ishii K., Förstermann U., Gorsky L., Kerwin J. F., Jr, Pollock J., Heller M. EDRF is an intracellular second messenger and autacoid to regulate cyclic GMP synthesis in many cells. Adv Second Messenger Phosphoprotein Res. 1990;24:441–448. [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Negro-Vilar A., Conte D., Valenca M. Transmembrane signals mediating neural peptide secretion: role of protein kinase C activators and arachidonic acid metabolites in luteinizing hormone-releasing hormone secretion. Endocrinology. 1986 Dec;119(6):2796–2802. doi: 10.1210/endo-119-6-2796. [DOI] [PubMed] [Google Scholar]
- Ojeda S. R., Negro-Vilar A., Arimura A., McCann S. M. On the hypothalamic mechanism by which prostaglandin E2 stimulates growth hormone release. In vivo and in vitro studies. Neuroendocrinology. 1980 Jul;31(1):1–7. doi: 10.1159/000123042. [DOI] [PubMed] [Google Scholar]
- Ojeda S. R., Negro-Vilar A., McCann S. M. Release of prostaglandin Es by hypothalamic tissue: evidence for their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology. 1979 Mar;104(3):617–624. doi: 10.1210/endo-104-3-617. [DOI] [PubMed] [Google Scholar]
- Ojeda S. R., Urbanski H. F., Junier M. P., Capdevila J. The role of arachidonic acid and its metabolites in the release of neuropeptides. Ann N Y Acad Sci. 1989;559:192–207. doi: 10.1111/j.1749-6632.1989.tb22609.x. [DOI] [PubMed] [Google Scholar]
- Ojeda S. R., Urbanski H. F., Katz K. H., Costa M. E., Conn P. M. Activation of two different but complementary biochemical pathways stimulates release of hypothalamic luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4932–4936. doi: 10.1073/pnas.83.13.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rettori V., Gimeno M. F., Karara A., Gonzalez M. C., McCann S. M. Interleukin 1 alpha inhibits prostaglandin E2 release to suppress pulsatile release of luteinizing hormone but not follicle-stimulating hormone. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2763–2767. doi: 10.1073/pnas.88.7.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]


