Supplemental Digital Content is available in the text
Keywords: albuminuria, diabetic kidney disease, glomerular filtration rate, risk factors, type 2 diabetes
Abstract
The identification of clinical predictors for the development of chronic kidney disease is a critical issue in the management of patients with type 2 diabetes mellitus.
We evaluated 27,029 patients with type 2 diabetes mellitus and estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 and normoalbuminuria from the database of the Italian Association of Clinical Diabetologists network. Primary outcomes were eGFR <60 mL/min/1.73 m2 and normoalbuminuria; albuminuria and eGFR ≥60 mL/min/1.73 m2; and eGFR <60 mL/min/1.73 m2 and albuminuria. Secondary outcomes were eGFR <60 mL/min/1.73 m2 and albuminuria. Measurements: eGFR from serum creatinine by chronic kidney disease epidemiology collaboration equation (CKD-EPI), urinary albumin excretion, HbA1c, triglycerides, high-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c), blood pressure, and body mass index.
Over a 4-year period, 33.2% of patients (n = 8973) developed chronic kidney disease, 10.3% (n = 2788) showed a decline in eGFR <60 mL/min/1.73 m2, 18.4% (n = 4978) developed albuminuria, and 4.5% (n = 1207) developed both features. Relative risk ratios (RRRs) for age (1.37, P < 0.001 by 5 years), sex (0.77, P < 0.001 for being male), body mass index (1.03, P < 0.001 by 1 kg/m2), triglycerides (1.02, P < 0.001 by 10 mg/dL), and LDL-c (0.97, P = 0.004 by 10 mg/dL) were independently related to the onset of eGFR reduction. Age (1.08, P < 0.001 by 5 years), sex (1.36, P < 0.001 for being male), body mass index (1.02, P < 0.001 by 1 kg/m2), triglycerides (1.01, P = 0.02 by 10 mg/dL), HDL-c, and LDL-c (0.97, P = 0.008 and 0.99, P = 0.003 by 5 and 10 mg/dL, respectively) were related to the onset of albuminuria. HbA1c and the intensity of antihypertensive treatment showed a weaker association with renal outcome.
Reduction in eGFR and albuminuria showed distinct sets of risk factors, suggesting that different mechanisms are involved in the development of these 2 components of diabetic kidney disease.
1. Introduction
Diabetes mellitus, mainly type 2, is a major health problem with global estimates exceeding >380 million peoples worldwide in the last year, thus representing 8.3% of the global adult population.[1] This number is expected to increase to 592 million by 2035.[2] Diabetes mellitus is currently the leading cause of chronic kidney disease (CKD).[3] In fact, approximately 40% of patients with diabetes develop diabetic kidney disease (DKD) resulting in albuminuria, reduction of glomerular filtration rate (GFR), or both.[4] DKD accounts for nearly half of all incident cases of end-stage renal disease in the United States and 5-year survival for patients with end-stage renal disease is <40%.[5]
The main features of DKD, that is, GFR <60 mL/min/1.73 m2 or albuminuria, are well-known independent predictors of mortality, mainly from cardiovascular complications. A recent meta-analysis has shown that the addition of GFR and urinary albumin-to-creatinine ratio significantly improved the prediction of cardiovascular outcomes and mortality beyond traditional risk factors in the general population[6] as well as in patients with diabetes mellitus,[7] and the improvement was greater with urinary albumin-to-creatinine ratio than with GFR. Accordingly, a new CKD classification, which includes both GFR and albuminuria stages, has been adopted to provide more accurate assessment of renal and cardiovascular outcomes.[8]
The typical natural history of diabetic nephropathy has been derived mainly from studies in patients with type 1 diabetes mellitus. In these patients, microalbuminuria is the first sign of renal damage and may eventually progress to macroalbuminuria, which predicts subsequent decline of GFR.[9] In contrast, the natural history of CKD in type 2 diabetes mellitus appears to be heterogeneous. We[10] and others[11,12] identified a subgroup of people with type 2 diabetes mellitus who had decreased GFR despite normoalbuminuria, whereas this particular renal feature is associated with an increased risk of mortality,[13,14] and its impact on the progression of kidney disease is still unclear. Furthermore, these observations suggest that the pathophysiology and risk factors for albuminuria and those for reduced GFR may be different.
The present prospective study was performed on a large cohort of patients with type 2 diabetes mellitus with preserved renal function and normoalbuminuria at baseline with the aim of looking for predictors of individual components of DKD and their relationship with traditional risk factors.
2. Methods
2.1. Study design, setting, patients, and data sources
In Italy, diabetes care is mainly provided by a public network of about 700 diabetes clinics where a team of specialists provide diagnostic confirmation, prevention, and treatment for diabetes and its complications through close patients’ follow-up and regular check-ups.[10,15,16]
In the present report, we analyzed a large cohort of patients with type 2 diabetes mellitus followed-up at 207 diabetes centers in Italy among those participating in the Italian Association of Clinical Diabetologists (Associazione Medici Diabetologi, AMD) initiative. The analysis was performed using the dataset of electronic medical records collected between January 1, 2004 and June 30, 2008. For the purpose of the analysis, we considered only patients who were aged 18 years or older and with a follow-up evaluation within 48 ± 6 months complete for data about estimated GFR (eGFR) and albuminuria. Of 68,611 patients identified, we excluded those with albuminuria, eGFR <60 mL/min/1.73 m2 or a previous eGFR value discordant (i.e., <60 mL/min/1.73 m2), or those with missing data of antidiabetic treatment. A total of 27029 patients from 118 clinics constitute the study population (see Fig. 1, Supplemental Content). The centers involved in the study include about one-third of all the Italian Centers for Diabetes, homogeneously distributed throughout the country.
2.2. Methods and data collection
As already reported,[10,15,16] the analysis of the database is an attempt by the Italian Association of AMD initiative to identify a set of indicators that can be used in the context of continuous quality improvement. Participating centers adopted the same software systems for everyday management of outpatients, whereas a specially developed software package allowed us to extract the information we intended to analyze from all the clinical databases (AMD Data File). Moreover, data from all participating centers were collected and centrally analyzed anonymously.[10,15,16] In the AMD database, data can be linked together by a unique anonymous identifier that is encrypted to protect patients’ privacy. Because this automated system precludes identification of individual patients, according to the Italian law, ethical committee approval and informed consent were not required. The results were internally approved by the AMD Annals Scientific Committee.
This initiative includes measuring and monitoring HbA1c, blood pressure (BP), low-density lipoprotein cholesterol (LDL-c), total and high-density lipoprotein cholesterol (HDL-c), and triglycerides. The use of specific classes of drugs (insulin, statins, and ≥2 antihypertensive agents) was also evaluated. Since normal ranges for HbA1c varied among centers, the percentage change with respect to the upper normal value (measured value/upper normal limit) was estimated and multiplied by 6.0 to allow comparisons among the centers. Kidney function was assessed by serum creatinine and urinary albumin excretion measurements. GFR was estimated for each patient using a standardized serum creatinine assay and the Chronic Kidney Disease Epidemiology Collaboration formula.[17] Increased urinary albumin excretion was diagnosed and defined as albuminuria if urinary albumin concentration was >30 mg/L or urinary albumin excretion rate was >20 μg/min or urinary albumin-to-creatinine ratio was >2.5 mg/mmol in men and >3.5 mg/mmol in women. At each participating center, all patients underwent physical examination and BP measurements according to a standardized protocol. BP was measured with the patient in the sitting position after a 5-minute rest, with a mercury sphygmomanometer. Systolic BP and diastolic BP were read to the nearest 2 mmHg. Disappearance of Korotkoff sounds (phase V) was the criterion for diastolic BP. Three measurements were taken at 2-minute intervals and the average value was used to define clinical systolic BP and diastolic BP. CKD was defined as diabetes with albuminuria or low eGFR (i.e., <60 mL/min/1.73 m2) or both.
2.3. Outcomes
The primary outcomes were eGFR <60 mL/min/1.73 m2 and normoalbuminuria; albuminuria and eGFR ≥60 mL/min/1.73 m2; and eGFR <60 mL/min/1.73 m2 and albuminuria. Secondary outcomes were eGFR <60 mL/min/1.73 m2 and albuminuria. Occurrence of prespecified endpoints was evaluated on a yearly basis over the 4-year study period. Patients were considered to have reached the study endpoints if, at any time during the study period, they met the above indicated criteria.
We have also evaluated the occurrence of a GFR decline >30% from baseline value.[18]
2.4. Statistical analysis
The data are given as mean value ± standard deviation; categorical variables are described as frequencies and percentages.
Statistical analysis was aimed to investigate factors associated to individual components of DKD. The main analysis was performed using a multinomial logistic regression model considering 4 outcome categories: eGFR ≥60 mL/min/1.73 m2 and normoalbuminuria; eGFR <60 mL/min/1.73 m2 and normoalbuminuria; GFR ≥60 mL/min/1.73 m2 and albuminuria; and eGFR <60 mL/min/1.73 m2 and albuminuria. In comparison to classic logistic regression analysis that evaluates a dependent variable with 2 alternative categories, the multinomial logistic regression estimates in a unique model the RRRs for observing a dependent variable with more categories (4 distinct DKD outcomes) as a function of independent covariates (baseline data).
The relative risk ratios (RRRs) were used to estimate the degree of association between baseline patients’ data and each single outcome considering those without DKD development as the reference category.
A multi-level mixed-effects logistic regression model was used to evaluate single outcome (i.e., eGFR <60 mL/min/1.73 m2 and albuminuria). Data were analyzed considering diabetes clinics as clusters of observations, so that possible differences in data across centers could be considered. RRRs were reported with their 95% confidence interval. The multivariate model was fitted including a missing indicator variable for patients with missing data. A complete-case analysis was performed including patients for which all data were observed. Multivariate analyses were performed adjusting for all baseline clinical characteristics. To derive a hierarchical tree of event risk, a logistic model for renal outcome (separately for eGFR <60 mL/min/1.73 m2 and albuminuria) was used to split the data recursively into subgroups selecting the variable with the minimum P value. Continuous variables were analyzed for all values from the 5th to 95th percentile selecting the best cut-point with the lowest P value. The tree-building process was stopped after 3 iterations to obtain 8 groups. The analyses were made using STATA software, Version 12 (StataCorp, College Station, TX). P values of <0.05 were considered statistically significant.
3. Results
The main clinical features of the study population (n = 27,029) at baseline, as a whole, and grouped by renal outcomes at 4-year follow-up are summarized in Table 1. Overall, the mean age was 64 ± 10 years, 56.4% of patients were males, and the mean duration of diabetes was 10 ± 8 years. Thirty-eight percent of patients were obese (i.e., they had a BMI ≥30 kg/m2). The glycemic, lipid, and BP control of participants was fairly good, being the mean values of HbA1c, LDL-c, and BP of 7.2% (55 mmol/mol), 111 mg/dL, and 139/80 mmHg, respectively. EGFR was 85 ± 13 mL/min/1.73 m2 (Table 1). By study design, all patients had normal urine albumin excretion and eGFR ≥60 mL/min/1.73 m2.
Table 1.
Baseline clinical characteristics by renal outcome.
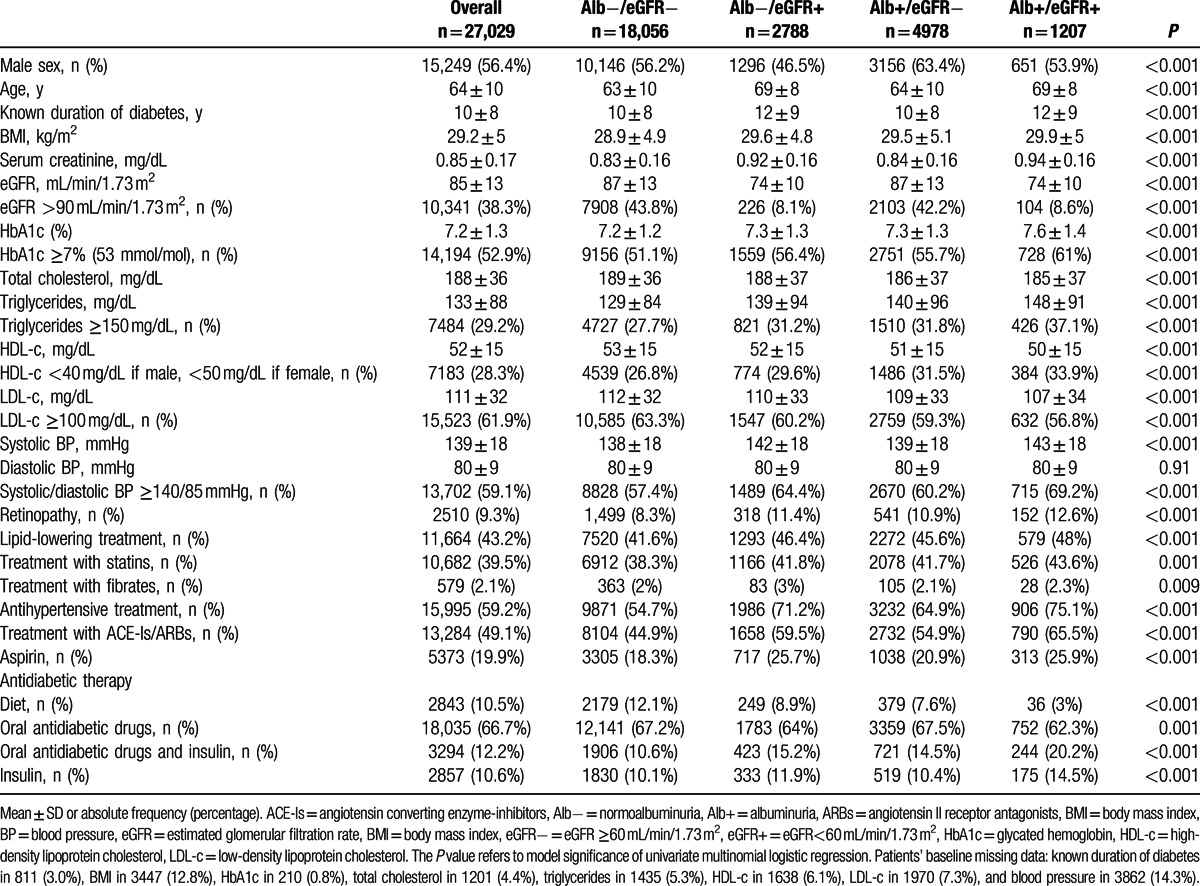
Over a 4-year follow-up period, a total of 33.2% of patients (n = 8973) developed CKD (i.e., eGFR <60 mL/min or albuminuria). According to the main aim of this work, we separately report the clinical features of patients in whom kidney function remained stable (n = 18,056, 66.8%) and of those who developed reduced eGFR alone (i.e., eGFR <60 mL/min/1.73 m2) (n = 2788, 10.3%), albuminuria alone (n = 4978, 18.4%) or both, reduced eGFR, and albuminuria (n = 1207, 4.5%) over a 4-year follow-up. Patients who developed reduced eGFR in the presence of normoalbuminuria represent 70% of the whole population who developed low eGFR (n = 3995) and 13% of those who remained normoalbuminuric at follow-up (n = 20,844). As compared to those whose kidney function remained stable at follow-up, they were more likely to be women and, on the average, older, with a longer duration of disease and a poor glycemic control. As expected, they had also a lower eGFR at baseline (74 vs. 87 mL/min/1.73 m2, patients who developed low eGFR and patients who remained with stable eGFR, respectively). Reduced eGFR at follow-up was associated also with a more atherogenic lipid profile (i.e., higher triglycerides and lower HDL-c) and higher systolic BP, the latter despite a parallel trend toward greater prevalence and intensity (i.e., number of drugs, data not shown) of antihypertensive treatment (Table 1).
Patients who developed albuminuria in presence of eGFR ≥60 mL/min/1.73 m2 (n = 4978) are 80% of the whole population who developed albuminuria (n = 6185) and 21.6% of those who remained with eGFR ≥60 mL/min/1.73 m2 at follow-up (n = 23,034). As compared to patients whose kidney function remained stable at follow-up, those who developed albuminuria were prevalently males and show a worse glycemic control. They clearly have a more atherogenic lipid profile. Systolic BP is only slightly increased in patients developing albuminuria, despite a parallel trend toward greater prevalence and intensity (i.e., number of drugs, data not shown) of antihypertensive treatment (Table 1).
Patients who developed both low eGFR and albuminuria (n = 1207) showed a worse cardiovascular risk factors profile when compared to patients who did not develop renal abnormalities. They were prevalently males and older, had a longer duration of disease, poor glycemic control, lower eGFR, more atherogenic lipid profile, and higher systolic BP values. Furthermore, a greater number of patients taking antihypertensive, lipid-lowering, and hypoglycemic drugs was also evident among these patients.
In the Table 4, Supplemental Content, we have reported the baseline clinical features of 2175 (8.1%) patients who develop a reduction of eGFR >30% from baseline value.
The relationship between the onset of DKD traits and traditional cardiovascular risk factors was further investigated by multivariate logistic analysis (Table 2, Fig. 1). Age, but not duration of disease, independently affects both features of CKD, with a 5-year increase in risk of 37% for the development of low eGFR and of 7.5% for albuminuria, respectively. Being female clearly represents an independent risk factor for developing normoalbuminuric renal impairment (i.e., isolated reduced eGFR), whereas males are at increased risk for developing albuminuria.
Table 2.
Multivariate relative-risk ratios for renal outcomes.
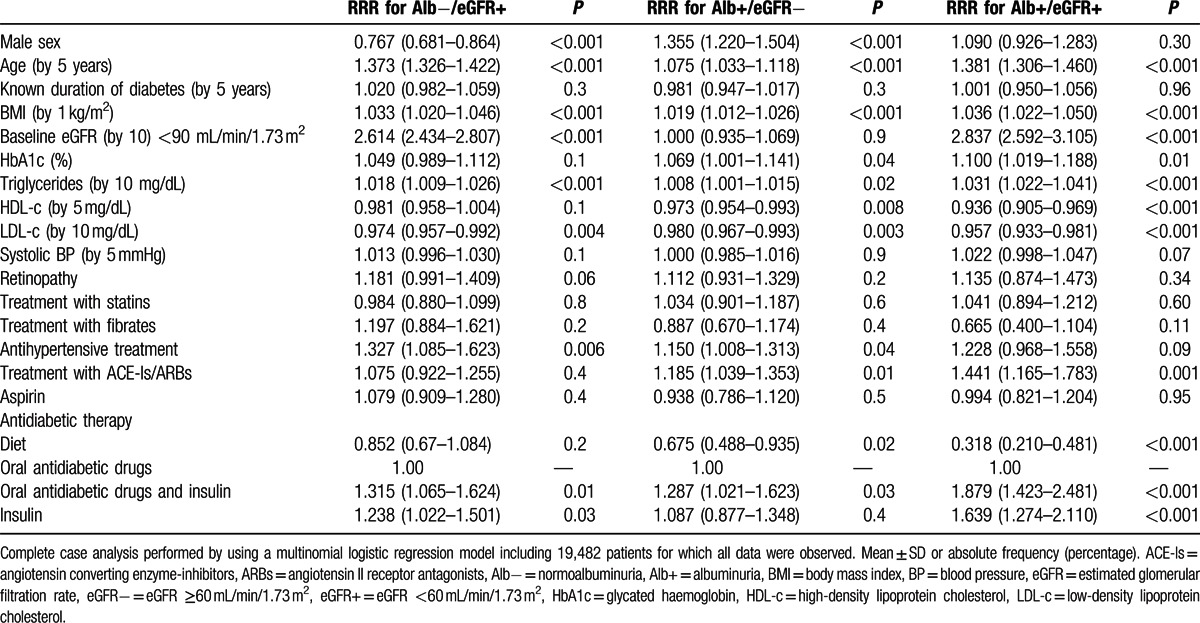
Figure 1.
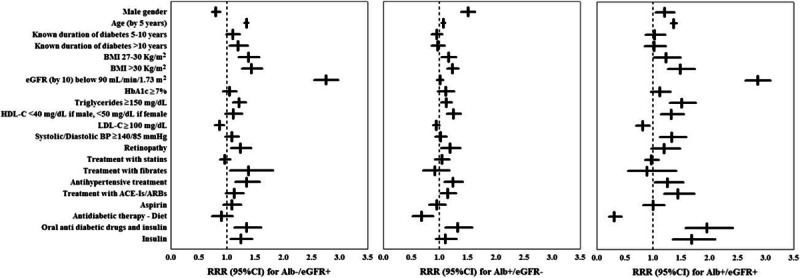
Multivariate relative risk ratios (RRRs) with their 95% confidence intervals (95% CIs) to develop estimated glomerular filtration rate <60 mL/min/1.73 m2 (Alb−/eGFR+) = albuminuria (Alb+/eGFR−) = or both (Alb+/eGFR+). Antidiabetic therapy was analyzed by using oral antidiabetic drugs as reference category. Analysis performed by using a multinomial logistic regression model with the missing indicator method for each incomplete variable.
Worse glycemic control also affected the onset of albuminuria with an increased risk of 7% for every 1% increase in HbA1c, while it would appear not to have any clinical relevance with regard to the risk of low eGFR. Higher BP levels predict the onset of albuminuria and low eGFR with albuminuria; in fact patients with uncontrolled BP (i.e., systolic/diastolic BP ≥140/85 mmHg) have a 33% increased risk of simultaneously developing both kidney dysfunctions traits (i.e., low eGFR and albuminuria) (Table 2).
DKD onset was also predicted by the typical atherogenic lipid profile. In fact, high levels of triglycerides were directly associated with an increased probability to develop reduced eGFR and/or albuminuria, whereas HDL-c levels inversely affected the risk of developing albuminuria (Table 2, Fig. 2).
Figure 2.
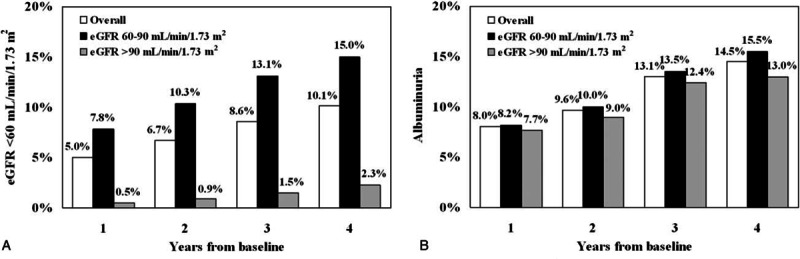
Proportion by year of patients with estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (panel A) and albuminuria (panel B) at the 4 post-baseline assessments. Number of evaluations were 27,029, 16,235, 17,591, 8019, and 27,029 at baseline, 1, 1, 3, and 4 year, respectively.
We also looked at patients who developed low eGFR or albuminuria, independently of each other. The cumulative incidence of low eGFR (i.e., eGFR <60 mL/min/1.73 m2) in the whole population or divided according to eGFR values >90 or <90 and >60 mL/min/1.73 m2 is reported in Fig. 2, panel A. As expected, the incidence of low eGFR increased progressively over the 4-year follow-up period and was higher among patients with a lower eGFR at baseline (i.e., 60–90 mL/min/1.73 m2). The cumulative incidence of albuminuria also showed a progressive increase during follow-up (Fig. 2, panel B).
Finally, by applying a tree analysis model, we identified 8 patient subgroups at different risk for developing eGFR <60 mL/min/1.73 m2 with the strongest variable in differentiating the risk being baseline eGFR value. In fact, patients with baseline eGFR ≥75 mL/min/1.73 m2, associated with age <65 years and male sex, showed the lowest incidence of eGFR <60 mL/min/1.73 m2. If this is considered as the reference class (class 8), the incidence of low eGFR progressively increases from class 8 to class 1, whose patients are more often females, have a lower eGFR and higher HbA1c values at baseline, and are prescribed more antihypertensive therapy (see Fig. 2 and Table 5, Supplemental Content).
As for albuminuria, 8 different subgroups were identified by tree analysis model. The strongest variable in differentiating the risk of developing albuminuria was HDL-c values. In detail, patients with HDL-c ≥50 mg/dL, age <69 years, and HbA1c <7.5% (58 mmol/mol) had the lowest incident rate of albuminuria (17.3%). If this is considered the reference class, the incidence of albuminuria progressively increases from class 8 to class 1 (44.1%), whose patients have lower HDL-c and eGFR values at baseline and are more often taking insulin therapy. In addition, a greater number of these latter patients are males, have higher triglycerides levels, and are taking antihypertensive therapy (see Fig. 3 and Table 6, Supplemental Content).
4. Discussion
Our study shows that in a real-life clinical setting, up to 33.2% of patients with type 2 diabetes mellitus develop CKD over a 4-year follow-up period. Overall, the incidence of renal outcome reported here is in line with what has been previously described[5,19–22] in patients with similar disease duration. The incidence of DKD we report here is similar to what has been previously described[23] and significantly higher than the figure recently reported in the general population,[18,24] making diabetes mellitus in its own right a tremendous risk factor for CKD.
We found a relatively greater incidence of renal events during the first study year, owing to disease progression in the subset of patients with GFR values only slightly above 60 mL/min/1.73 m2 at baseline. Subsequently, the average yearly rate of progression was 0.5% to 1.0% for GFR reduction and 1% to 2% for albuminuria onset, independently of baseline GFR. The study cohort, which had a mean GFR value at baseline of 85 mL/min/1.73 m2, showed a yearly decrement of 1 mL/min/1.73 m2 (P < 0.001). More patients developed albuminuria (18%) rather than renal impairment (10% eGFR <60 mL/min/1.73 m2), whereas a minority (i.e., 5%) developed both features of CKD. A total of 8.1% of study patients reached the secondary endpoint of a GFR reduction >30% compared to baseline. However, the vast majority of these patients (i.e., 81%) also reached the primary endpoint of an eGFR <60 mL/min/1.73 m2. Determinants of GFR reduction in this subgroup were similar to those identified for the primary endpoints. The considerable discordance we found in the incidence of these 2 renal outcomes is in line with previously published longitudinal[19–22,25] and cross-sectional studies[26,27] and adds to the concept that renal involvement is a rather heterogeneous condition in type 2 diabetes mellitus, and therefore differs significantly from the traditional clinical paradigm observed in type 1 diabetes mellitus. Whereas albuminuria and renal impairment shared a number of risk factors, such as age, BMI, lipid profile, and the amount of antihypertensive and glucose-lowering treatment, there also was a distinct set of variables, which predicted one but not the other. In fact, reduction of GFR below 60 mL/min/1.73 m2 was more likely to be associated with female sex and triglycerides levels, while albuminuria was more frequently observed in men and in patients with higher HbA1c levels (and low HDL-c). These results support the concept that albuminuria and renal impairment may not necessarily reflect the same underlying pathophysiological mechanism in type 2 diabetes mellitus.
Owing to the stringent selection criteria of the study, our results cannot provide information on the potential role of baseline albuminuria on the loss of GFR overtime. However, by looking at a rather homogenous cohort of patients who, after a mean 10-year disease duration, presented no sign of renal involvement at baseline, we could better investigate the role of hypertension, lipid profile, and hyperglycemia as renal disease promoters. In fact, the majority of patients with diabetes-unrelated causes of renal damage were likely excluded from our study cohort on the basis of selection criteria.
As most of our study patients were already under hypertensive treatment at baseline, the lack of a relationship between BP and renal outcomes is not surprising. We found, however, a strong relationship between antihypertensive treatment, especially the use of renin-angiotensin system inhibiting drugs, and renal outcome (Table 2 and Fig. 1), probably because of indication bias. Indirectly, we take this as a sign of the unfavorable influence of the severity of hypertension on renal outcome.
As for the role of glycometabolic control, we found that HbA1c was related to albuminuria but not to the development of low GFR. This is partly at variance with what has been previously reported[22,25] but in line with data from UKPDS[20] and other more recent studies.[28,29] Such discrepancies may be attributable to differences in the inclusion criteria or ethnicity, as well as possible differences in definitions used for outcomes. In addition, one should bear in mind that our cohort had, on average, a relatively good glycometabolic control, with HbA1c values well below 7% (53 mmol/mol) in >50% of cases.
A greater incidence of renal endpoints was positively associated with triglycerides levels and negatively with HDL-c. The predictive power of these lipid abnormalities, traditionally considered a surrogate for the insulin resistance state, together with the observed relationship between BMI and subsequent development of albuminuria, renal impairment or both, supports a role for the metabolic syndrome and insulin resistance in the progression of renal damage.[30,31] Accordingly, Penno et al have, very recently, confirmed in a cross-sectional study the independent association between hypertriglyceridemia and CKD among patients with type 2 diabetes mellitus.[32]
The tree analysis allowed us to investigate also the interaction between several clinical variables and their hierarchical impact on the incidence of reduced eGFR or albuminuria. The results of this analysis show that patients with lower GFR values, older age, and longer duration of diabetes have a 49% risk of developing eGFR <60 mL/min/1.73 m2. Moreover, features of the metabolic syndrome, mainly low HDL-c levels, and high triglycerides entail a 4-fold greater risk of developing albuminuria.
Our study has some limitations as well as several strengths that should be mentioned. Among the first, we must acknowledge that laboratory parameters, including serum creatinine, were not measured in a single centralized laboratory and this may have led to some variability, especially in GFR estimation. In addition, we have information on albuminuria only as a categorical trait and this, together with some heterogeneity in the techniques used to measure urinary albumin concentration in different laboratories, may have contributed to variability in the outcome measure. Moreover, data regarding the entire 4-year follow-up period were available for most but not all patients and therefore caution should be taken not to generalize our findings, as mortality from competitive risk was not positively collected in the missing subgroup. Baseline clinical features of the subgroup with missing values, however, were similar to that of the entire cohort. Furthermore, even when less stringent selection criteria were applied to our study cohort, our main results remained unchanged. Finally, our data may not be applicable to the population of patients with type 2 diabetes mellitus at large, as the vast majority of participants were of white origin and ethnicity has previously been shown to bear some impact on the risk of developing renal complications.[33] However, we should mention the large size of the study cohort and the homogeneous geographical distribution of the recruiting centers as well as the relatively long follow-up period, which certainly contribute in making the study cohort a good representation of real-life clinical practice.
In conclusion, our 4-year prospective study of a large, real-life cohort of patients with type 2 diabetes mellitus with normal renal function at baseline showed a 33.2% cumulative incidence of CKD, with nearly 23% of patients developing albuminuria and about 15% renal impairment. Albuminuria and reduction in GFR show, at least in part, distinct sets of risk factors suggesting that different pathophysiological mechanisms are involved in the development of these renal outcomes.
Supplementary Material
Footnotes
Abbreviations: AMD = Associazione Medici Diabetologi, BP = blood pressure, CKD = chronic kidney disease, DKD = diabetic kidney disease, eGFR = estimated glomerular filtration rate, HDL-c = total and high density lipoprotein cholesterol, LDL-c = low-density lipoprotein cholesterol.
Authorship: SDC and RP designed the study, analyzed and interpreted data, and wrote the manuscript; FV and AP analyzed data, wrote the manuscript, and contributed to discussion; AC, SG, and CG analyzed data and reviewed the manuscript; PG analyzed data and contributed to discussion; GR, MCR, and AN contributed to discussion and reviewed the manuscript. All the authors approved the final version for publication. SDC is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- 1.IDF. Diabetes Atlas. 6th ed.2013; Brussels, Belgium: International Diabetes Federation, http://www.idf.org/diabetesatlashttp://www.idf.org/diabetesatlas. Accessed July 16, 2015. [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103:137–149. [DOI] [PubMed] [Google Scholar]
- 3.US Renal Data System. Renal Data US System USRDS 2014 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: US Renal Data System; 2014. [Google Scholar]
- 4.National Kidney Foundation. KDOQI clinical practice guidelines for Diabetes and CKD: 2012 update. Am J Kidney Dis 2012; 60:850–886. [DOI] [PubMed] [Google Scholar]
- 5.De Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305:2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015; 3:514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012; 380:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 9.Krolewski AS, Warram JH, Christlieb AR, et al. The changing natural history of nephropathy in type I diabetes. Am J Med 1985; 78:785–794. [DOI] [PubMed] [Google Scholar]
- 10.De Cosmo S, Rossi MC, Pellegrini F, et al. Kidney dysfunction and related cardiovascular risk factors among patients with type 2 diabetes. Nephrol Dial Transplant 2014; 29:657–662. [DOI] [PubMed] [Google Scholar]
- 11.Thomas MC, MacIsaac RJ, Jerums G, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (National Evalution of the Frequency of Renal Impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 2009; 32:1497–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwyer JP, Parving HH, Hunsicker LG, et al. Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: results from the DEMAND study. Cardiorenal Med 2012; 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Cosmo S, Lamacchia O, Pacilli A, et al. Normoalbuminuric renal impairment and all-cause mortality in type 2 diabetes mellitus. Acta Diabetol 2014; 51:687–689. [DOI] [PubMed] [Google Scholar]
- 14.Fox CS, Matsushita K, Wooward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012; 380:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolucci A, Rossi MC, Arcangeli A, et al. Four-year impact of a continuous quality improvement effort implemented by a network of diabetes outpatient clinics: the AMD-Annals initiative. Diabet Med 2010; 27:1041–1048. [DOI] [PubMed] [Google Scholar]
- 16.De Cosmo S, Viazzi F, Pacilli A, et al. Serum uric acid and risk of chronic kidney disease in type 2 diabetes: the AMD Annals initiative. CJASN 2015. CJN.03140315. [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311:2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler AI, Stevens RJ, Manley SE, et al. on behalf of the UKPDS GROUP Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63:225–232. [DOI] [PubMed] [Google Scholar]
- 20.Retnakaran R, Cull CA, Thorne KI, et al. UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006; 55:1832–1839. [DOI] [PubMed] [Google Scholar]
- 21.Bruno G, Merletti F, Biggeri A, et al. Progression to overt nephropathy in type 2 diabetes: the Casale Monferrato Study. Diabetes Care 2003; 26:2150–2155. [DOI] [PubMed] [Google Scholar]
- 22.Rossing K, Christensen PK, Hovind P, et al. Progression of nephropathy in type 2 diabetic patients. Kidney Int 2004; 66:1596–1605. [DOI] [PubMed] [Google Scholar]
- 23.USRDS Annual report 2015. Available at: http://www.usrds.org/adr.aspx (accessed May 22, 2016). [Google Scholar]
- 24.Shastri S, Katz R, Shlipak MG, et al. Cystatin C and albuminuria as risk factors for development of CKD stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2011; 57:832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoppini G, Targher G, Chonchol M, et al. Predictors of Estimated GFR Decline in Patients with Type 2 Diabetes and Preserved Kidney Function. Clin J Am Soc Nephrol 2012; 7:401–408. [DOI] [PubMed] [Google Scholar]
- 26.Parving HH, Lewis JB, Ravid M, et al. DEMAND Investigators.. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006; 69:2057–2063. [DOI] [PubMed] [Google Scholar]
- 27.Rossi MC, Nicolucci A, Pellegrini F, et al. Identifying patients with type 2 diabetes at high risk of microalbuminuria: results of the DEMAND (Developing Education on Microalbuminuria for Awareness of reNal and cardiovascular risk in Diabetes) Study. Nephrol Dial Transplant 2008; 23:1278–1284. [DOI] [PubMed] [Google Scholar]
- 28.Takagi M, Babazono T, Uchigata Y. Differences in risk factors for the onset of albuminuria and decrease in glomerular filtration rate in people with type 2 diabetes mellitus: implications for the pathogenesis of diabetic kidney disease. Diabet Med 2015; 32:1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penno G, Solini A, Bonora E, et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 2011; 29:1802–1809. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med 2004; 140:167–174. [DOI] [PubMed] [Google Scholar]
- 31.De Cosmo S, Menzaghi C, Prudente S, et al. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant 2013; 28:29–36. [DOI] [PubMed] [Google Scholar]
- 32.Penno G, Solini A, Zoppini G, et al. Hypertriglyceridemia is independently associated with renal, but not retinal complications in subjects with type 2 diabetes: a cross-sectional analysis of the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicenter Study. PLoS One 2015; 10:e0125512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhalla V, Zhao B, Azar KM, et al. Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care 2013; 36:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


