Supplemental Digital Content is available in the text
Keywords: breast milk, cytokine, Epstein–Barr virus, HIV-1, subclinical mastitis
Abstract
Epstein–Barr virus (EBV) in breast milk and subclinical mastitis (SCM) are both associated with human immunodeficiency virus (HIV) shedding and possibly with postnatal HIV transmission. The objective of this nested case–control study was to investigate the interplay between SCM and EBV replication in breast milk of HIV-infected mothers.
The relationships between EBV deoxyribonucleic acid (DNA) shedding, HIV-1 ribonucleic acid (RNA) level, and SCM were explored in breast milk samples of Zambian mothers participating in the ANRS 12174 trial. Mammary gland inflammation was defined as a breast milk sodium to potassium ratio (Na+/K+) greater than 0.6 and further subclassified as either “possible SCM” (Na+/K+ ratio 0.6–1.0) or SCM (Na+/K+ ratio ≥ 1.0). Breast milk interleukin 8 (IL-8) was measured as a surrogate marker of mammary gland inflammation.
EBV DNA was detected in breast milk samples from 42 out of 83 (51%) participants and was associated with HIV-1 shedding in breast milk (P = 0.006). EBV DNA levels were higher in samples with SCM and “possible SCM” compared to non-SCM breast milk samples (P = 0.06; P = 0.007). An EBV DNA level of >200 copies/mL was independently associated with SCM and “possible SCM” (OR: 2.62; 95%: 1.13–6.10). In patients with SCM, higher EBV replication in the mammary gland was associated with a lower induction of IL-8 (P = 0.013). Resistance to DNase treatment suggests that EBV DNA in lactoserum is encapsidated.
SCM and decreased IL-8 responses are associated with an increased EBV shedding in breast milk which may in turn facilitate HIV replication in the mammary gland.
1. Introduction
Breastfeeding is one of the main guaranties of an infant's health and normal development.[1,2] While encompassing most essential nutrients, vitamins and immunologically active components, breast milk still remains a source of human immunodeficiency virus (HIV) mother-to-child transmission (MTCT) especially in resource-limited countries.[3] Studies in Africa have shown that antiretroviral therapy (ART) significantly reduces HIV MTCT through breast milk.[4,5] Currently ART is recommended for all HIV-positive lactating mothers regardless of CD4 T cell counts and clinical stage of the disease.[6]
Mastitis, an inflammation of the breast tissue is a well-established risk factor for postnatal HIV MTCT.[7,8] One suggested explanation is that inflammation increases the permeability of mammary gland epithelia by which leukocytes and plasma, with cell-associated and cell-free HIV, respectively, leak into the breast milk. Inflammation also affects local electrolyte balance in breast milk resulting in elevated sodium (Na+) relative to potassium (K+) concentration. Increased breast milk Na+ concentration or Na+/K+ ratio are used to detect the clinically silent form of mastitis, termed subclinical mastitis (SCM). Assessment of interleukin 8 (IL-8) levels, leukocyte counts, and enzymes in breast milk have also been used to evaluate and diagnose SCM.[9–12] SCM is quite common; Nussenblatt et al[12] have reported a 27% prevalence of SCM in HIV infected Malawian breastfeeding mothers. Mixed breastfeeding (combination of breast milk and other foods) may result in more frequent SCM.[10]
Virus coinfections such as cytomegalovirus (CMV) and/or Epstein–Barr virus (EBV) also contribute to HIV shedding in breast milk. CMV deoxyribonucleic acid (DNA) is persistently found in breast milk and high CMV DNA levels are generally observed in HIV-infected mothers.[13] Furthermore, we recently reported that high breast milk CMV DNA levels are associated with HIV MTCT independently of HIV ribonucleic acid (RNA) levels.[14] Breast milk EBV DNA has also been shown to be associated with RNA shedding of HIV-1 in breast milk,[15] and EBV shedding is more frequently detected in the breast milk of mothers transmitting HIV postnatally as compared to nontransmitters.[14]
EBV is a gamma herpesvirus infecting epithelial cells and memory B cells.[16] More than 90% of the adult human population are EBV carriers. Primary infection occurring via virus present in the saliva is usually asymptomatic in children and causes infectious mononucleosis in adolescents and adults. Although viral persistence is generally asymptomatic, EBV infection is involved in development of B cell lymphomas. EBV establishes a lifelong latent cycle in memory B cells, periodically entering in lytic replication and virus shedding in oropharyngeal mucosa.[16,17] HIV infection and malaria exposure are involved in dysregulation of EBV persistence.[18] B cell activation and terminal differentiation into plasma cell is a requisite to initiate EBV lytic replication from latently infected cells.[19]
Here, we investigated the interplay between SCM and EBV shedding in the mature breast milk of HIV-infected mothers. We also examined the relationships between EBV DNA shedding and IL-8 levels, EBV DNA and HIV-1 RNA shedding, and the presence of encapsidated EBV in breast milk.
2. Materials and methods
2.1. Study population and samples
The source population for this nested study was the cohort of 1273 HIV-infected mothers who participated in a multicenter randomized controlled trial (PROMISE-PEP/ANRS 12174, NCT00640263) conducted in Burkina Faso, South Africa, Uganda, and Zambia.[20,21] Women included were not eligible for ART based on World Health Organization (WHO) recommendations at the time (enrollment between 2009 and 2012).
Mothers were advised to exclusively breastfeed their infants during the first 6 months postpartum and were thereafter advised to introduce complementary foods and stop breastfeeding by week 49.
The main trial protocol and results have been described elsewhere.[21] Substudies from this trial including the one presented herein have been approved by the scientific PROMISE-PEP committee in April 2014.
After providing an informed consent, mature breast milk samples from HIV-infected Zambian mothers were collected at 38th week postpartum, the first available point after the end of exclusive breastfeeding. Mothers with at least unilateral SCM or “possible SCM” were randomly selected as cases (n = 33) and mothers without SCM were selected as controls (n = 50) for this nested study. Breast milk was expressed manually in sterile 50 mL conical tubes. The specimens were stored at −80°C before testing. Lactoserum was separated from the cellular pellet after 5 minutes centrifugation at 5000 rpm.
2.2. Na+/K+ ratio measurement
Presence of SCM was evaluated by the Na+/K+ ratio. SCM was defined as a Na+/K+ ratio ≥ 1.0, “possible SCM” by a Na+/K+ ratio ranging from 0.6 to 1.0, and non-SCM samples a Na+/K+ ratio < 0.6.[22,23]
Stored lactoserum was diluted 101-fold in deionized water prior to testing. Na+ and K+ concentrations in the aqueous fraction were assessed using a PFP7 flame photometer (Jenway, Staffordshire, United Kingdom) as previously described.[23]
2.3. Assessment of breast milk IL-8
Stored aliquots of lactoserum were used. IL-8 levels were measured by an enzyme-linked immunosorbent assay (Human Standard ELISA Development Kit, PeproTech, Inc., CT), according to the manufacturer's instructions, and read out using a microplate reader (Multiskan FC, Thermo Scientific, Vantaa, Finland).
2.4. Monitoring CD4 T cell counts and HIV-1 RNA loads in plasma and breast milk
CD4 T-lymphocyte counts were measured by flow cytometry using a fluorochrome-conjugated mAb (FACSCalibur, BD, San Jose, CA). Breast milk HIV RNA was extracted from 1.0 mL of sample using QIAamp UltraSens Virus Kit (Qiagen, Hilden, Germany). Plasma HIV RNA was extracted by QIAamp mini viral RNA mini kit using 200 μL of sample. HIV-1 RNA was quantitated using a commercial real-time RNA PCR test (Generic HIV Charge Virale, Biocentric, Bandol, France) with a low detection limit of 300 HIV RNA copies/mL in plasma[24] and 50 HIV RNA copies/mL for lactoserum. Breast milk HIV-1 RNA levels below the threshold, but not 0, were arbitrarily assigned a value equal to half of the threshold (25 copies/mL).
2.5. Quantification of EBV DNA and human cell-free DNA
DNA was extracted from 200 μL of lactoserum using an automated QIAamp DNA Mini QIAcube Kit according to the manufacturer's protocol (Qiagen). EBV DNA was amplified using primers within the BamHI-W region of the EBV genome and described elsewhere.[25] A calibration curve was plotted using serial dilutions of first WHO international EBV standards (National Institute for Biological Standards and Control (NIBSC) reference number 09/260). A threshold set at 200 EBV DNA copies/mL of breast milk was used to define moderate/high viral load in breast milk, based on results of previous studies.[14,26]
Human cell-free DNA was quantified in lactosera to control the DNA extraction, control the deoxyribonuclease (DNase) I assay efficacy, and quantify lysed cells in acellular part of breast milk. For this purpose β globin was used as a target gene as described.[27] A calibration curve was plotted using serial dilutions of human genomic DNA (Biocentric), with a reference value of 6.6 pg of DNA per human diploid cell.[28] Results of β globin PCR were expressed as human cell genome equivalents (GEs) per mL.
2.6. DNase I assay for assessment of encapsidated Epstein–Barr virus
EBV positive breast milk samples were treated with the DNase I enzyme (DNase recombinant I, RNase free, Roche, Mannheim, Germany). Briefly, 100 μL of lactoserum was exposed to 100 U of DNase I, 20 μL of 10× incubation buffer and RNase-free water in a total volume of 200 μL and incubated at 37°C for 2 hours. A DNase I concentration of 100 U was chosen as it destroys more than 90% of the human cell-free DNA (β globin) in lactosera. DNase I was then inactivated at 75°C for 10 minutes. EBV viral loads were determined by qPCR as described above, comparing loads pre- and postexposure to DNase I; the presence of encapsidated DNA was defined as a decrease in EBV DNA of <1.0 log10 following DNase I treatment.
2.7. Statistical analysis
The Mann–Whitney test for nonparametric data was used to compare viral loads in different groups and the Spearman correlation coefficient was used to describe the correlations between left and right breast milk EBV DNA loads. Wilcoxon matched-pairs signed rank test was used to compare EBV DNA loads between 2 breast milk samples from mothers with unilateral mastitis. Frequencies of viral shedding in different groups were compared using 2-tailed Fisher exact test. Odds ratio (OR) was calculated for quantifying the association between HIV-1 RNA and EBV DNA in lactoserum, and the relation between moderate/high EBV DNA and SCM. Generalized Estimating Equation (GEE) variant of logistic regression analysis was used to evaluate the potential impact of SCM and “possible SCM” on moderate/high EBV loads in breast milk. The described model was chosen as it computed the regression coefficients assuming potential nonindependence between left and right breast milk samples. Maternal age, parity, breast milk HIV-1 RNA, and blood CD4 T cell counts were included in the analysis to evaluate the independency of associations. Factors having P values more than 0.2 in crude estimates were not further evaluated in adjusted estimates. Receiver operating characteristic (ROC) curves were plotted to assess the diagnostic potential of human cell-free DNA (β globin) and IL-8 as markers of SCM. Except the GEE model, where both breast milk variables were combined per mother, in all other analyses breast milk data are analyzed separately. All viral loads were log transformed and undetectable viral loads were transformed to logarithmic zeros for analyses. Statistical analyses and graphs were performed by GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA) and IBM SPSS statistics 20 software (SPSS, Inc., Chicago, IL).
3. Results
3.1. Clinical characteristics of participants and SCM
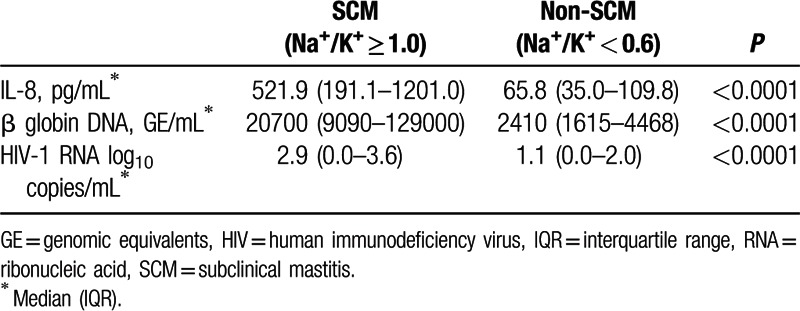
This study included 163 breast milk samples from 83 HIV-infected mothers (80 bilateral samples and 3 unilateral). Sociodemographic, obstetrical, and clinical characteristics are presented in Table 1. SCM and “possible SCM” were detected in 40% of the mothers. Notably, breast milk from specimens with SCM contained significantly higher levels of IL-8, human cell-free DNA, and HIV-1 RNA as compared to non-SCM samples (Table 2, P < 0.0001).
Table 1.
Clinical characteristics of study participants and samples.
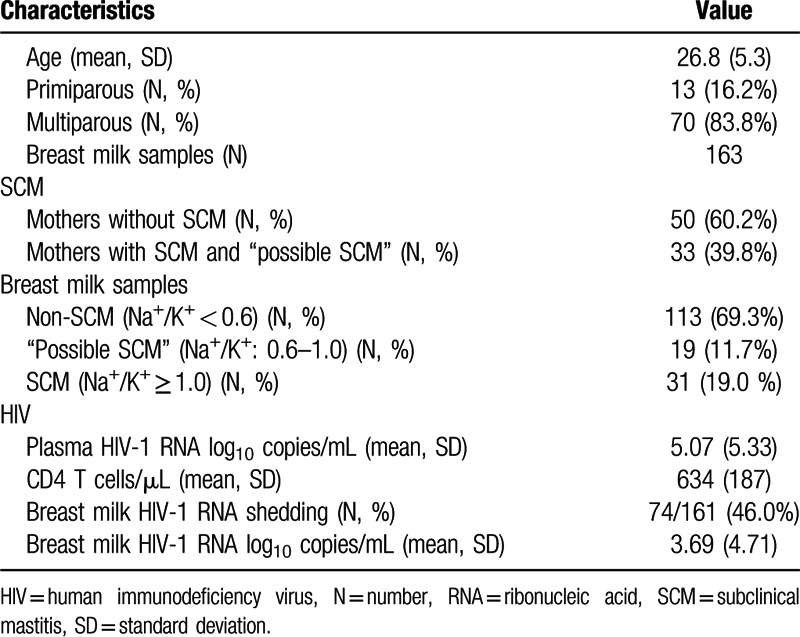
Table 2.
IL-8, β globin, and HIV-1 RNA levels in lactosera of SCM and non-SCM samples.
Human cell-free DNA in lactosera, reflecting lysed breast milk cells, was assessed as a surrogate marker for SCM detection. ROC plots demonstrated an area under the curve (AUC) of 0.96 (95% confidence interval [CI], 0.93–0.99) for cell-free DNA and 0.90 (95% CI, 0.82–0.97) for IL-8, as a means of identifying patients with SCM. The use of human cell-free DNA as a marker of SCM showed a sensitivity and specificity of 90.3 % and 90.2%, respectively, at a threshold level of 7,220 GE/mL (Supplementary Fig. 1).
3.2. Link between SCM and EBV DNA shedding in breast milk
Breast milk EBV DNA was detected in 51% of mothers (42/83), and 39% of breast milk samples (64/163). EBV DNA levels ranged from 0 to 88,300 copies/mL. Twenty-two mothers (27%) tested positive for EBV DNA bilaterally. EBV DNA loads were correlated between left and right breast milk samples (Rho = 0.65; P < 0.0001) (Fig. 1A). HIV-1 RNA shedding was more frequent in EBV-positive breast milk samples as compared with EBV-negative samples (59.3% vs 37.1%, P = 0.006) with OR: 2.48 (95% CI, 1.3–4.73; Fig. 1B). HIV-1 RNA loads were also higher in EBV-positive as compared to EBV-negative breast milk samples (median: 79.6 copies/mL; IQR: 7.0–338.6 vs 25.0 copies/mL; IQR: 0–208.1; P = 0.008).
Figure 1.
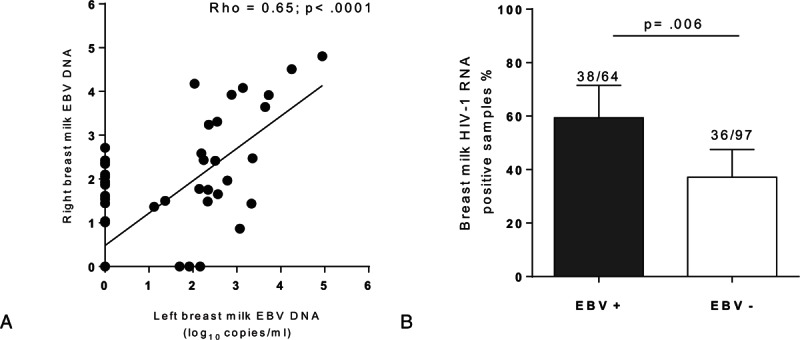
EBV and HIV shedding in breast milk. (A) Most women (n = 83) have similar levels of EBV DNA being secreted from both breasts. (B) More HIV RNA can be detected in milk when EBV DNA is also present in the milk.
EBV DNA was detected in 51.0% of samples with SCM (16/31), and 34.5% of non-SCM samples (39/113; P = 0.097). Furthermore, moderate/high breast milk EBV viral loads (>200 copies/mL) were more frequently observed in SCM samples (10/31; 32.2%) than in non-SCM samples (16/113; 14.1%; P = 0.033). The median values and interquartile ranges (IQRs) of EBV DNA levels in EBV-positive samples were: 2.48 (1.92–3.30) log10 copies/mL in SCM samples; 2.51 (2.35–4.58) log10 copies/mL in “possible SCM” samples and 2.04 (1.48–2.78) log10 copies/mL in non-SCM samples, respectively (Fig. 2A).
Figure 2.
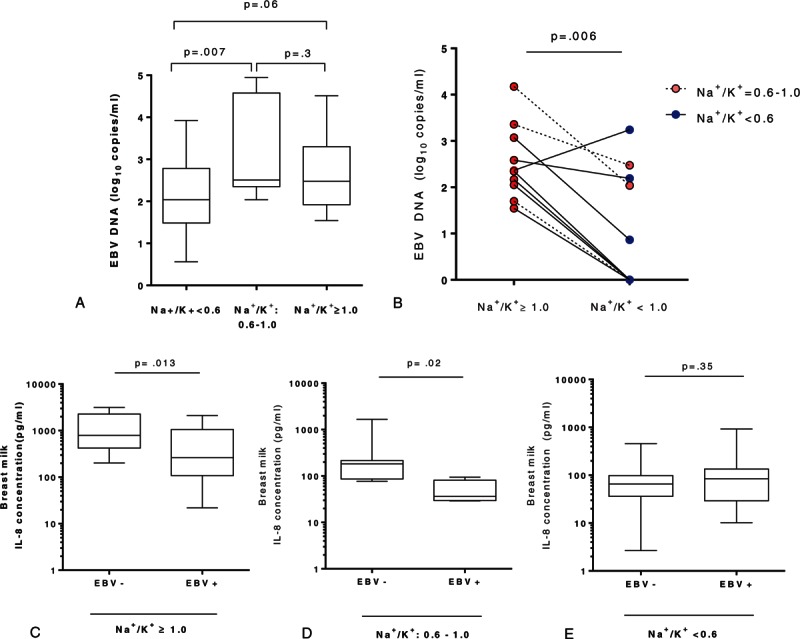
Relationship between EBV DNA level in breast milk and SCM. (A) Within EBV positive breast milk samples those with SCM and “possible SCM” have higher EBV load compared to non-SCM samples. (B) In mothers with unilateral SCM higher breast milk EBV DNA levels are detected in the site of SCM. Each pair represents samples collected at the same time from the 2 mammary glands of 1 mother. (C–E) IL-8 concentration according to breast milk EBV DNA shedding. In SCM (C) and “possible SCM” samples (D) IL-8 concentrations are significantly higher in EBV DNA negative breast milk samples compared to EBV DNA positive samples, whereas in non-SCM samples (E) IL-8 concentrations are not different. SCM = subclinical mastitis.
To evaluate the association between SCM and EBV levels within an individual breastfeeding mother, EBV DNA levels were compared in paired samples collected from mothers with unilateral SCM. In 9 of 10 mothers, EBV shedding was higher in milk samples from the breast with SCM (median: 2.35 log10 copies/mL; IQR: 1.96–3.14) as compared with the contralateral side (median: 0.43 log10 copies/mL; IQR: 0.0–2.26; P = 0.006; Fig. 2B).
Within samples with SCM, EBV shedding was also associated with the presence of human cell-free DNA. The frequencies of EBV-positive samples within samples with human cell-free DNA ≥7220 GE/mL were 50% (23/46), whereas only 33% (39/117) were positive in those samples with a level of human cell-free DNA <7220 GE/mL (P = 0.07). Furthermore, the levels of EBV DNA within the former group were significantly higher (median: 2.54 log10 copies/mL; IQR: 2.02–4.10) than in the latter group (median: 2.12 log10 copies/mL; IQR: 1.50–2.81; P = 0.01; Supplementary Figure 2A).
Based on the same human cell-free DNA threshold (7220 GE/mL), 15 mothers with unilateral mastitis showed evidence of EBV shedding. Within this group, 12 of 15 mothers presented with a higher EBV DNA load (median: 2.36 log10 copies/mL; IQR: 1.91–3.07) in the breast milk samples with higher human cell-free DNA as compared with contralateral breast milk samples (median: 1.03 log10 copies/mL; IQR: 0.0–2.42; P = 0.012; Supplementary Figure 2B).
3.3. Association of IL-8 levels with EBV shedding in SCM breast milk samples
Interestingly, when assessed all together, IL-8 concentrations did not differ between EBV-positive and EBV-negative breast milk specimens. Nevertheless, when IL-8 levels were evaluated in SCM and “possible SCM” samples, they were significantly lower in the EBV-positive as compared to the EBV-negative samples (median (IQR): 263 pg/mL (107–1059) vs 793 pg/mL (421–2273) in SCM samples (P = 0.013) and 36.1 pg/mL (29.6–81.0) vs 181.5 (85.9–215.3) in “possible SCM” samples (P = 0.02); Fig. 2C and D). In marked contrast, IL-8 levels were not significantly different in EBV-positive and EBV-negative non-SCM samples (84.0 pg/mL (29.3–134.8) vs 65.2 pg/mL (36.2–97.9); P = 0.35; Fig. 2E).
3.4. EBV in breast milk: free or encapsidated DNA?
DNase exposure prior to DNA extraction was used to assess whether EBV DNA was encapsidated and therefore protected from enzymatic digestion. Beta globin DNA quantification was used as a human cell-free DNA control. DNase I treatment resulted in a dramatic decrease in β globin DNA with a median (IQR) change from 14,500 GE/mL (10,850–25,075) before DNase treatment to 0.0 GE/mL (0.0–971.3) after DNase treatment. Among 12 EBV positive breast milk samples 4 (33%) showed a lack or negligible decrease of EBV DNA after DNase exposure with median (IQR) change from 2.78 log10 copies/mL (2.68–2.98) before DNase exposure to 2.48 log10 copies/mL (2.44–2.54) after DNase exposure suggesting that EBV DNA was protected from DNase of samples (data not shown). The remaining 8 samples became undetectable (from 2.63 to 0.0 log10 copies/mL) (data not shown).
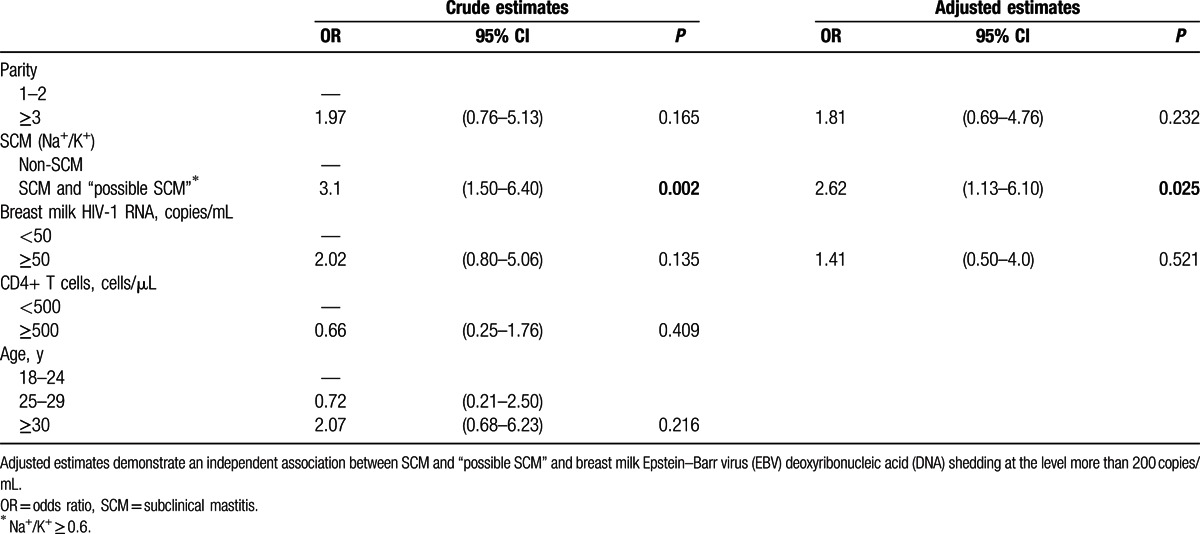
3.5. Multivariate analysis
Multivariate analysis was used to evaluate the potential impact of SCM and “possible SCM” on EBV replication in breast milk (Table 3). We found that SCM as well as “possible SCM” were independently associated with EBV replication at levels >200 copies/mL in breast milk (OR: 2.62; 95% CI: 1.13–6.10).
Table 3.
Generalized Estimating Equation version of logistic regression model.
4. Discussion
Our results evidence the association between SCM and EBV replication in mature breast milk of HIV-1 positive mothers, with local immunological factors such as IL-8 interacting with EBV replication in the mammary gland.
EBV was detected in lactoserum of 51% of the mothers tested in this study. Notably, the frequency of HIV-1 RNA shedding and viral load was significantly higher in EBV-positive as compared to EBV-negative breast milk samples. These data are in agreement with a similar report showing that the association between breast milk EBV DNA and HIV-1 RNA is independent of plasma HIV-1 RNA level.[15]
EBV DNA levels were higher in SCM as compared to non-SCM breast milk samples. Furthermore, assessment of breast milk samples from the 2 mammary glands of mothers with unilateral SCM confirmed the link between SCM and EBV DNA shedding independently of host systemic factors. Specifically, EBV DNA levels were significantly higher in breast milk from the mammary gland with SCM than from the contralateral side. However, EBV detection was not more frequent in SCM samples, suggesting that low levels of EBV DNA are a frequent finding in the breast milk of HIV-infected mothers, as previously observed.[15] Multivariate analyses showed that a level of EBV DNA of >200 copies/mL is associated with SCM and “possible SCM.”
SCM in humans is a recently proposed entity, characterized by increased mammary gland permeability and leakage of plasma components and leucocytes into breast milk.[23] The prevalence of SCM in lactating mothers has been reported to range between 10% and 45%[29,30] and this prevalence does not appear to be altered in HIV-infected mothers.[31] SCM is generally defined by high Na+ concentration or Na+/K+ ratio in breast milk.[32,33] Breast milk cell count and secretory leukocyte protease inhibitor activity can also be used to detect SCM.[8,12] As cellular DNA is released during apoptosis and necrosis[34] we measured human cell-free DNA in breast milk to explore lysed cells. Our data show that the quantification of human cell-free DNA in breast milk may serve as a surrogate marker for SCM. Moreover, SCM has also been associated with increased production of proinflammatory mediators such as IL-8, RANTES, and lactoferrin[9,10,35,36] and in our study as well, high IL-8 levels strongly correlated with this condition.
EBV is frequently detected in the breast milk of women with chronic EBV infection,[36] irrespective of HIV infection. Junker et al[37] reported an overall 46% prevalence of EBV DNA in the cellular fraction of breast milk in the general population. Further studies also demonstrated frequent EBV DNA in the acellular breast milk fraction, called lactoserum or whey.[26] EBV shedding in breast milk is higher during the first weeks of breastfeeding and decreases in mature breast milk.[26,37] Notably though, EBV is rarely detected in the plasma of healthy people[38] and even in HIV-infected individuals, EBV DNA is confined to the cellular fraction of the blood.[39,40]
The replication of EBV depends on the activation and maturational stage of the infected B cell.[19,41] An EBV-specific cellular response is pivotal in the protective immunity against EBV reactivation.[42] EBV-infected memory B cells that undergo plasma cell differentiation are able to switch from a latent to lytic cycle.[19] We previously reported an association between persistent B-cell stimulation and EBV, HIV,[43,44] and HCV[45] replication in HIV-infected patients on ART. The shedding of EBV in mucosal areas raises the question of the local factors driving EBV replication in breast milk. During lactation, mammary glands constitute an effector compartment of the mucosal associated lymphoid tissue.[46] Breast milk B cells display a phenotype strikingly different from blood B cells with a mucosal homing profile similar to cells located in gut-associated lymphoid tissue and a higher percentage of large-sized B cells, plasmablasts, and plasma cells (CD19, CD20 low, CD27 high, CD138)[47] that are essential for EBV replication. This suggests that a significant proportion of B cells infected by EBV can initiate replication in the lactating mammary gland. In this study if SCM occurs or HIV was detectable in breast milk then increased EBV shedding was observed. The immune response induced by SCM and HIV infection may fuel B cell activation, triggering EBV replication in latently infected cells. Interestingly, we observed a positive association between SCM and IL-8, but an inverse association between IL-8 concentration and the quantity of EBV DNA in samples with SCM and “possible SCM.” This result suggests that a robust cytokine response during SCM may limit EBV shedding.
To distinguish complete EBV from naked DNA DNase I treatment was used as previously described for EBV in plasma and saliva.[48,49] The low impact of DNase exposure on EBV DNA concentration in some breast milk samples suggested that a significant proportion of EBV DNA is encapsidated in the breast milk. Our observation is in line with a recent study performed in breast milk samples of mothers from high-risk malaria region.[26] However, we have not performed infectivity assay to verify the virulent potential of breast milk EBV.
This study highlights the interplay between SCM and EBV replication in breast milk. Although asymptomatic, SCM is characterized by significant changes in the breast milk environment, altering the host-virus interplay. EBV shedding in breast milk is likely to be regulated by immune factors that control local EBV reactivation. The results presented here suggest that HIV replication in breast milk as well as SCM facilitate EBV replication in the mammary gland.
Further studies will be needed to assess the consequences of high EBV exposure on children during breastfeeding.
Supplementary Material
Acknowledgments
We are grateful to the participants. We thank to Naomi Taylor and Valerie Zimmermann for critical evaluation of the manuscript and codirection of the A. Sanosyan's doctoral study, and to the ANRS 12174 trial group: University of Montpellier 1 (France): Roselyne Vallo, Valerie Marechal, Dorine Neveu, Vincent Foulongne, Michel Segondy; University of Paris V (France): Stephane Blanche, Jean-Marc Treluyer, Deborah Hirt; Makerere University (Uganda): James K. Tumwine, Grace Ndeezi, Charles Karamagi, Philippa Musoke, Proscovia M. Mugaba, Mary Kwagala, Joan Murungi, Hawa Nabuuma Muweesi, Evelyn Ninsiima, Simon Baryeija, Frederic Juma, Caleb Bwengye Kata, Stuart Katushabe; University of Ouagadougou (Burkina Faso): Nicolas Meda, Rasmata Ouedraogo, Diarra Ye, Eric Some, Hugues A. Traore, Christelle Nadembega, Justin Konate, Arsene Zongo, Abass Ouedraogo, Desire Neboua, Aissatou Belemvire, Armel Bambara, Justine Boncoungou; Danielle University of Western Cape (South Africa): Cheryl Nikodem, Justus Hofmeyr, Kim Harper, Debra Jackson, David Sanders, Mandisa Singata, Amwe Aku, Collins Okegbe-Eze, Xoliswa Williams, Nolundi Mshweshwe, Vatiswa Henge, Fikiswa Gomba, Tapiwa Gundu, Oswell Khandwa; University of Zambia (Zambia): Mildred Lusaka, Mary Chizyuka, Mary Phiri, Billies Imakando, Mwenechanya Musaku, Monica Kapasa, Gondwe Clement, Hilton Mwila Mwaba, Japhet Matoba, Chafye Siuluta, Katai Chola, Patricia Mwamutanda; University of Bergen (Norway): Halvor Sommerfelt, Ingunn Engebretsen, Jorn Klungsoyr, Jan van den Broeck, Jorn Blume; INSERM-ANRS (France): Claire Rekacewicz.
Footnotes
Abbreviations: ART = antiretroviral therapy, AUC = area under the curve, CI = confidence interval, CMV = cytomegalovirus, DNA = deoxyribonucleic acid, DNase = deoxyribonuclease, EBV = Epstein–Barr virus, GE = genome equivalent, GEE = Generalized Estimating Equation, HIV = human immunodeficiency virus, IL-8 = interleukin 8, IQR = interquartile range, MTCT = mother-to-child transmission, RANTES = Regulated on Activation, Normal T cell Expressed and Secreted, RNA = ribonucleic acid, ROC = receiver operating characteristic, SCM = subclinical mastitis, WHO = World Health Organization.
AS and DGR contributed equally to the article.
Funding: This study was supported by France National Agency for Research on AIDS & Hepatitis (ANRS), European Developing Countries Clinical Trials Partnership (EDCTP) and Research Council of Norway, and Erasmus-Mundus was financing A. Sanosyan's doctoral scholarship.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.WHO. Long-term effects of breastfeeding: a systematic review. http://www.who.int/maternal_child_adolescent/documents/breastfeeding_long_term_effects/en/ Accessed November 16, 2015. [Google Scholar]
- 2.Hassiotou F, Geddes DT. Immune cell-mediated protection of the mammary gland and the infant during breastfeeding. Adv Nutr Bethesda Md 2015; 6:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van de Perre P, Rubbo P-A, Viljoen J, et al. HIV-1 reservoirs in breast milk and challenges to elimination of breast-feeding transmission of HIV-1. Sci Transl Med 2012; 4:143sr3. [DOI] [PubMed] [Google Scholar]
- 4.de Vincenzi I. Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 2011; 11:171–180. [DOI] [PubMed] [Google Scholar]
- 5.Shetty AK, Maldonado Y. Antiretroviral drugs to prevent mother-to-child transmission of HIV during breastfeeding. Curr HIV Res 2013; 11:102–125. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/ Accessed September 14, 2015. [Google Scholar]
- 7.Lunney KM, Iliff P, Mutasa K, et al. Associations between breast milk viral load, mastitis, exclusive breast-feeding, and postnatal transmission of HIV. Clin Infect Dis 2010; 50:762–769. [DOI] [PubMed] [Google Scholar]
- 8.Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci 2000; 918:156–162. [DOI] [PubMed] [Google Scholar]
- 9.Semba RD, Kumwenda N, Taha TE, et al. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol 1999; 6:671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willumsen JF, Filteau SM, Coutsoudis A, et al. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol 2000; 478:211–223. [DOI] [PubMed] [Google Scholar]
- 11.Gantt S, Shetty AK, Seidel KD, et al. Laboratory indicators of mastitis are not associated with elevated HIV-1 DNA loads or predictive of HIV-1 RNA loads in breast milk. J Infect Dis 2007; 196:570–576. [DOI] [PubMed] [Google Scholar]
- 12.Nussenblatt V, Lema V, Kumwenda N, et al. Epidemiology and microbiology of subclinical mastitis among HIV-infected women in Malawi. Int J STD AIDS 2005; 16:227–232. [DOI] [PubMed] [Google Scholar]
- 13.Slyker J, Farquhar C, Atkinson C, et al. Compartmentalized cytomegalovirus replication and transmission in the setting of maternal HIV-1 infection. Clin Infect Dis 2014; 58:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viljoen J, Tuaillon E, Nagot N, et al. Cytomegalovirus, and possibly Epstein-Barr virus, shedding in breast milk is associated with HIV-1 transmission by breastfeeding. AIDS 2015; 29:145–153. [DOI] [PubMed] [Google Scholar]
- 15.Gantt S, Carlsson J, Shetty AK, et al. Cytomegalovirus and Epstein-Barr virus in breast milk are associated with HIV-1 shedding but not with mastitis. AIDS 2008; 22:1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young LS, Rickinson AB. Epstein–Barr virus: 40 years on. Nat Rev Cancer 2004; 4:757–768. [DOI] [PubMed] [Google Scholar]
- 17.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 2004; 350:1328–1337. [DOI] [PubMed] [Google Scholar]
- 18.Reynaldi A, Schlub TE, Chelimo K, et al. Impact of plasmodium falciparum coinfection on longitudinal Epstein-Barr virus kinetics in Kenyan children. J Infect Dis 2016; 213:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol 2005; 79:1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagot N, Kankasa C, Meda N, et al. Lopinavir/Ritonavir versus Lamivudine peri-exposure prophylaxis to prevent HIV-1 transmission by breastfeeding: the PROMISE-PEP trial Protocol ANRS 12174. BMC Infect Dis 2012; 12:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagot N, Kankasa C, Tumwine JK, et al. Extended pre-exposure prophylaxis with lopinavir–ritonavir versus lamivudine to prevent HIV-1 transmission through breastfeeding up to 50 weeks in infants in Africa (ANRS 12174): a randomised controlled trial. Lancet 2016; 387:566–573. [DOI] [PubMed] [Google Scholar]
- 22.Arsenault JE, Aboud S, Manji KP, et al. Vitamin supplementation increases risk of subclinical mastitis in HIV-infected women. J Nutr 2010; 140:1788–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantarci S, Koulinska IN, Aboud S, et al. Subclinical mastitis, cell-associated HIV-1 shedding in breast milk, and breast-feeding transmission of HIV-1. J Acquir Immune Defic Syndr 1999 2007; 46:651–654. [DOI] [PubMed] [Google Scholar]
- 24.Rouet F, Ekouevi DK, Chaix M-L, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol 2005; 43:2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadinoto V, Shapiro M, Greenough TC, et al. On the dynamics of acute EBV infection and the pathogenesis of infectious mononucleosis. Blood 2008; 111:1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daud II, Coleman CB, Smith NA, et al. Breast milk as a potential source of Epstein-Barr virus transmission among infants living in a malaria-endemic region of Kenya. J Infect Dis 2015; 212:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orozco AF, Jorgez CJ, Horne C, et al. Membrane protected apoptotic trophoblast microparticles contain nucleic acids. Am J Pathol 2008; 173:1595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiki RK, Gelfand DH, Stoffel S, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988; 239:487–491. [DOI] [PubMed] [Google Scholar]
- 29.Aryeetey RNO, Marquis GS, Timms L, et al. Subclinical mastitis is common among Ghanaian women lactating 3 to 4 months postpartum. J Hum Lact 2008; 24:263–267. [DOI] [PubMed] [Google Scholar]
- 30.Flores M, Filteau S. Effect of lactation counselling on subclinical mastitis among Bangladeshi women. Ann Trop Paediatr 2002; 22:85–88. [DOI] [PubMed] [Google Scholar]
- 31.Gomo E, Filteau SM, Tomkins AM, et al. Subclinical mastitis among HIV-infected and uninfected Zimbabwean women participating in a multimicronutrient supplementation trial. Trans R Soc Trop Med Hyg 2003; 97:212–216. [DOI] [PubMed] [Google Scholar]
- 32.Filteau Lietz, Mulokozi G, Bilotta S, et al. Milk cytokines and subclinical breast inflammation in Tanzanian women: effects of dietary red palm oil or sunflower oil supplementation. Immunology 1999; 97:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semba RD, Neville MC. Breast-feeding, mastitis, and HIV transmission: nutritional implications. Nutr Rev 1999; 57:146–153. [DOI] [PubMed] [Google Scholar]
- 34.Gahan PB, Swaminathan R. Circulating nucleic acids in plasma and serum. Recent developments. Ann N Y Acad Sci 2008; 1137:1–6. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen LBW, Hansen DH, Kaestel P, et al. Milk enzyme activities and subclinical mastitis among women in Guinea-Bissau. Breastfeed Med 2008; 3:215–219. [DOI] [PubMed] [Google Scholar]
- 36.Glenn WK, Whitaker NJ, Lawson JS. High risk human papillomavirus and Epstein Barr virus in human breast milk. BMC Res Notes 2012; 5:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Junker AK, Thomas EE, Radcliffe A, et al. Epstein-Barr virus shedding in breast milk. Am J Med Sci 1991; 302:220–223. [DOI] [PubMed] [Google Scholar]
- 38.Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. J Mol Diagn 2008; 10:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens SJC, Blank BSN, Smits PHM, et al. High Epstein-Barr virus (EBV) DNA loads in HIV-infected patients: correlation with antiretroviral therapy and quantitative EBV serology. AIDS 2002; 16:993–1001. [DOI] [PubMed] [Google Scholar]
- 40.Stevens SJC, Pronk I, Middeldorp JM. Toward standardization of Epstein-Barr virus DNA load monitoring: unfractionated whole blood as preferred clinical specimen. J Clin Microbiol 2001; 39:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callan MFC. The immune response to Epstein–Barr virus. Microbes Infect 2004; 6:937–945. [DOI] [PubMed] [Google Scholar]
- 42.Landais E, Saulquin X, Houssaint E. The human T cell immune response to Epstein-Barr virus. Int J Dev Biol 2005; 49:285–292. [DOI] [PubMed] [Google Scholar]
- 43.Ouedraogo DE, Tuaillon E, Rubbo P-A, et al. Close relationship between immunoglobulin secreting-cells and Epstein-Barr virus reservoir in patients infected with HIV. J Med Virol 2014; 86:30–37. [DOI] [PubMed] [Google Scholar]
- 44.Ouedraogo DE, Makinson A, Vendrell J-P, et al. Pivotal role of HIV and EBV replication in the long-term persistence of monoclonal gammopathy in patients on antiretroviral therapy. Blood 2013; 122:3030–3033. [DOI] [PubMed] [Google Scholar]
- 45.Casanova M-L, Makinson A, Eymard-Duvernay S, et al. Monoclonal gammopathy in HIV-1-infected patients: factors associated with disappearance under long-term antiretroviral therapy. J Acquir Immune Defic Syndr 1999 2015; 70:250–255. [DOI] [PubMed] [Google Scholar]
- 46.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr 2010; 156 (suppl. 2):S8–S15. [DOI] [PubMed] [Google Scholar]
- 47.Tuaillon E, Valea D, Becquart P, et al. Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. J Immunol 2009; 182:7155–7162. [DOI] [PubMed] [Google Scholar]
- 48.Ryan JL, Fan H, Swinnen LJ, et al. Epstein-Barr virus (EBV) DNA in plasma is not encapsidated in patients with EBV-related malignancies. Diagn Mol Pathol 2004; 13:61–68. [DOI] [PubMed] [Google Scholar]
- 49.Hadinoto V, Shapiro M, Sun CC, et al. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog 2009; 5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


