Supplemental Digital Content is available in the text
Keywords: antidiabetic medication, diabetes type 2, dipeptidyl peptidase-4 inhibitor, insulin, metformin, National Health Insurance, sulfonylurea
Abstract
This study investigated trends in the prescription of antidiabetic medications for patients with type 2 diabetes, focusing on changing patterns of prescriptions and the cost of drugs during the last 10 years. Retrospective data on patients with type 2 diabetes aged 30 years or older were analyzed using information from the National Health Information Database collected by the National Health Insurance Service in Korea from January 2002 to December 2013. We identified patients with type 2 diabetes who had at least one service claim in each year during the study period. The prescribing information was collected and fixed-dose combination tablets were counted as each of their constituent classes. The total number of adults with type 2 diabetes who were treated using antidiabetic agents increased from 0.87 million in 2002 to 2.72 million in 2013 in Korea. Among antidiabetic medications in 2002, sulfonylurea (SU) was the most commonly used agent (87.2%), and metformin was the second (52.9%). However, in 2013, the use of metformin increased to 80.4% of the total antidiabetic prescriptions. The use of dipeptidyl peptidase-4 (DPP-4) inhibitor increased remarkably after release in late 2008 and composed one-third of the market share with 1 million prescriptions (38.4%) in 2013. Among the prescriptions for monotherapy, only 13.0% were metformin in 2002, but the amount increased to 53.2% by 2013. In contrast, the use of SU declined dramatically from 75.2% in 2002 to 30.6% in 2013. Dual and triple combinations steadily increased from 35.0% and 6.6% in 2002 to 44.9% and 15.5% in 2013, respectively. In 2013, SU with metformin (41.7%) and metformin with DPP-4 inhibitor (32.5%) combination were most frequently prescribed. The total antidiabetic medication cost increased explosively from U.S. $70 million (82.5 billion won) in 2002 to U.S. $4 billion (480 billion won) in 2013.
The use of antidiabetic agents and their costs have been increasing steadily. Metformin is the most commonly used drug recently. The use of DPP-4 inhibitor increased significantly over the past decade, whereas the use of SU decreased. However, SUs still remain the most commonly prescribed second-line agents with metformin in 2013.
1. Introduction
According to the recently published report on the Korea National Health and Nutritional Examination survey (KNHANES) 2011, four million Koreans aged 30 years or older have diabetes, and the prevalence of diabetes and prediabetes were 12.4% and 38.3%, respectively.[1] This remarkably increased number of patients with diabetes is inevitably accompanied by diabetic vascular complications and diabetes-related morbidity and mortality, leading to an enormous economic burden.[2] In the U.S. population, the rates of diabetes-related complications have declined steadily in the past two decades, but a large burden of the disease, such as acute myocardial infarction, stroke, end-stage renal disease, or lower-extremity amputation, persists because of the continued increase in the prevalence of diabetes.[3] In 2010, diabetes was ranked as the fifth cause of death in Korea and the most common cause of renal replacement therapy.[4,5]
Many large epidemiological studies have shown that maintenance of glucose levels within the target range is the only viable alternative for the prevention of these diabetic complications.[6] With this background, clinical practice guidelines from many organizations including the Korean Diabetes Association (KDA) recommend that HbA1c can be used as a marker of glycemic control status, and should be maintained below 6.5% or 7.0%.[7–10] To achieve this goal, various oral antidiabetic agents and insulin should be actively used, matching the individual's situation. However, the data from KNHANES V (2010–2012) demonstrated that only 27% reached the HbA1c goal of 6.5%, and only 45.6% achieved the goal even if it was set at 7.0%.[11] Considering the importance of type 2 diabetes as a national health care issue, the accurate estimation of the use of antidiabetic medication, prescription patterns, and pharmacy expenditures would be valuable information for the establishment of health policies. Of note, this analysis would not available if a nationwide health information database did not exist.
In Korea, the national health care program named National Health Insurance Service (NHIS) covers the entire Korean population as a social insurance benefits scheme. Since February 1999, all medical insurance societies were integrated into a single insurer; that is, the National Health Insurance Corporation, in 2000.[12] Based on the NHI program, the computerized database contains all of the claim data, including drug prescriptions, diagnostic codes for the coding system of disease, the International Classification of Disease (ICD), and claimed treatment details.[12,13]
There have been rapid and revolutionary changes in the marketplace for antidiabetic medications since 2000. Around 2008, one of the thiazolidinediones (TZDs), that is, rosiglitazone, received an FDA warning of increased cardiovascular risk, and long-acting insulin or insulin analogs and incretin-based treatment [glucagon-like peptide 1 (GLP-1) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors] have been introduced sequentially in Korea. In addition, KDA published and updated clinical practice guidelines regularly in 2007, 2011, and 2013.[9] With this trend, insurance benefit policies for antidiabetic medications in subjects with type 2 diabetes have been changed during the last decade. The introduction of new classes of antidiabetic agents and dissemination of clinical recommendations for diabetes care changed the trend of market share comprised of sulfonylurea (SU) and metformin during the last 7 years.[14–16]
The aim of this study was to investigate trends in the prescription of antidiabetic agents in adult patients with type 2 diabetes and medication costs of antidiabetic drugs from 2002 to 2013 using the national health insurance claim database maintained by NHIS in Korea.
2. Materials and methods
2.1. Source of database
In this study, we used the National Health Information database maintained by the Korean NHIS, a government-affiliated agency under the Korean Ministry of Health and Welfare that supervises all medical services in Korea. Retrospective data for adult patients with type 2 diabetes aged 30 years or older were extracted from January 2002 through December 2013 using the Korean NHIS database. The database consists of four categories, such as general information on specifications, consultation statements, diagnosis statements classified by the ICD 10th revision (ICD-10), and detailed information about prescriptions.[12] Among all of the beneficiaries of NHI, the source population consisted of patients over the age of 30 years who visited clinics or hospitals with a diagnostic code (ICD-10) of type 2 diabetes (E11-E14) more than once in a given year from January 2002 to December 2013. Type 1 diabetes, gestational diabetes, and subjects with missing data were excluded.
The NHIS contains information on the patients’ demographics, medical use/transaction information, and deduction and claim database, as well as the insurers’ payment coverage.[17] We analyzed the information for each individual with an unidentifiable code including age, gender, diagnosis, prescribed drugs, and pharmacy expenditures. In Korea, patients who visit clinics or hospitals for medical care are issued prescriptions, and then they visit a pharmacy to get medication. Ambulatory visits and any reason for hospitalizations were included in this analysis if the patients had a disease code of type 2 diabetes.
To validate the database and screen for information accuracy, the expert committee from the KDA reviewed the database regularly during this analysis. The committee decided the suitability of the dataset and results of the analysis. This study was approved by the institutional review board of the Korean National Institute for Bioethics Policy (P01–201504–21–005). Informed consent was exempt by the board.
2.2. Definition of diabetes and anti-diabetic medications
A diagnosis of type 2 diabetes based on ICD-10 codes included principal diagnosis and up to four additional accompanying diagnoses, in order of clinical significance in the current condition. Patients were classified as having type 2 diabetes when they had at least one service claim with a diagnosis of type 2 diabetes, either in outpatient or inpatient care, and were prescribed at least one antidiabetic drug anytime in a given year to exclude prediabetes or non-diabetic subjects.
Antidiabetic drugs dispensed in the pharmacy during the study period in Korea consisted of seven classes (i.e., SU, biguanide, alpha-glucosidase inhibitor, TZD, DPP-4 inhibitors, meglitinide, and insulin; Supplement Table S1). The GLP-1 agonist was introduced in Korea by the end of 2008; however, health insurance coverage was available only in November 2010 with strict conditions. Therefore, the GLP-1 agonist was not included in this analysis. We obtained the annual number of prescriptions for all antidiabetic drug classes, including insulin, during the study period.
For information on prescriptions, the name of the drug, date prescribed, days of supply, quantity dispensed, and price of each tablet or injection were collected. Insulin was not classified as intermediate-, short-, or long-acting forms, but was counted as one class of antidiabetic medication. If the patients took more than two different classes of antidiabetic drugs, either as a fixed-dose combination or different pills, they were defined as receiving combination therapy.[15,16] We classified the medication as monotherapy, dual therapy, and triple therapy.
To investigate drug adherence, the medication possession ratio (MPR) was used and defined as a cumulative medication adherence of more than 80% (292 days) per year among patients with type 2 diabetes prescribed in a given year and included data only on antidiabetic drug prescriptions dispensed from pharmacies.[18,19] For evaluation of medication costs, we used the database of pharmacy claims, and confined them to the cost of antidiabetic drugs.
2.3. Statistical analysis
Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). We used descriptive statistics to investigate trends in the total number and cost of antidiabetic drugs. Data were expressed as numbers and as a frequency percentage (%). We used the generalized linear model to test for linear trends of each prescription rate over time after adjustment for sex and age. P < 0.05 was considered significant.
3. Results
3.1. Changes in prescription patterns of antidiabetic medications
The number of adult type 2 diabetic patients treated with antidiabetic agents increased from 0.87 million in 2002 to 2.72 million in 2013 (Table 1). This corresponded to 3.3% and 8.03% of all of the beneficiaries of NHI in 2002 and 2013, respectively. The proportion of men among type 2 diabetes was 50.3% in 2002 and 53.9% in 2013. Also, the proportion of patients aged 70 years and older among total type 2 diabetic patients increased from 17.7% in 2002 to 32.5% in 2013 (Table 1).
Table 1.
The number of adult type 2 diabetic patients treated with antidiabetic agents.

The use of different types of antidiabetic medications among people with diagnosed type 2 diabetes is shown in Fig. 1. Among 0.87 million patients with type 2 diabetes in 2002, SU was the most commonly used agent (87.2%), and metformin held the second rank (52.9%). At that time, alpha-glucosidase inhibitors (a-GI) were used in 24.3% of total antidiabetic prescriptions.
Figure 1.
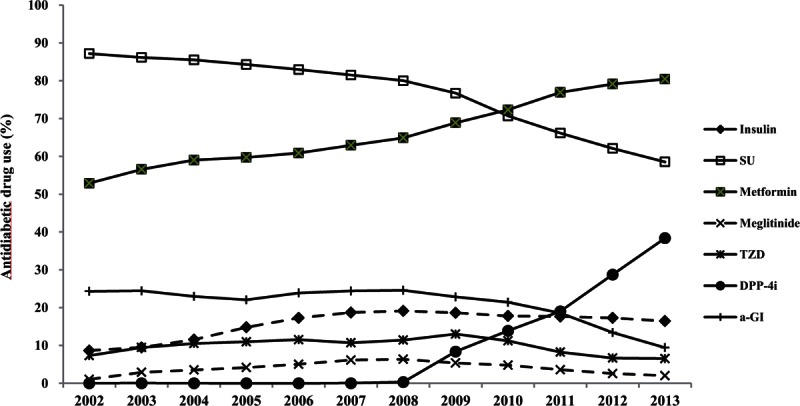
The use of antidiabetic medications among people with diagnosed type 2 diabetes. SU = sulfonylurea, TZD = thiazolidinedione, DPP4i = Dipeptidyl peptidase-4 inhibitor, a-GI = alpha-glucosidase inhibitor.
During the past decade, the use of metformin increased by 52.9%–80.4% of total antidiabetic drug prescriptions in 2013, and became the most frequently prescribed glucose-lowering agent in Korea in 2013. However, the use of SU steadily decreased from 87.2% in 2002 to 58.5% in 2013. Before 2010, SU was the most commonly used drug for diabetic patients, but metformin overtook SU in 2010 (P for trend < 0.0001, Fig. 1, Supplementary Table S2).
Remarkably, the use of a DPP-4 inhibitor was characterized by a steep increase from 2008. The DPP-4 inhibitors were introduced at the end of 2008, then increased dramatically since 2009, and composed one-third of market share, with 1 million prescriptions (38.4%) in 2013 in Korea. The TZD market increased from 7.3% in 2002 to 13.0% in 2009, but it decreased to 6.5% in 2013, to the level in 2002. Regarding insulin, its prescription increased to 19.1% of all the patients with type 2 diabetes in 2008 and then was maintained as a constant over time.
Among prescriptions confined to monotherapy, metformin made up only 11.7% of the total prescriptions in 2002, but this value increased strikingly to 56.4% in 2013. In contrast, SU use steadily declined from 76.7% in 2002 to 28.3% in 2013 as monotherapy (P for trend < 0.0001, Fig. 2, Supplementary Table S3). SU comprised 75.2% of antidiabetic drug use in 2002 and 30.6% in 2013, whereas insulin comprised 6.5 % in 2002 and 10.8% in 2013 as monotherapy. Other classes, including a-GI, TZD, and meglitinide, were used in just 5.3% of the cases in 2002 and 5.4% in 2013 as monotherapy.
Figure 2.

Changing pattern of monotherapy prescription. a-GI = alpha-glucosidase inhibitor, DPP-4i = dipeptidyl peptidase-4 inhibitors, SU = Sulfonylurea, TZD = Thiazolidinediones.
3.2. Dual and triple combination therapy
Dual and triple combination therapy steadily increased from 35.0% and 6.6% in 2002 to 44.9% and 15.5% in 2013, respectively (P for trend < 0.0001, Fig. 3). In 2002, SU with metformin was the most frequently used dual therapy combination (68.7%). Next, SU with a-GI accounted for 17.4% of all dual combination prescriptions. In 2013, SU with metformin (41.7%) and metformin with DPP-4 inhibitors (32.5%) dual combination therapies were most frequently prescribed. In addition, 6.3% of the total prescriptions for dual therapy accounted for insulin combinations (Table 2). Confined to dual therapy containing metformin, 49.7% were used with SU and 38.8% were concomitant use with DPP-4 inhibitors in 2013. However, in 2002, 92.5% were used with SU, and 3.6% were concomitant use with a-GI.
Figure 3.

Changes pattern of dual and triple combination therapy.
Table 2.
Dual combination therapy in 2002 and 2013.

3.3. Trends of medication adherence and medication costs
The dispensing rate of prescriptions of antidiabetic agents also dramatically increased during the last decade. In 2002, only 24.1% of prescriptions had been dispensed at a pharmacy, however, 67.2% of prescriptions for patients with type 2 diabetes were filled in 2013. The MPR was only 0.45 in 2013 (P for trend < 0.0001, Fig. 4).
Figure 4.

Dispensing rate and medication possession ratio. Dispensing rate = (Number of claims dispensed from pharmacy)/(Number of prescription claims) × 100. Medication possession ratio (MPR) = (Numbers of days of medication dispended within the refill interval)/(Numbers of days in refill interval).
Total antidiabetic pharmacy expenditures explosively increased from U.S. $71.1 million (83 billion won) in 2002 to U.S. $417.6 million (582 billion won) in 2013, corresponding to an increase of 5.8-fold (Table 3). In particular, DPP-4 inhibitors were ranked third in terms of prescription frequency; however, their medication cost ranked top in 2013 (U.S. $175.1 million, 239 billion won), occupying 34.6% of the total antidiabetic medication costs. Metformin (U.S. $140.4 million, 192 billion won) and SU (U.S. $99.2 million, 135 billion won) followed next in order (Table 3). A mean payment per patient per year for antidiabetic medication was U.S. $82.0 in 2002 and U.S. $153.5 in 2013.
Table 3.
Trends in medication costs, 2002–2013.

4. Discussion
In this analysis of a national database of health insurance claims in Korea, we found substantial and significant changes in the prescription patterns for type 2 diabetes during the past decade. According to increases in the number of patients with type 2 diabetes, use of antidiabetic agents and their costs have been increasing dramatically. Metformin has been the most commonly used antidiabetic drug recently (80.4% in 2013). The use of DPP4 inhibitors increased significantly during the last 5 years, while the use of SU has decreased. However, SUs remained the most commonly prescribed class of second-line agents.
The trend of gradual and steady growth in metformin prescriptions is not confined only to Korea. Usage of metformin increased 34.8% in 1999 to 53.8% in 2010 in NHANES, and metformin was the most common medication prescribed for diabetes in 2003–2010 in the United States.[20] In addition, a nationally representative audit of ambulatory physician practice in the United States, metformin use increased from 23% in 1997 to 53% in 2012.[15] Our data also agreed with this trend. This seems to be the effect of clinical practice guidelines to use metformin as a first-line therapy.[7,8] Metformin is recommended as first-line therapy due to its efficacy, effects in weight reduction or cardiovascular mortality, cost effectiveness, and low risk of hypoglycemia.[21,22]
Clinical practice guideline for type 2 diabetes of KDA recommended that any class of antidiabetic drugs can be used in newly diagnosed diabetes as initial treatment after lifestyle modifications.[9,23] However, the KDA updated the guideline in 2015 and changed the algorithm for metformin as a first-line therapy. Though it needs to be observed, we suggest that metformin use would increase more with the release of Clinical Practice Guideline 2015 in the future.
Starting with sitagliptin, DPP-4 inhibitors were introduced in Korea in late 2008 and have grown in use steeply during the last five years. Compared to the prescription events in 2009, it increased more than 4-fold in 2013 (38.4% of total antidiabetic prescription). This contributed one-third of the total market share for antidiabetic medication; however, total medication costs of DPP-4 inhibitors ranked at the top in 2013 (U.S. $175 million). This increase was more prominent than the market share in the United States, which was 21% of treatment visits by 2012.[15] Accordingly, a decreased trend for SU is prominent among antidiabetic medication use, and the portion of SU seems to have been replaced by DPP4 inhibitors. Conversely, the TZD prescription rate was strongly associated with the publication and FDA warning of increased cardiovascular risks with rosiglitazone.[15] TZD use increased from 7.3% in 2002 to 13.0% in 2009, but its use decreased to the level of the prescription rate of 2002 at 2013.
Due to the appearance of long-acting insulin by early 2005 and emphasis on strict glycemic control, insulin use increased about 2-fold during the last decade (from 8.6% in 2002 to 16.4% in 2013). This proportion of people treated with insulin was somewhat different between Korea and America. In the United States, insulin use accounted for 26% of ambulatory treatment visits for type 2 diabetes in 2012.[15,16] Remarkably, 60.4% of the patients with type 2 diabetes are treated with more than two classes of antidiabetic agents. This might contribute to the growth of fixed-dose combination products. Fixed-dose combination products were first introduced in 2003 in Korea, and their use has been increasing gradually. In the United States, 15% of treatment visits in 2004 and 13% of treatment visits in 2007 were associated with oral combination products, which were predominantly combinations of metformin with either sitagliptin or glyburide.[16] A considerable number of patients with type 2 diabetes needs more than 1 class of antidiabetic drugs to achieve glycemic goal and the beneficial effects on drug compliance or cost effectiveness, the use of a fixed-dose combination is recommended.[24–26]
The health costs for diabetes care are enormous. According to the Centers for Disease Control and Prevention, the estimated total (direct and indirect) cost of diabetes in the United States has increased from U.S. $174 billion in 2007 to U.S. $245 billion in 2012, and among them, spending on antidiabetic medications accounted for U.S. $18.3 billion in 2012.[2,15,27] In Japan, the estimated number of adults with suspected diabetes was approximately 9.5 million in 2012 and diabetes accounted for 6% of the healthcare budget.[28,29] In Switzerland, annual diabetes costs to the mandatory health insurance program increased from EUR 5036 per patient in 2006 to EUR 5331 per patient in 2011 with the overall prevalence of diabetes at 3.9% in 2006 and 4.9% in 2011.[30] In 2007, the total medical costs in the adult diabetes population aged 20–79 years old in Korea was approximately one-fifth (19.2%) of the total cost for the entire adult population.[31] The total medical cost per person in patients with diabetes was 4.6 times higher than the general population.[32]
Medication adherence is also very important for the prevention of associated complications or morbidity and mortality. In this study, the MPR was only 0.449 even in 2013. The adherence rate to antihypertensive medication in the general population was reported as only 57.4% in Korea.[32] Adherence to long-term therapy for chronic illnesses in developed countries averages only 50%.[33,34] Data from different studies showed that adherence to oral hypoglycemic agents ranged from 36% to 93% in patients remaining on treatment for 6–24 months.[19] To maintain good glycemic control and prevent or delay diabetic complications, strategies to improve medication adherence are urgently needed at the government level.
The most powerful strength of this study was that these data were based on a nationwide Korean population covering nearly 100% of Korean patients with type 2 diabetes, which provided evidence regarding real-world clinical practice. In our NHIS database, all antidiabetic drugs available in Korea were included.
There were some limitations to this analysis. First, because health insurance coverage is confined to the allowable range for recommendations of antidiabetic drug combinations made by the KHIRA Service in Korea, prescriptions not covered by health insurance were missed in this analysis. In addition, the NHIS database does not contain uninsured prescriptions or over-the-counter drugs without prescription. Moreover, Medical Aid beneficiary information has been incorporated into a single NHIS database only since 2006; therefore, the NHIS database included only information of NHI beneficiaries, not MA beneficiaries, from 2002 to 2005. In addition, between 2002 and 2005, health insurance coverage for some antidiabetic drugs was limited more so than in recent years. For these reasons, the dispensing rate before 2006 could be underestimated. Second, subjects with disease codes E11–14 who were not on medication (any of the insulin or oral hypoglycemic agents) were not included in this analysis. Third, discrepancy between actual diagnosis and claim data might be possible. Our reported number may not be the actual number of diabetic patients because not every patient who had type 2 diabetes uses antidiabetic medication, and not all patients who had a prescription visited the pharmacy and got the medication. Fourth, this analysis relied only on claims data, we therefore could not obtain clinical information on glucose or HbA1c levels, diabetic duration, or reason for nonadherence.
In conclusion, metformin is the most commonly prescribed antidiabetic drug, and 60% of subjects with type 2 diabetes are treated with more than two classes of antidiabetic medication in Korea, and its use will be increased or maintained at top levels in the meantime. Recently, DPP-4 inhibitors have been taking the place of SU, and the medication expenditure for type 2 diabetes seems to be dependent on DPP-4 inhibitors and new classes of drugs in the future.
Acknowledgments
This work was performed by the cooperation with National Health Insurance Service (NHIS), and the National Health Information Database made by NHIS was used (No. NHIS-2015–4–008).
Supplementary Material
Footnotes
Abbreviations: ADA = American Diabetes Association, CPG = Clinical practice guidelines, DPP-4 = dipeptidyl peptidase-4, EASD = European Association for the Study of Diabetes, GLP-1 = Glucagon-like peptide 1, KDA = Korean Diabetes Association, KNHANES = Korea National Health and Nutritional Examination survey, NHIS = National Health Insurance Service, SU = Sulfonylurea, TZD = Thiazolidinediones.
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article.
References
- 1.Jeon JY, Ko SH, Kwon HS, et al. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J 2013; 37:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013; 36:1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 2014; 370:1514–1523. [DOI] [PubMed] [Google Scholar]
- 4.Statistics Korea 2013. Available from: http://kostat.go.kr/portal/korea/index.action.(latest update 2015 Dec 7). [Google Scholar]
- 5.Jin DC. Major changes and improvements of dialysis therapy in Korea: review of end-stage renal disease registry. Korean J Intern Med 2015; 30:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes--2015. Diabetes Care 2015; 38 Suppl 1:S41–48. [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140–149. [DOI] [PubMed] [Google Scholar]
- 9.Ko SH, Kim SR, Kim DJ, et al. Committee of Clinical Practice Guidelines, Korean Diabetes Association. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J 2011; 35:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014; 104:1–52. [DOI] [PubMed] [Google Scholar]
- 11.Jeon JY, Kim DJ, Ko SH, et al. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J 2014; 38:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J 2014; 38:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo BK, Lee CH, Yang BR, Hwang SS, Choi NK. The incidence and prevalence of diabetes mellitus and related atherosclerotic complications in Korea: a National Health Insurance Database Study. PLoS One 2014; 9:e110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Arch Intern Med 2008; 168:2088–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner LW, Nartey D, Stafford RS, Singh S, Alexander GC. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care 2014; 37:985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care 2014; 37:1367–1374. [DOI] [PubMed] [Google Scholar]
- 17.Lee YH, Han K, Ko SH, Ko KS, Lee KU. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data Analytic Process of a Nationwide Population-Based Study Using National Health Information Database Established by National Health Insurance Service. Diabetes Metab J 2016; 40:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaer TL, Sclar DA, Robison LM, Markowski DJ, Won JK. Effect of pharmaceutical formulation for diltiazem on health care expenditures for hypertension. Clin Ther 1993; 15:905–911. [PubMed] [Google Scholar]
- 19.Garcı’a-Pe’rez LE, Alvarez M, Dilla T, Gil-Guille’n V, Orozco-Beltra’n D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther 2013; 4:175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong HK, Ong KL, Cheung CL, Cheung BM. Utilization of glucose, blood pressure, and lipid lowering medications among people with type II diabetes in the United States. Ann Epidemiol 2014; 24:516–521. [DOI] [PubMed] [Google Scholar]
- 21.UK., Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865. [PubMed] [Google Scholar]
- 22.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med 2011; 154:602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon KH, Shin JA, Kwon HS, et al. Comparison of the efficacy of glimepiride, metformin, and rosiglitazone monotherapy in Korean drug-naïve type 2 diabetic patients: the practical evidence of antidiabetic monotherapy study. Diabetes Metab J 2011; 35:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab 2014; 16:410–417. [DOI] [PubMed] [Google Scholar]
- 25.Wainstein J, Katz L, Engel SS, Xu L, et al. Initial therapy with the fixed-dose combination of sitagliptin and metformin results in greater improvement in glycaemic control compared with pioglitazone monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 2012; 14:409–418. [DOI] [PubMed] [Google Scholar]
- 26.Blonde L, San Juan ZT, Bolton P. Fixed-dose combination therapy in type 2 diabetes mellitus. Endocr Pract 2014; 20:1322–1332. [DOI] [PubMed] [Google Scholar]
- 27.Herman WH. The economic costs of diabetes: is it time for a new treatment paradigm? Diabetes Care 2013; 36:775–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Health, Labour and Welfare 2012. Reference data of Healthy Japan 21 (in Japanese). Available at: http://www.mhlw.go.jp/bunya/kenkou/dl/kenkounippon21_02.pdf. [Google Scholar]
- 29.Neville SE, Boye KS, Montgomery WS, Iwamoto K, Okamura M, Hayes RP. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009; 25:705–716. [DOI] [PubMed] [Google Scholar]
- 30.Huber CA, Schwenkglenks M, Rapold R, Reich O. Epidemiology and costs of diabetes mellitus in Switzerland: an analysis of health care claims data, 2006 and 2011. BMC Endocr Disord 2014; 14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park IeB, Kim J, Kim DJ, et al. Task Force Team for Basic Statistical Study of Korean Diabetes Mellitus of Korean Diabetes Association.Diabetes epidemics in Korea: reappraise nationwide survey of diabetes “diabetes in Korea 2007”. Diabetes Metab J 2013; 37:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin DW, Park JH, Park JH, et al. Antihypertensive medication adherence in cancer survivors and its affecting factors: results of a Korean population-based study. Support Care Cancer 2011; 19:211–220. [DOI] [PubMed] [Google Scholar]
- 33.Vervloet M, van Dijk L, Santen-Reestman J, van Vlijmen B, Bouvy ML, de Bakker DH. Improving medication adherence in diabetes type 2 patients through Real Time Medication Monitoring: a randomised controlled trial to evaluate the effect of monitoring patients’ medication use combined with short message service (SMS) reminders. BMC Health Serv Res 2011; 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Adherence to long-term therapies. Evidence for action. Geneva, World Health Organisation 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


