Abstract
Myocarditis is a common complication of severe dengue infection. However, data about prevalence and characterization of myocarditis in dengue are still lacking. In 2014, the worst outbreak of dengue in the last two decades in China occurred. In this study, we described the clinical and laboratory diagnostic features of dengue with myocarditis. Totally, 1782 diagnosed dengue patients were admitted from August to October, 2014, all of whom were subjected to electrocardiogram, ultrasound cardiogram, and cardiac enzyme test. About 201 cases of dengue patients were diagnosed with myocarditis and the prevalence of myocarditis in hospitalized dengue was 11.28%. The prevalence of myocarditis in nonsevere dengue with warning signs and severe dengue [NSD(WS+)/SD] and nonsevere dengue without warning signs [NSD(WS–)] was 46.66% and 9.72%, respectively. The NSD(WS+)/SD patients with myocarditis presented with higher incidence of cardiac symptoms, supraventricular tachycardia (14.29% vs. 0%, P < 0.001), atrial fibrillation (25.71% vs. 10.24%, P = 0.019) and heart failure compared with NSD (WS–) patients with myocarditis. About 150 cases of dengue patients without myocarditis in the same period of time in department of Cardiology were recruited as control group. The proportion of NSD(WS+)/SD in dengue patients with and without myocarditis was 17.41% and 2.53%, respectively. Dengue patients with myocarditis experienced longer hospital stay than those without myocarditis (7.17 ± 4.64 vs. 5.98 ± 2.69, P = 0.008). There was no difference between patients with and without myocarditis in the proportion of symptoms, auxiliary methods abnormality, arrhythmia, and heart failure on the discharge day. Our study demonstrates the prevalence of myocarditis in worst outbreak of dengue in China was 11.28% and the incidence of myocarditis increased with the severity of dengue. The NSD(WS+)/SD patients with myocarditis presented with higher incidence of cardiac complication compared with NSD (WS–) patients with myocarditis. The prognosis of dengue patients with and without myocarditis had no significant difference even if myocarditis patients experienced longer hospital stay.
Keywords: arrhythmia, dengue fever, dengue virus, heart failure, myocarditis
1. Introduction
Dengue is mainly prevalent in tropical and subtropical parts of the world, and has spread to many geographic areas previously unaffected.[1] Clinical manifestations of dengue fever range from asymptomatic, or mild, flu-like symptoms to plasma leakage, thrombocytopenia, bleeding, and/or severe shock.[2] At present, it mainly depends on the prevention of mosquito bites and the symptomatic treatment to reduce the morbidity and mortality, since there is neither effective antiviral treatment nor vaccine available for dengue prevention.[3,4]
Myocarditis is an especially common cause of death in patients with severe dengue.[5–8] However, study of myocarditis in dengue is still very lacking, and no data of large sample study are available about the incidence of myocarditis in dengue, mainly due to the wide variety of clinical manifestations of myocarditis as well as the inconveniency of endomyocardial biopsy (EMB) as a deterministic diagnostic method.[9–11] There are some sporadic case reports of myocarditis in dengue,[12–14] and only 3 studies conducted the cross-sectional or cohort study during dengue virus infection. Unfortunately, the sample size of all the 3 studies were very small (11, 12, and 16 cases of dengue with myocarditis, respectively) and the prevalence of myocarditis in dengue was reported from 9% to 15%.[15–17] To better understand the clinical pathogenesis and diagnostic characteristics of dengue myocarditis, which is important for better prevention and treatment of this disease, large sample systematic study are urgently needed. In 2014, the worst outbreak of dengue in the last two decades in China attacked the city and surrounding areas of Guangzhou. Before December 15, at least 45,171 dengue patients have been confirmed by the Health Department of Guangdong province, 82.69% cases of which were from Guangzhou.[18] We conducted a systematic studies on the dengue patients in Guangzhou 8th People's Hospital from August to October in 2014, described the clinical and laboratory diagnostic features of dengue with myocarditis.
2. Methods
2.1. Patients
All data of this study were obtained from the admitted patients in Guangzhou 8th People's Hospital from August to October 2014.
According to the World Health Organization (WHO) 2009 criteria, clinically suspected dengue patients were subjected to serum rapid test to detect the nonstructural protein 1 (NS1) and/or immunoglobulin M (IgM)/immunoglobulin G (IgG) antibody to determine dengue infection.[19] According to the diagnostic criteria from European Society of Cardiology (ESC, 2013), dengue patients were subjected to electrocardiogram (ECG), ultrasound cardiogram (UCG), and cardiac enzyme test (CET) to make the diagnosis of myocarditis.[20] Patients with a history of myocarditis, myocardial ischemia, myocardial infarction, heart failure, coronary artery disease, rheumatic heart disease, cardiac neurosis, valvular heart disease, and congenital heart disease were excluded.[21–23] Patients with acute and chronic toxicity, autoimmune diseases, renal failure, and pregnant women were also excluded. The study was approved by the local research ethics committee. All patients signed written informed consent to participate; for those critically ill patients or pediatric patients, informed consent was obtained from their relatives or legal guardians.
2.2. Study design
Dengue IgM/IgG Capture enzyme-linked immunosorbent assay (ELISA; Daan Gene Co, Ltd. of Sun Yat-sen University, China), a rapid detection of NS1 (ELISA kit InBios, seattle, WA) and reverse transcription polymerase chain reaction (RT-PCR) were used to detect dengue virus infection in clinically suspected patients.[24] Viral ribonucleic acid (RNA) was extracted from serum samples using QIAamp Viral RNA kit (Qiagen, Inc, Valencia, CA) according to the manufacturer's instructions. RT-PCR was performed using the QuantiTect Virus Kit (Qiagen, Germantown, MA) directly from 5 μL of RNA extract.
Patients with epidemiological history and clinical manifestations of dengue were diagnosed with positive specific serum IgM antibodies and/or NS1 antigen and/or positive dengue RT-PCR. Secondary infections were defined as the presence of positive RT-PCR and/or positive NS1 associated with positive IgG during the acute phase (<7 days’ disease duration) and an IgM/IgG ratio of less than 1.2 during the convalescent phase (≥7 days’ disease duration).[19,24]
According to the WHO (2009) classification, dengue can be divided into: nonsevere dengue without warning signs [NSD(WS–)]; nonsevere dengue with warning signs [NSD(WS+)] and severe dengue (SD).[19] The two latter groups are more danger and need more medical attention compared to the NSD(WS–) as a whole group.
Auxiliary diagnosis methods, including ECG, UCG, and CET were performed in dengue patients, no matter whether the patients had symptoms of myocarditis. ECG was performed immediately and UCG was performed within 3 days after patient's admission and was repeated on the discharge day. In addition, ECG was repeated at any time if patients had new emerging cardiac symptoms. CET was performed daily during hospitalization. Amino-terminal probrain natriuretic peptide (NT-proBNP) was determined daily by AQT90 NT-proBNP (Radiometer, Denmark), creatine kinase-MB(CK-MB) by Arquitect CK-MB Liquid and cardiac troponin I (cTnI) by Arquitect STAT Troponin-I (Abbott Diagnostics).[25] Complaints and symptoms were collected instantly on admission, and were added if any changes of patient's condition.
12 lead ECG was considered abnormal with any of the following: sinus arrest, atrioventricular block, or bundle branch block, intraventricular conduction delay (widened QRS complex, which is the combination of the Q wave, R wave and S wave and represents ventricular depolarization), atrial fibrillation, supraventricular tachycardia, frequent premature beats ventricular tachycardia or fibrillation and asystole, ST/T wave change (ST elevation or non-ST elevation, T wave inversion), reduced R wave height, abnormal Q waves, and low voltage.[20,26–29]
Functional and structural abnormalities on UCG were defined as following: ventricular dilatation, increased wall thickness, regional wall motion or global systolic or diastolic function abnormality, pericardial effusion, endocavitary thrombi, left ventricular ejection fraction (LVEF) less than 55%, valvular regurgitation and valvular vegetation.[20]
Cardiac enzyme was considered to be elevated and abnormal if CK-MB more than 25 U/L and/or cTnI more than 0.02 ng/mL and/or NT-proBNP more than 450 ng/L (age < 50 years), 900 ng/L (age 50–75 years), and 1800 ng/L (age > 75 years).
According to the diagnostic criteria from ESC (2013), myocarditis was diagnosed if 1 or more clinical presentation and 1 or more auxiliary diagnosis method; 2 or more auxiliary diagnosis method should be met if the patient is asymptomatic.[20] Clinical presentation includes chest pain, dyspnea at rest or exercise, palpitation, syncope, cardiac shock, and sudden cardiac death.[30]
2.3. Statistical analysis
Analyses were carried out using IBM SPSS 19.0.1 for Windows (IBM, NY). Qualitative variables are defined by number of cases and percentage. Quantitative variables are expressed as means and standard deviation. In quantitative non-Gaussian variables, median, and interquartile range (P25–P75) was used instead of mean. Comparisons of means were done using unpaired Student's t-test for variables with Gaussian distribution. Comparison of categorical variables was made using Chi-square test. A P value of less than 0.05 was considered to be significant in all analyses.
3. Results
3.1. Classification of diagnosis and patients composition
We totally found out 449 cases of ECG abnormality, 247 cases of CET abnormality, 139 cases of UCG abnormality from 1782 admitted dengue patients. Combined with clinical manifestations, 201 cases of dengue were diagnosed with myocarditis. The prevalence of myocarditis in hospitalized dengue patients was 11.28% in our study. The diagnostic-type distribution of all 201 dengue patients with myocarditis is shown in the Venn diagram (Fig. 1A).
Figure 1.
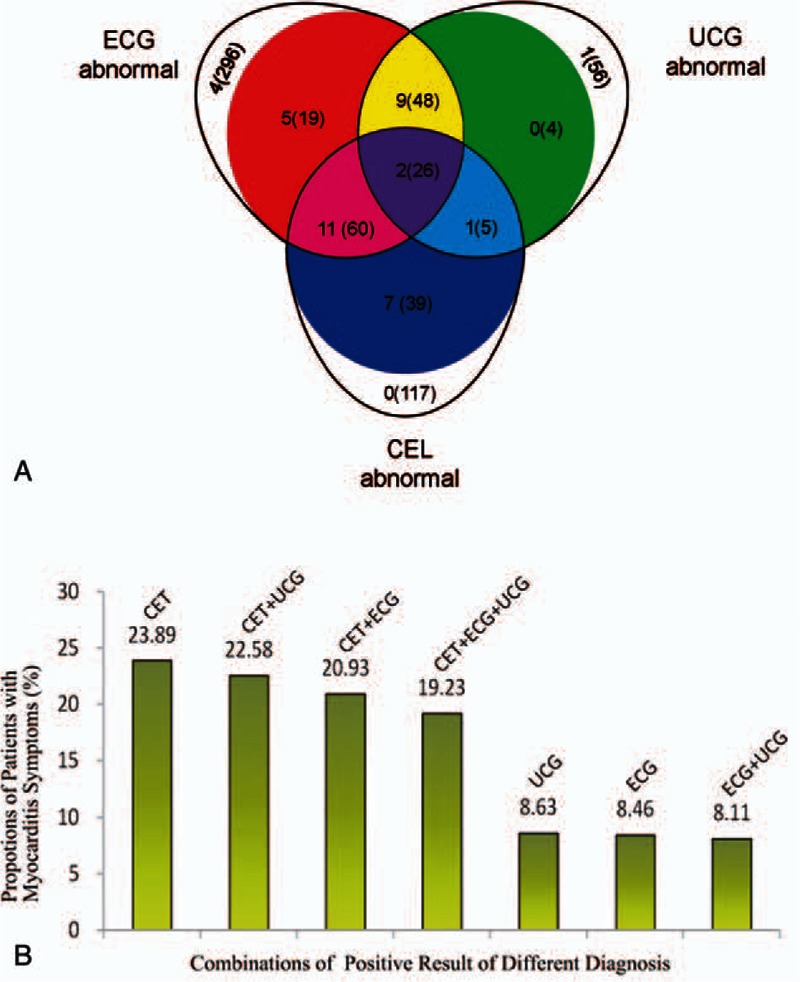
Classification of diagnosis and patients composition. (A) Three egg-shaped area in the Venn diagram represent the patients with indicated abnormal in different clinical or laboratory tests: the ECG abnormal is on the upper left, the UCG abnormal is on the upper right, and the CET abnormal is on the lower middle. The solid areas with different colors represent the different group of dengue patients with myocarditis, whereas the open crescent areas represent the dengue patients without any cardiac symptom but one single abnormal diagnostic criteria. The number of dengue with warning signs and severe dengue (out braces) or the number of all dengue (in braces) have been indicated on the diagram. (B) The proportion (shown as percentage) of dengue patients with cardiac symptom in different population of dengue patients with diversified positive results of the myocarditis diagnostic method as ECG, UCG, and CET. For example, CET means all dengue patients with CET abnormal with or without ECG/UCG abnormal. CET+UCG means all dengue patients with both CET and UCG abnormal with or without ECG abnormal.
In all 201 cases of dengue patients with myocarditis, ECG abnormal, CET abnormal and UCG abnormal were 153, 83, and 130 cases, respectively. Among these 3 auxiliary diagnosis methods, ECG was most sensitive, which covered about 76.12% (153 in 201 patients) dengue cases diagnosed with myocarditis.
The proportion of NSD(WS+)/SD in dengue myocarditis patients with UCG, ECG, and CET abnormal was 14.46% (12 in 83 patients), 17.65% (27 in 153 patients), and 16.15% (21 in 130 patients), respectively (Fig. 1A). The percentages of patients with symptoms of myocarditis using different combinations of auxiliary diagnostic including ECG, UCG, and CET were shown in Fig. 1B. Among them, we found that the percentages in patients with positive results of CET were higher than others, which can reach to the highest percentage (23.89%) in the patients with CET abnormal without considering the results of ECG or UCG.
3.2. Manifestation in dengue patients with myocarditis
All the 201 cases of dengue patients with myocarditis were admitted in department of Cardiology and totally 150 dengue patients without myocarditis in the same period of time were hospitalized in department of Cardiology, all of whom were recruited as control. Characteristics of dengue patients with and without myocarditis are shown in Table 1. There were no significant differences in age, sex, common clinical symptoms, and secondary dengue infection between these two groups. We found shock in patients of dengue with myocarditis was increased significantly (6.97% vs. 0.67%, P = 0.004). However, there were no significant differences in the prevalence of bleeding, convulsion, coma, and aphasia (Table 1).
Table 1.
Comparison between patients with and without myocarditis.
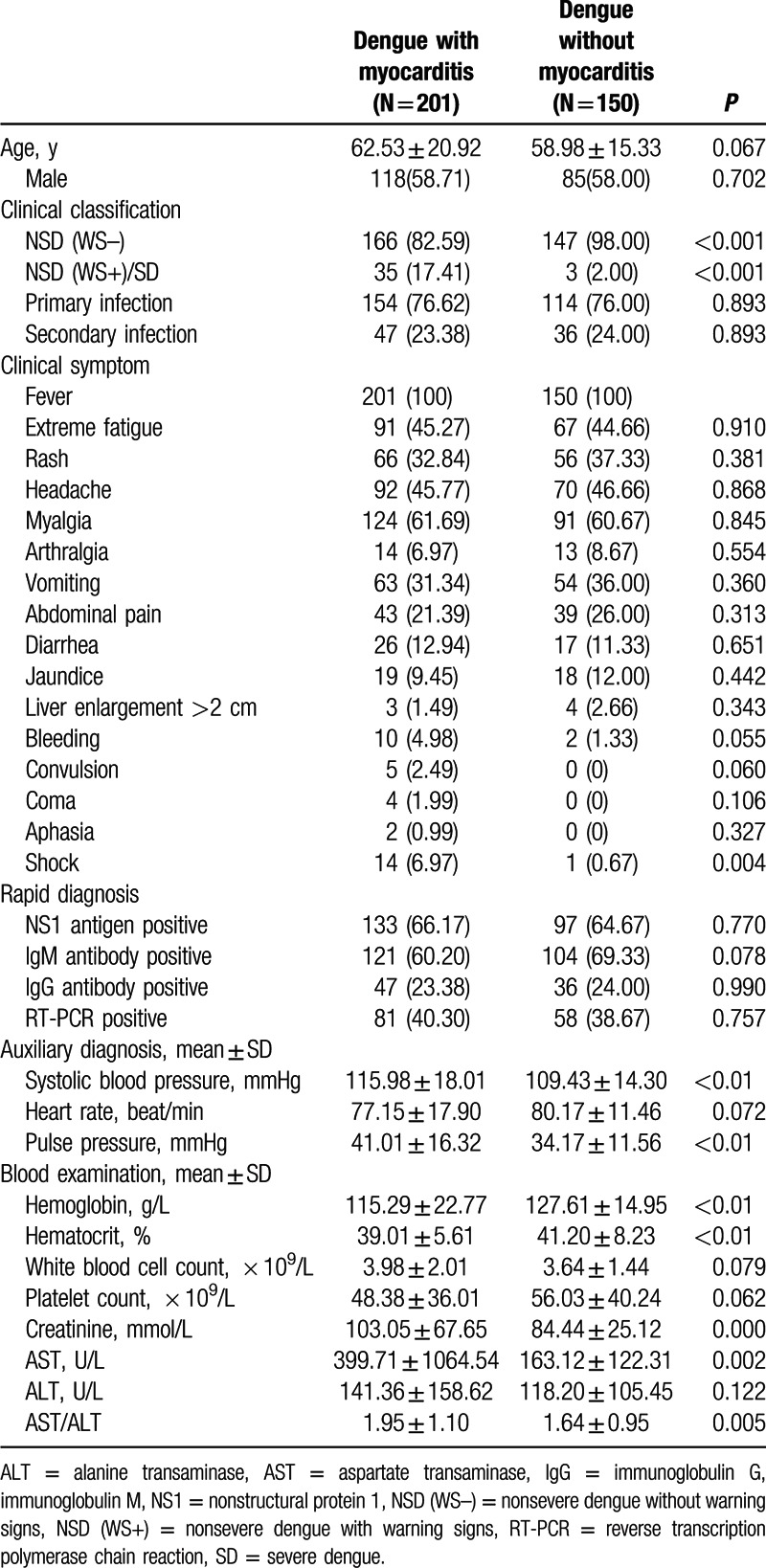
We found 75 cases of NSD(WS+)/SD and 1707 cases of NSD(WS–) in all the 1782 dengue patients, with 35 cases of myocarditis in the former and 166 cases of myocarditis in the latter. The prevalence of myocarditis in NSD(WS+)/SD and NSD(WS–) was 46.66% and 9.72%, respectively. The proportion of NSD(WS+)/SD in dengue patients with myocarditis is 17.41% and 2.53% in those without myocarditis, indicating the prevalence of warning signs or severe dengue was significantly higher in dengue patients with than those without myocarditis (Table 1).
We also observed significantly higher aspartate transaminase (AST) and AST/alanine transaminase (ALT) in the group with myocarditis compared with patients without myocarditis (399.71 ± 1064.56 vs. 163.12 ± 122.31, P = 0.002; 1.95 ± 1.10 vs. 1.64 ± 0.95, P = 0.005). Dengue patients with myocarditis had lower hemoglobin (115.29 ± 22.77 vs. 127.61 ± 14.95; P < 0.01), and hematocrit (39.01 ± 5.61 vs. 41.20 ± 8.23; P < 0.01) as well as higher levels of systolic blood pressure (115.98 ± 18.01 vs. 109.43 ± 14.30; P < 0.01) and creatinine (103.05 ± 67.65 vs. 84.44 ± 25.12; P < 0.0001) than those without myocarditis.
3.3. Clinical outcomes and prognosis in dengue patients with and without myocarditis
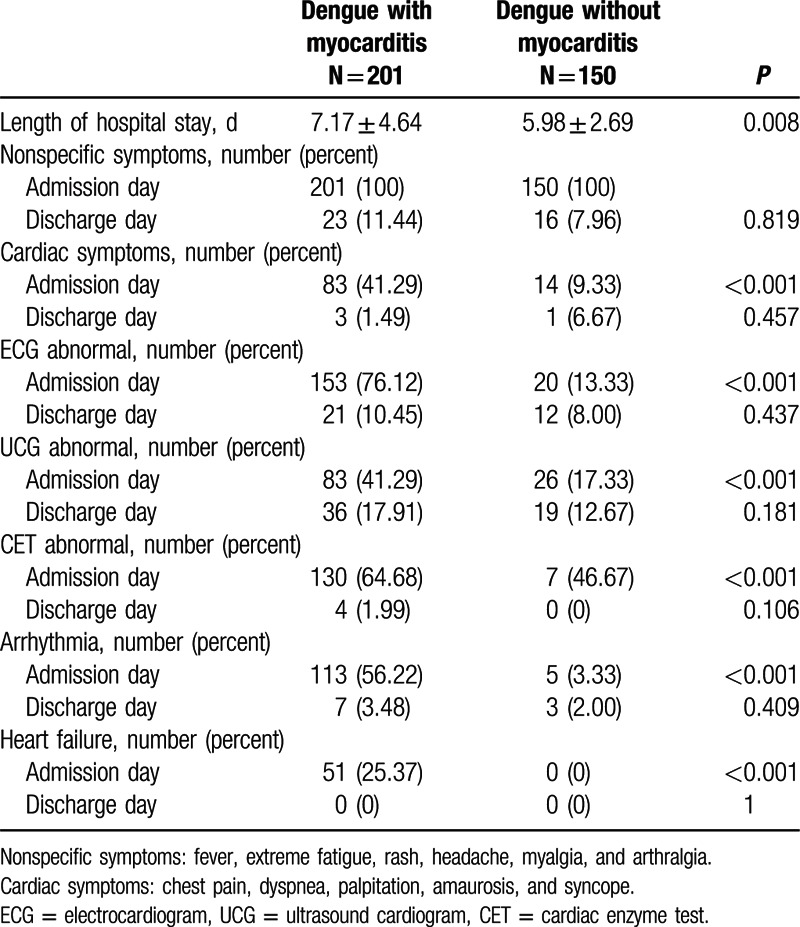
Dengue patients with myocarditis experienced longer hospital stay than patients without myocarditis (7.17 ± 4.64 vs. 5.98 ± 2.69, P = 0.008). However, there were no differences in the proportion of symptoms, auxiliary diagnosis methods abnormality, arrhythmia, and heart failure on the discharge day (Table 2).
Table 2.
Overview of dengue patients with and without myocarditis on admission and discharge day.
3.4. Difference between NSD(WS+)/SD and NSD(WS–) in dengue patients with myocarditis
Clinical symptoms, myocardial enzymology, ECG, and ultrasonic imaging characteristics of the 35 NSD(WS+)/SD and 166 NSD(WS–) patients with myocarditis are shown in Table 3.
Table 3.
Comparison between NSD (WS+)/SD and NSD(WS–) in dengue patients with myocarditis.
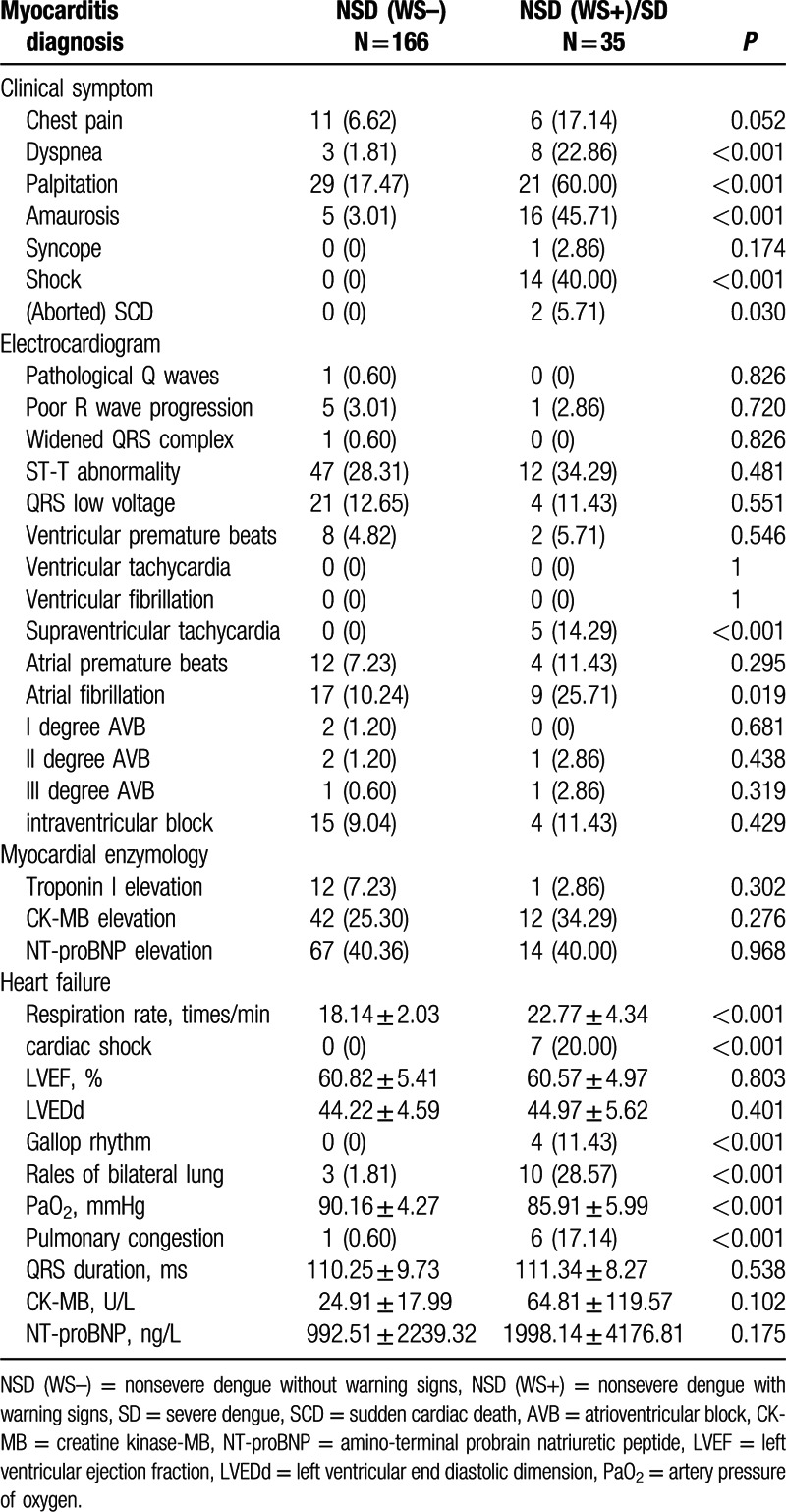
The NSD(WS+)/SD patients presented with higher incidence of clinical symptoms, including chest pain, dyspnea, palpitation, amaurosis, and syncope, which suggested the patients experienced more discomfort in NSD(WS+)/SD than NSD(WS–) (Table 3). However, the differences of myocardial enzymology, ECG, and ultrasonic imaging characteristics between these two groups were not statistically significant.
Ventricular arrhythmias, supraventricular arrhythmias, and conduction blockade were found in both groups of patients. Supraventricular tachycardia (0% vs. 14.29% P < 0.001) and atrial fibrillation (10.24% vs. 25.71%, P = 0.019) were more common in the NSD(WS+)/SD group than that in NSD(WS–) group. There were no significant differences for other types of arrhythmia between the two groups.
Most indexes associated with cardiac failure were increased in myocarditis patients with NSD(WS+)/SD compared with NSD(WS–). For an example, gallop rhythm was 4 (11.43%, P < 0.001) cases in NSD(WS+)/SD versus 0 cases in NSD(WS–). However, LVEF, left ventricular end diastolic dimension, NT-proBNP were not significant different in the 2 groups (Table 3).
There were 2 mortal cases in the 1782 patients, who were all the NSD(WS+)/SD dengue patients. One of them was a case of sudden death with unclear reason (his relatives refused an autopsy). Another one died from severe heart failure, combined with sinus bradycardia, sinus arrest, and paroxysmal supraventricular tachycardia.
4. Discussion
Dengue virus, which is a newly discovered pathogen of viral myocarditis, belongs to the Flaviviridae family, and Dengue is the most rapidly spreading mosquito-borne viral disease in the world.[31] So far very few studies have reported that dengue virus directly acted on myocardiocytes, probably due to the lower tropism of dengue virus on myocardium.[32]
According to our results, the prevalence of myocarditis in hospitalized dengue patients is 11.28% with the ESC myocarditis criteria, which is consistent with previous reports (9%–15%).[15,16,17] However, our sample size was significantly larger than previous studies and was more representative. The prevalence of myocarditis in NSD(WS+)/SD was 46.67%, much more higher than that in whole dengue patients, which suggests that the incidence of myocarditis is associated with the severity of dengue. Furthermore, our data showed that the proportion of NSD(WS+)/SD in myocarditis (17.41%) was significantly higher than in whole worldwide dengue without considering myocarditis(1.31%–7.49%).[19] These data suggest that the severity of dengue infection was increased when companied with myocarditis.
4.1. Clinical diagnosis of dengue patients with myocarditis
EMB is the gold standard method for diagnosis of myocarditis, but it is difficult to popularize. Currently, the internationally accepted criteria is the combination of other indicators as an alternative of EMB. According to the ESC's new criteria,[20] we combined the symptoms, electrocardiography, cardiac enzymology, and cardiac imaging methods for the diagnosis of dengue patients with myocarditis. Taking economic cost and operational simplicity into account, we chose UCG as the imaging diagnosis method of myocarditis, rather than cardiovascular magnetic resonance.
Most dengue patients with myocarditis were ECG-positive, which suggested that the suspected patients should be subjected to ECG examination firstly due to its high sensitivity. Furthermore, it is obviously elaborated that the patients with positive result of CET were more likely to have the symptoms of myocarditis (Fig. 1B), suggesting the importance of CET in diagnosis of dengue with myocarditis.
We found higher prevalence of NSD(WS+)/SD in dengue patients with myocarditis than those without myocarditis, and the former group included more shock patients and experienced longer hospital stay. Fortunately, under the early detection, intensive care, and treatment, the prognosis of dengue patients with myocarditis was similar with those without myocarditis.
4.2. Cardiac arrhythmia and heart failure
Dengue complicated with myocarditis has different diagnostic and laboratory performance ranging from mild increase of cardiac markers to death. Cardiac arrhythmia and heart failure were common in severe dengue and the targeted therapy is critical for recovery.[33,34] However, there are few detailed data about the incidence of cardiac arrhythmia and heart failure in dengue infection in previous studies.[35,36] Carlos found 6 cases of acute heart failure, 5 cases of LVEF depression, and 3 cases of wall motion abnormalities in 12 cases of dengue patients with elevated cardiac biomarker.[17]
In this study, different parameters have been adopted for determining cardiac arrhythmia and heart failure in the dengue patients with myocarditis. Diverse types of arrhythmia were found in the patients; however, neither any type of arrhythmia, nor any change of ECG were significantly different between the NSD(WS+)/SD and NSD(WS–) groups, except for supraventricular tachycardia and atrial fibrillation. It could be deduced that severe dengue are more likely to be complicated with tachyarrhythmia. We also found that several indexes of heart failure were significantly higher in NSD(WS+)/SD than NSD(WS–) patients, including respiratory rate, rales of bilateral lung, gallop rhythm, and pulmonary congestion in chest X-ray, which suggested that myocardial damages resulted from dengue infection were significantly increased with the aggravation of dengue fever disease.
4.3. Limitations
In the present study, it is inevitable that some of the methodological limitations exist. First, the age composition is not balanced, for example, older people are more in all groups. In addition, some social risk factors, such as being single or living far from the hospital, are important for dengue management. Missing of these data may affect the diagnosis of dengue type and the correlation analysis between dengue and myocarditis. Second, it was impossible to completely exclude coinfections, such as enteroviruses in our study. Third, some patients with past history of cardiac disease (such as continuous or repeated cardiac arrhythmias resulted from other reasons than myocarditis) may be included in the present study, so some of the clinical evidence of dengue fever (such as palpitation, premature beats, etc.) would lead to the diagnosis of myocarditis according to the new ESC criteria. Fourth, we did not classify the serotypes distribution in dengue myocarditis. So far, we could not determine whether susceptibility to myocarditis is related to some specific serotype predominance. Finally, there were too few outpatients with mild symptoms included in the study, but that should not be a problem of our study as those patients with severe symptoms are the ones need more medical care.
5. Conclusion
In conclusion, this study demonstrates the clinical and diagnostic characteristics of dengue associated myocarditis and it correlation with dengue severity. Clinical manifestations of dengue myocarditis primarily are arrhythmias and heart failure, which can often lead to some serious consequences, such as shock and death. In order to reduce the complications in clinical practice, we need to pay more attention on the importance of the diagnosis of dengue with myocarditis.
Acknowledgments
The authors are grateful for the vital contribution of the participating volunteer patients in the study. The authors would like to thank the local research ethics committee and the individual researchers, clinicians and nurses who participated in this study. The authors also thank Professor Lifang Jiang for the gift of antidengue antibody. The authors also thank Ningning Wang for his contributions to the statistical analyses.
Footnotes
Abbreviations: ALT = alanine transaminase, AST = aspartate transaminase, CET = cardiac enzyme test, CK-MB = creatine kinase-MB, cTnI = cardiac troponin I, ECG = electrocardiogram, ELISA = enzyme-linked immunosorbent assay, EMB = endomyocardial biopsy, ESC = European Society of Cardiology, IgG = immunoglobulin G, IgM = immunoglobulin M, LVEF = left ventricular ejection fraction, NS1 = nonstructural protein 1, NSD (WS–) = nonsevere dengue without warning signs, NSD (WS+) = nonsevere dengue with warning signs, NT-proBNP = amino-terminal probrain natriuretic peptide, RNA = ribonucleic acid, RT-PCR = reverse transcription polymerase chain reaction, SD = severe dengue, UCG = ultrasound cardiogram, WHO = World Health Organization.
All authors contributed substantially to the conception, design, analysis, and interpretation of the data presented. All authors had full access to the data and were involved in critical revision of the manuscript for important intellectual content. The corresponding author was responsible for manuscript submission.
Financial Support. None.
All authors declare that they have neither financial nor nonfinancial competing interests. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
The authors declare that they have no conflict of interest.
References
- 1.Simmons CP, Farrar JJ, Nguyen v V, Wills B. Dengue. New Engl J Med 2012; 366:1423–1432. [DOI] [PubMed] [Google Scholar]
- 2.Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B. Cardiovascular manifestations of the emerging dengue pandemic. Nat Rev Cardiol 2014; 11:335–345. [DOI] [PubMed] [Google Scholar]
- 3.Low JG, Sung C, Wijaya L, et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect Dis 2014; 14:706–715. [DOI] [PubMed] [Google Scholar]
- 4.Webster DP, Farrar J, Rowland-Jones S. Progress towards a dengue vaccine. Lancet Infect Dis 2009; 9:678–687. [DOI] [PubMed] [Google Scholar]
- 5.Cooper LT., Jr Myocarditis. New Engl J Med 2009; 360:1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaratnam N, Siripala K, de Silva N. Arbovirus (dengue type) as a cause of acute myocarditis and pericarditis. British Heart J 1973; 35:204–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhi S, Jayashree M. Dengue shock syndrome: at the heart of the issue. Pediatr Crit Care Med 2007; 8:583–584. [DOI] [PubMed] [Google Scholar]
- 8.Wiwanitkit V. Dengue myocarditis, rare but not fatal manifestation. Int J Cardiol 2006; 112:122. [DOI] [PubMed] [Google Scholar]
- 9.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007; 116:2216–2233. [DOI] [PubMed] [Google Scholar]
- 10.da Fonseca BA, Fonseca SN. Dengue virus infections. Curr Opin Pediatr 2002; 14:67–71. [DOI] [PubMed] [Google Scholar]
- 11.Gulati S, Maheshwari A. Atypical manifestations of dengue. Trop Med Int Health 2007; 12:1087–1095. [DOI] [PubMed] [Google Scholar]
- 12.Deepak D, Garg R, Pawar M, Banerjee N, Solanki R, Maurya I. Filgrastim as a rescue therapy for persistent neutropenia in a case of dengue hemorrhagic Fever with acute respiratory distress syndrome and myocarditis. Case Rep Anesthesiol 2011; 2011:896783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee IK, Lee WH, Liu JW, Yang KD. Acute myocarditis in dengue hemorrhagic fever: a case report and review of cardiac complications in dengue-affected patients. Int J Infect Dis 2010; 14:e919–e922. [DOI] [PubMed] [Google Scholar]
- 14.Marques N, Gan VC, Leo YS. Dengue myocarditis in Singapore: two case reports. Infection 2013; 41:709–714. [DOI] [PubMed] [Google Scholar]
- 15.Neeraja M, Iakshmi V, Teja VD, et al. Unusual and rare manifestations of dengue during a dengue outbreak in a tertiary care hospital in South India. Arch Virol 2014; 159:1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salgado DM, Eltit JM, Mansfield K, et al. Heart and skeletal muscle are targets of dengue virus infection. Pediatr Infect Dis J 2010; 29:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miranda CH, Borges Mde C, Matsuno AK, et al. Evaluation of cardiac involvement during dengue viral infection. Clin Infect Dis 2013; 57:812–819. [DOI] [PubMed] [Google Scholar]
- 18.http://www.gdwst.gov.cn/a/yiqingxx/2014121512665.html, accessed Feb 29th, 2016. [Google Scholar]
- 19.Trent D, Shin J, Hombach J, Knezevic I, Minor P. WHO Working Group on technical specifications for manufacture and evaluation of dengue vaccines, Geneva, Switzerland, 11-12 May 2009. Vaccine 2010; 28:8246–8255. [DOI] [PubMed] [Google Scholar]
- 20.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34:2636–2648.48a-48d. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Teo C, Low AF. Fulminant dengue myocarditis masquerading as acute myocardial infarction. Int J Cardiol 2009; 136:e69–71. [DOI] [PubMed] [Google Scholar]
- 22.Neo HY, Wong RC, Seto KY, Yip JW, Yang H, Ling LH. Noncompaction cardiomyopathy presenting with congestive heart failure during intercurrent dengue viral illness: importance of phenotypic recognition. Int J Cardiol 2006; 107:123–125. [DOI] [PubMed] [Google Scholar]
- 23.Patra S, Bhardwaj G, Manohar JS, Srinivasa KH, Kharge J, Manjunath CN. Acute myocardial infarction being the presentation of dengue myocarditis. J Cardiovasc Dis Res 2013; 4:159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro-Jorge LA, Machado PR, Favero CA, et al. Clinical evaluation of the NS1 antigen-capture ELISA for early diagnosis of dengue virus infection in Brazil. J Med Virol 2010; 82:1400–1405. [DOI] [PubMed] [Google Scholar]
- 25.Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 2006; 27:330–337. [DOI] [PubMed] [Google Scholar]
- 26.Mahmod M, Darul ND, Mokhtar I, Nor NM, Anshar FM, Maskon O. Atrial fibrillation as a complication of dengue hemorrhagic fever: non-self-limiting manifestation. Int J Infect Dis 2009; 13:e316–e318. [DOI] [PubMed] [Google Scholar]
- 27.Matthias AT, Indrakumar J, Gunatilake SB. Ventricular trigeminy in a patient with serologically confirmed dengue haemorrhagic fever. Int Arch Med 2014; 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengupta SP, Nugurwar A, Jaju R, Khandheria BK. Left ventricular myocardial performance in patients with dengue hemorrhagic fever and thrombocytopenia as assessed by two-dimensional speckle tracking echocardiography. Indian Heart J 2013; 65:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav DK, Choudhary S, Gupta PK, et al. The Tei index and asymptomatic myocarditis in children with severe dengue. Pediatr Cardiol 2013; 34:1307–1313. [DOI] [PubMed] [Google Scholar]
- 30.Wiwanitkit V. Acute shock dengue myocarditis. Rev Esp Cardiologia 2014; 67:502. [DOI] [PubMed] [Google Scholar]
- 31.Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future. Lancet Infect Dis 2014; 14:1271–1280. [DOI] [PubMed] [Google Scholar]
- 32.Miranda CH, Borges Mde C, Schmidt A, et al. A case presentation of a fatal dengue myocarditis showing evidence for dengue virus-induced lesion. Eur Heart J Acute Cardiovasc Care 2013; 2:127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohli U, Sahu J, Lodha R, Agarwal N, Ray R. Invasive nosocomial aspergillosis associated with heart failure and complete heart block following recovery from dengue shock syndrome. Pediatr Crit Care Med 2007; 8:389–391. [DOI] [PubMed] [Google Scholar]
- 34.La-Orkhun V, Supachokchaiwattana P, Lertsapcharoen P, Khongphatthanayothin A. Spectrum of cardiac rhythm abnormalities and heart rate variability during the convalescent stage of dengue virus infection: a Holter study. Ann Tropic Paediatr 2011; 31:123–128. [DOI] [PubMed] [Google Scholar]
- 35.Salgado DM, Panqueba CA, Castro DMRV, Rodriguez JA. Myocarditis in children affected by dengue hemorrhagic fever in a teaching hospital in Colombia. Rev Dalud Publica 2009; 11:591–600. [DOI] [PubMed] [Google Scholar]
- 36.Wali JP, Biswas A, Chandra S, et al. Cardiac involvement in Dengue Haemorrhagic Fever. Int J Cardiol 1998; 64:31–36. [DOI] [PubMed] [Google Scholar]


