Supplemental Digital Content is available in the text
Keywords: cohort study, gastric cancer, prognosis, review, signet ring cell, survival
Abstract
Although signet ring cell cancer (SRCC) has long been regarded as an adverse prognostic factor of gastric cancer, the findings of existing studies on this issue are inconsistent. We conducted a retrospective cohort study of 2199 consecutive patients with gastric cancer treated in a tertiary cancer hospital in Beijing, China, 1994 to 2013. The characteristics of SRCC and non-SRCC were compared. The prognostic effects of SRCC and other important clinicopathological factors on overall survival were evaluated by both univariate and multivariate Cox regression analyses and expressed as hazard ratio (HR) with 95% confidence interval (CI). SRCC accounted for 16.1% of gastric cancer, increasing from 6% to 20% over the last 2 decades, and was associated with younger age, female sex, poor differentiation, diffuse type, and distal location. SRCC (HR: 1.387, 95% CI: 1.177–1.634), stage (HR: 1.752, 95% CI: 1.458–2.106), surgery (palliative resection: HR: 0.712, 95% CI: 0.590–0.859; curative resection: HR: 0.490, 95% CI: 0.380–0.633), performance status (HR: 1.849, 95% CI: 1.553–2.201), and age (HR: 1.070, 95% CI: 1.001–1.143) were independent prognostic factors for gastric cancer, whereas time period of diagnosis, sex, and tumor location were not statistically significantly associated with overall survival. Subgroup analyses showed that the prognostic value of SRCC did not vary much with age, sex, performance status, stage, and surgery and chemotherapy status. As compared with non-SRCC, SRCC accounted for increasingly more of gastric cancer and was associated with younger age, female sex, poor differentiation, diffuse type, and distal location. It was an independent prognostic factor associated with worse survival in gastric cancer.
1. Introduction
Worldwide, gastric cancer is the fourth commonest cancer in terms of incidence and the third commonest cause of cancer-related deaths, with an estimated 952,000 new cases and 723,000 deaths every year.[1] Despite the advances in diagnosis and treatment options, the prognosis of gastric cancer has not been improved much over the last 2 decades,[2,3] with a 5-year survival rate of 25% to 30%.[4–6] Additionally, although the incidence of gastric cancer is decreasing, the proportion of signet ring cell cancer (SRCC) in gastric cancer was reported to be increasing in recent years.[7–10]
SRCC is a histologic diagnosis based on the microscopic characteristics of tumor, according to the World Health Organization classification.[11] It is defined by the presence of signet ring cell, which contains abundant intracytoplasmic mucin pushing nucleus to the periphery, in more than 50% of the tumor. The classifications by Lauren, Ming, and Sugano designate SRCC as “diffuse type,” “infiltrative type,” and “undifferentiated type,” respectively.[12–14] Many studies have shown that the biological behavior of SRCC is distinct from that of other subtypes.[15] Due to its potential to infiltrate gastric wall and disseminate in the peritoneal cavity, SRCC has long been considered as an adverse prognostic factor of gastric cancer.[16,17]
However, the results of existing studies on the prognostic value of SRCC are inconsistent. For example, Shridhar et al[18] and Piessen et al[19] found that SRCC had shorter overall survival as compared with other patients (multivariate hazard ratio [HR]: 1.218, 95% confidence interval [CI]: 1.073–1.381 and 1.5, 95% CI: 1.1–2.0, respectively), while Kim et al[20] (multivariate HR: 0.948, 95% CI: 0.746–1.245) and Taghavi et al[17] (multivariate HR: 1.05, 95% CI: 0.96–1.11) found no differences in overall survival between SRCC and non-SRCC. Interestingly, some studies found that the independent prognostic value of SRCC may vary with the stage of cancer. For example, Jiang et al[21] found that among those with early gastric cancer SRCC was associated with significantly better survival as compared with non-SRCC (multivariate HR for non-SRCC vs SRCC: 2.366, 95% CI: 1.221–4.586), but it was not an independent predictive factor in patients with advanced disease (multivariate HR for non-SRCC vs SRCC: 1.171, 95% CI: 0.979–1.400).
China suffers from a heavy burden of gastric cancer, with the fourth highest incidence of gastric cancer among all countries.[22] Although some studies on the prognostic role of SRCC are available from China, most of them are limited by such problems as small sample size,[23,24] no multivariate analysis,[23] and being restricted to specific patient groups, for example, those who had undergone surgery.[21,24–26] We therefore recruited a large sample of patients to further investigate the prognostic value of SRCC in gastric cancer through a multivariate approach, adjusting for a number of important clinicopathological characteristics, with subgroup analyses according to age, gender, performance status, stage, surgery and chemotherapy. We aimed to examine the independent prognostic value of SRCC in relation to those of other variables. We also wanted to know if its prognostic value, if any, would vary with the status of selected clinicopathological factors.
2. Methods
2.1. Patients and data collection
Two thousand one hundred ninety-nine consecutive patients diagnosed with gastric cancer admitted to the Department of GI Medical Oncology, Peking University Cancer Hospital & Institute, Beijing, China during January 1994 to July 2013 were included in the present study. From their medical records the data on date of diagnosis, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, tumor location at diagnosis, stage of cancer, histological type (SRCC or non-SRCC), differentiation, Lauren classification, surgical procedure, neoadjuvant therapy (if applicable), chemotherapy regimens (if any), and the outcome of our interest, that is, overall survival, were extracted anonymously.
For convenience of analysis, date of diagnosis was categorized into 4 groups: 1994 to 1998, 1999 to 2003, 2004 to 2008, and 2009 to 2013. Age was also divided into 4 groups: <40, 40 to 49, 50 to 59, and ≥60 years. ECOG performance status is recommended by the World Health Organization to measure patients’ functional performance, whose score ranges from 0 to 5, with 0 representing the best status.[27] In this study, ECOG performance status was divided into 2 groups, that is, 0 to 1 and ≥2, for analysis. Tumor location at diagnosis included fundus, esophagogastric junction, body, antrum, and multisite. The 7th edition of the American Joint Committee on Cancer staging system was employed to describe the pathological stage of cancer.[28]
The World Health Organization classification of histological types was used to define SRCC. This criterion has not been changed through the whole study and all tissues were examined by experienced pathologists. Lauren classification included intestinal, diffuse, and mixed types. As it was not routinely used in Peking University Cancer Hospital until recently, Lauren classification was available for only a small part of the patients included for this study. Surgical procedure included curative resection (radical operation) and palliative operation, and neoadjuvant therapy was used only for the patients who received radical operation. First-line chemotherapy regimens, if any, were divided into regimen consisting of 1 to 2 drugs and that with more than 2 drugs. Also, the type of drugs was recorded, such as platinum based, taxane based, both, and others. Whether second-line chemotherapy was given to patients was also recorded. Overall survival was defined as the time from diagnosis to all-cause death or the last date of follow-up, whichever earlier. Informed consent was obtained from each patient at the time of admission to hospital. The study was approved by the Ethics Committee of Peking University Cancer Hospital.
2.2. Statistical analysis
Patient characteristics were compared between SRCC and non-SRCC groups using Chi-square test. Overall survival rates were computed and survival curves generated by using the Kaplan–Meier method. Both univariate and multivariate analyses were conducted to evaluate the prognostic value of each clinicopathological factor mentioned above. Log-rank test was adopted to detect the difference between survival curves. Cox regression model including all clinically relevant variables as listed above was used to do multivariate analyses to control the potential confounding. The prognostic effects of patient characteristics on overall survival were expressed with HR and 95% CI. Sensitivity analyses were conducted to test the robustness of main results when only the carcinomas containing 100% signet ring cells were counted as SRCC.[29] Subgroup analyses according to age, sex, ECOG performance status, stage of cancer, surgery and chemotherapy status to see if the prognostic effect of SRCC, if any, would change with these important clinical factors. The level of statistical significance was set at α = 0.05, except for the test for subgroup difference, for which the level was α = 0.10. All analyses were conducted using the IBM SPSS Statistics 22.
3. Results
3.1. Patient characteristics
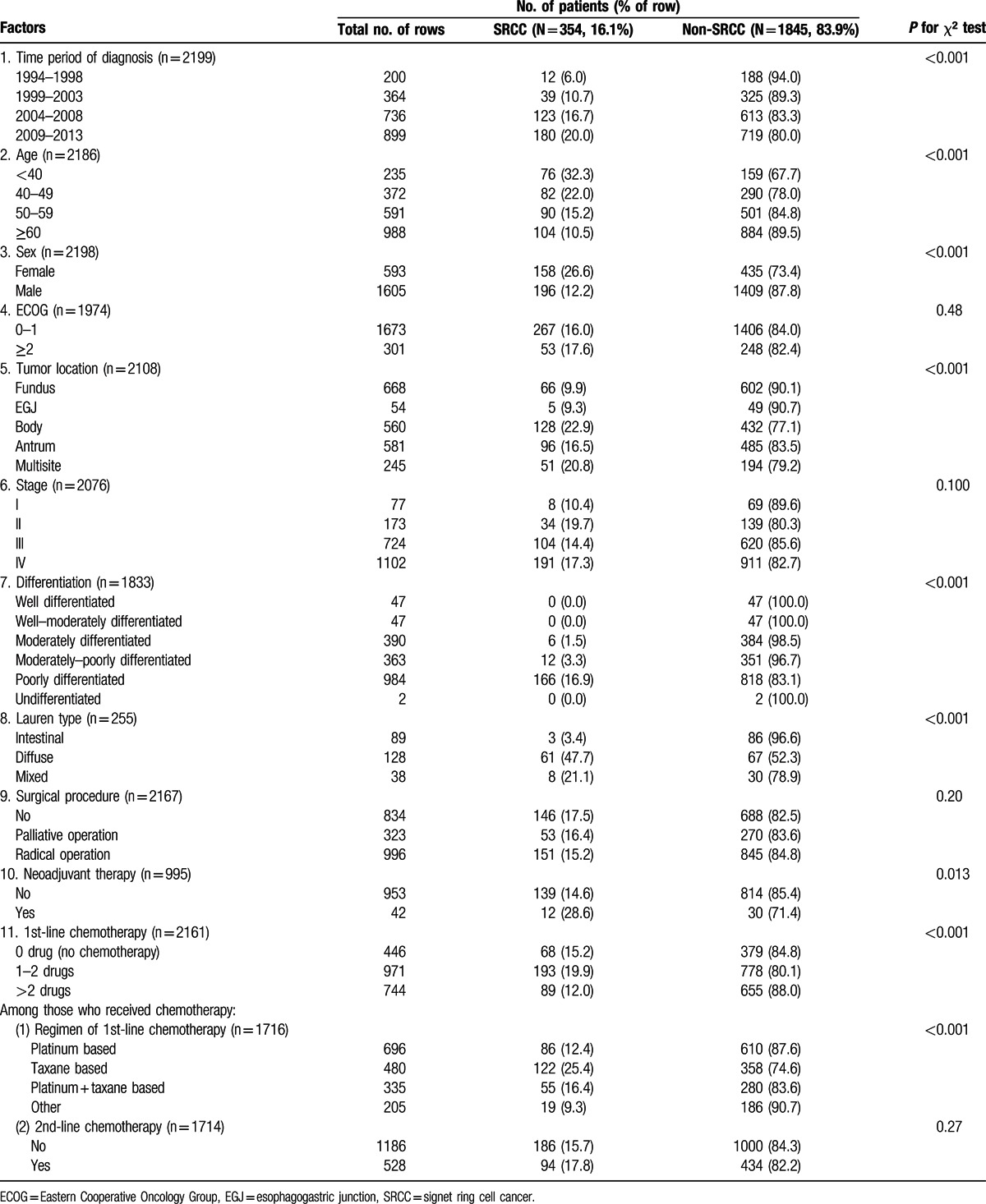
Patient characteristics are shown in Table 1. Of the 2199 patients included for analysis, 354 (16.1%) had SRCC and 1845 (83.9%) had non-SRCC. The proportion of SRCC in gastric cancer increased steadily from 6.0% in the period of 1994 to 1998 to 20.0% in the period of 2009 to 2013 (P < 0.001). SRCC was more frequently seen in younger patients than in older ones, with a proportion of 32.3% in those aged <40 years decreasing to 10.5% in those aged ≥60 years (P < 0.001). Female were more likely to have SRCC than male (26.6% vs 12.2%, P < 0.001). The male-to-female ratio in SRCC was 1.24, as compared to the ratio of 3.24 in non-SRCC. Carcinomas located in the fundus or esophagogastric junction part of stomach were less likely to be SRCC than those in other sites (P < 0.001). Although Lauren classification was available for only 255 patients, statistically significance was observed between different types, with SRCC much more commonly seen in diffuse (47.7%) and mixed types (21.1%) than in intestinal type (3.4%) (P < 0.001). The majority of SRCC were poorly differentiated. Stage I cancer appeared to have less SRCC, but the difference between stages was not statistically significant. The distribution of SRCC did not vary considerably in the subgroups defined by ECOG performance status and surgery status. Among the patients who received radical operation, 4.2% (42/995) received neoadjuvant therapy, and there were more SRCC (28.6%) in the group with neoadjuvant therapy than in the one without (14.6%) (P = 0.013). Regarding palliative chemotherapy, there were more SRCC in the group receiving 1 to 2 drugs than in those receiving none or more than 2 drugs, and the taxane-based treatment group had more SRCC than other groups.
Table 1.
Patient characteristics.
3.2. Overall survival and prognostic factors
Kaplan–Meier survival curves are shown in Fig. 1. Of the 2199 cases, 1274 (57.9%) died during follow-up. The median overall survival of all patients as a whole was 20.8 (95% CI: 19.5–22.1) months (Fig. 1A). For SRCC and non-SRCC, the median overall survival was 15.9 (95% CI: 14.1–17.8) and 22.1 (95% CI: 20.7–23.5) months, respectively (Fig. 1B, log-rank test: P = 0.002). The results of univariate and multivariate Cox regression analyses to evaluate the prognostic value of various factors are shown in Table 2. Multivariate analyses showed that SRCC was an independent prognostic factor associated with worse survival (HR: 1.387, 95% CI: 1.177–1.634). Sensitivity analyses by changing the definition of SRCC (only the carcinomas containing 100% SRCC cells were counted as SRCC) did not change the results much (HR: 1.377, 95% CI: 1.081–1.754). We did not include differentiation in the multivariate analyses, as the majority of SRCC were poorly differentiated and thus inclusion of differentiation into the model would cause multicollinearity problem, in which case the prognostic effect of SRCC could be masked by differentiation. Lauren classification was not included in multivariate analyses either, because it was available for only a few patients and its inclusion in the model would severely reduced the statistical power.
Figure 1.
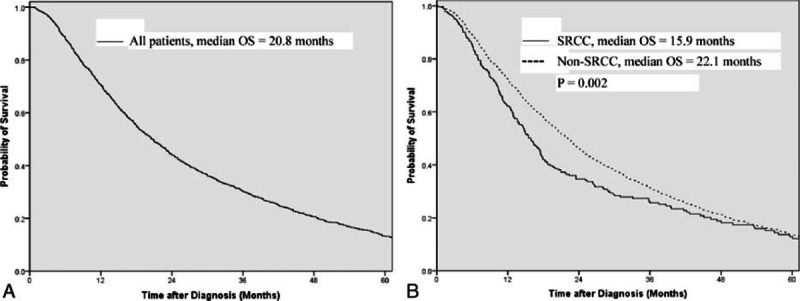
Survival curves for (A) all patients; (B) SRCC vs non-SRCC.
Table 2.
The prognostic value of patient characteristics.
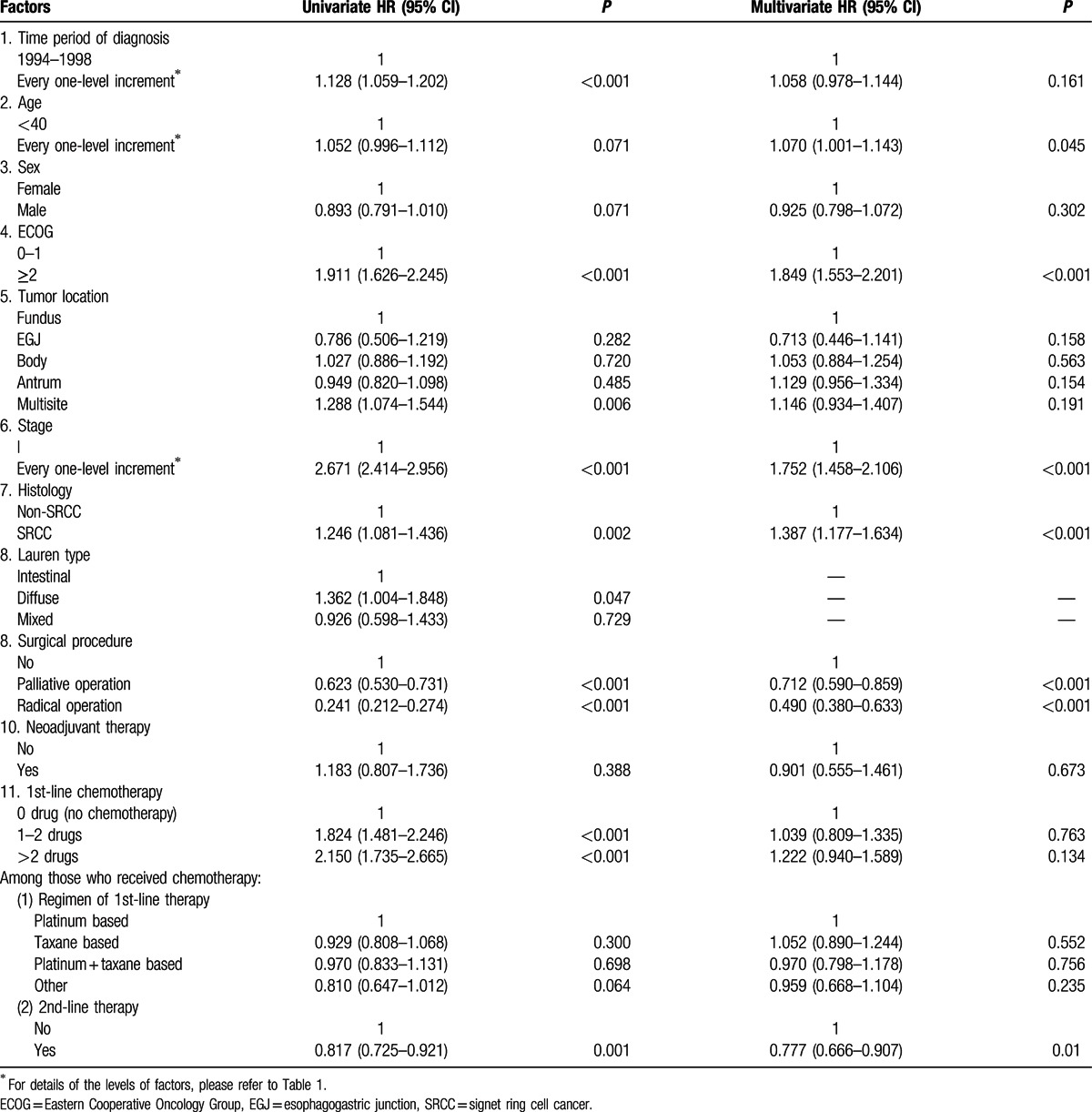
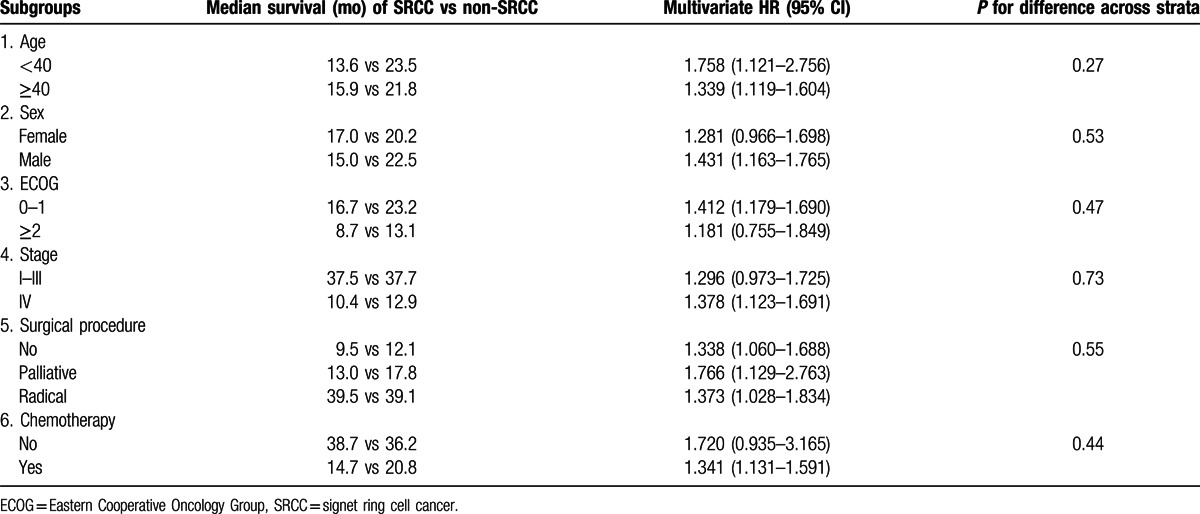
Apart from SRCC, older age at diagnosis (HR: 1.070, 95% CI: 1.001–1.143), poorer ECOG performance status (HR: 1.849, 95% CI: 1.553–2.201), and more advanced stage (HR: 1.752, 95% CI: 1.458–2.106) were also independent prognostic factors associated with worse overall survival, whereas surgery, including palliative resection (HR: 0.712, 95% CI: 0.590–0.859) and curative resection (HR: 0.490, 95% CI: 0.380–0.633), was independent prognostic factor associated with better overall survival. In this cohort, no evidence was found that the time period of diagnosis, sex, tumor location, neoadjuvant therapy, and first-line chemotherapy, regardless of the number and types of drugs used, had prognostic value, while second-line chemotherapy (HR: 0.777, 95% CI: 0.666–0.907) was associated with better survival. Subgroup analyses (Table 3) showed that the prognostic value of SRCC did not vary with sex, performance status, stage, and surgery and chemotherapy status.
Table 3.
Subgroup analyses for the prognostic value of SRCC.
4. Discussion
Although SRCC has long been regarded as an adverse prognostic factor of gastric cancer, the findings of existing studies on this issue are inconsistent (see Appendix 1 in the “Supplementary Appendix 1.docx,”). Here we tried to compare the characteristics of SRCC with those of non-SRCC, examine its prognostic value with control fro confounding, compare it with other major prognostic factors, and also take into account the potential interaction of SRCC with important clinicopathological factors in a single study.
SRCC was found to account for 16.1% of all patients with gastric cancer, falling in the range reported by previous studies.[30,31] Over the last 2 decades, the proportion of SRCC increased from 6% to 20%, which was consistent with the findings of previous studies from Western countries like France and the United States.[8–10,32] Of note, this trend of proportion does not necessarily mean that SRCC was increasing in incidence, because the overall incidence of gastric cancer has been decreasing. For example, the U.S. national surveillance data showed that the incidence of intestinal type decreased consistently from 1978 to 2005, whereas the incidence of diffuse type, which is significantly associated with SRCC, increased through 2000 but then declined in recent years.[33] In this study, SRCC, as compared with non-SRCC, was found to be more frequent in younger patients, female, diffuse type, and other sites than the fundus of stomach, such as the middle and lower thirds of stomach. These findings are in line with previous studies from China, other Asian countries, as well as Western countries.[17,34,35] The association of SRCC with stage of gastric cancer has been controversial. Some studies found that SRCC was more frequent in early stage gastric cancer, which might be due to its depressed lesions and characteristic appearance that make it easily detected,[36] while others found more SRCC in advanced gastric cancer, arguing that it was because most SRCC were located in gastric corpus, which led to the late onset of clinical symptoms.[31] In the present study, SRCC appeared to be slightly more in advanced stage, but the difference was not statistically significant. Thus, this issue remains to be further investigated.
A lot of studies have evaluated the prognostic value of various factors in gastric cancer. Two studies from the United States[17,37] found that Asian people had better prognosis than African American, whereas white people and Hispanic people lay in between them with comparable risk of mortality. Gill et al[38] found that the benefit from surgery was greater in Asians than non-Asians. Older age was consistently found to be associated with worse prognosis in previous studies,[8,17,34,37,39,40] which was replicated in the present one. However, as the way age was treated in regression models differed across studies (e.g., age as a continuous or ordinal variable), the results from different studies are not directly comparable. To our knowledge, no studies have found significant association between sex and overall survival of gastric cancer in multivariate analysis, although Dittmar et al[41] found that female had shorter recurrence-free survival than male. In this study, we found no evidence for the prognostic value of sex either. There was no statistically significant association between time period of diagnosis and overall survival, indicating that the prognosis of gastric cancer has not been improved much over the last 2 decades, similar to the situation in Western countries.[2,3] Not surprisingly, stage at diagnosis, surgery (especially curative resection) and ECOG performance status were found to be the 3 most powerful prognostic factors for gastric cancer, while the impact of age at diagnosis was modest. We did not find that tumor location was associated with overall survival, contrary to previous studies showing that proximally located cancer had worse prognosis than those located in antrum or pylorus.[37,38,42]
As mentioned previously in the Introduction section, the prognostic value of SRCC has long been controversial, with its relation to the stage of cancer being especially heavily investigated. Difference in statistical methods might partly explain the heterogeneity of results from existing studies. For example, some studies conducted univariate analyses only, while others employed a multivariate approach. Studies doing both kinds of analysis have shown that the statistically significant association observed in univariate analyses could disappear in multivariate analyses after control for confounding.[20,43] However, even the results of multivariate analyses from different studies still contradicted with each other in some cases. Several hypotheses have been proposed to explain the difference.[15,44] In this study, SRCC was found to be significantly associated with worse survival in gastric cancer, which did not vary with age, sex, ECOG performance status, stage of cancer, and surgery and chemotherapy status. This may be because SRCC has greater potential for infiltrative growth, lymph node metastasis, and distant metastasis characterized by peritoneal dissemination, which are all associated with poor prognosis.[19,45] Our findings support the notion that SRCC may represents a disease biologically distinct from gastric adenocarcinoma.[46] In fact, some gene expression data also supported this concept.[47] Thus, the results of this study have important implications for the clinical management of SRCC, including the type of surgery to be conducted, use of adjuvant therapy and follow-up strategy.
Randomized controlled trials and meta-analyses showed that chemotherapy was effective and could prolong the overall survival by approximately 6 months as compared with best supportive alone in patients with advanced gastric cancer.[48] Interestingly, first-line chemotherapy was not associated with better survival in the present study. The 3-drug regimens even seemed to have statistically nonsignificant detrimental effects. It is not impossible that these results were confounded by stage and surgery; however, we have actually adjusted for these factors in the multivariate analyses. In addition, our post hoc stratified analyses showed that the results did not differ much between the patients receiving surgery and those who did not. Thus, it is unlikely that the results have been distorted by the surgery status of patients. We propose that the difference between our results and those of randomized trials favoring chemotherapy could be due to the following reasons among others. First, overall survival in randomized trials usually starts from the time of randomization, while in this study it starts from the time of first diagnosis of gastric cancer. The former approach excludes the survival time from first diagnosis to randomization, and could not reflect the prognostic impact of chemotherapy relative to that of other factors such as surgery. Second, patients included in randomized trials are generally in better condition, more homogeneous, and more likely to respond to chemotherapy than those treated in routine clinical settings where the efficacy of chemotherapy is greatly diluted by various factors. Third, the present study is a retrospective one and may suffer from unmeasurable bias. For example, there might be some important confounding factors that were not adjusted for by multivariate analyses.
Apart from the retrospective nature of study as just mentioned, another limitation of this study is that we obtained limited data on Lauren classification and no data on tumor size, lymphovascular invasion, lymph node metastasis, distant metastasis and time to recurrence, which are important to describe the behaviors of tumor and could help better explain the adverse prognostic effect of SRCC. Despite these problems, we think this study still has its own merit due to the relatively large sample size, comprehensive data analyses, and systematic summary of literature. In the future, specific strategies most suitable for the treatment and management of SRCC may be worthy of further investigation.
In conclusion, we found that SRCC, as compared with non-SRCC, accounted for increasingly more of gastric cancer and was associated with younger age, female sex, poor differentiation, diffuse type, and distal location. It was an independent prognostic factor associated with worse survival in gastric cancer. Early detection and aggressive treatments for the disease are thus warranted.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, ECOG = Eastern Cooperative Oncology Group, HR = hazard ratio, SRCC = signet ring cell cancer.
ML and ZY contributed equally to this study.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012—stomach cancer. World Heal Organ. 2015. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Accessed October 5, 2015. [Google Scholar]
- 2.Lau M, Le A, El-Serag HB. Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in incidence and survival. Am J Gastroenterol 2006; 101:2485–2492.doi:10.1111/j.1572-0241.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 3.Le A, Berger D, Lau M, et al. Secular trends in the use, quality, and outcomes of gastrectomy for noncardia gastric cancer in the United States. Ann Surg Oncol 2007; 14:2519–2527.doi:10.1245/s10434-007-9386-8. [DOI] [PubMed] [Google Scholar]
- 4.Fitzmaurice C, Dicker D, Pain A, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015; 1:505–527.doi:10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson LA, Tavilla A, Brenner H, et al. Survival for oesophageal, stomach and small intestine cancers in Europe 1999–2007: results from EUROCARE-5. Eur J Cancer 2015; 51:2144–2157.doi:10.1016/j.ejca.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Huang C-M, Wang J-B, et al. Survival and surgical outcomes of cardiac cancer of the remnant stomach in comparison with primary cardiac cancer. World J Surg Oncol 2014; 12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 8.Arsène D, Chomontowski J, Pottier D, et al. Epidemiology and prognosis of gastric carcinomas at the province of Calvados. A 10-year study. Gastroenterol Clin Biol 1995; 19:797–803. [PubMed] [Google Scholar]
- 9.Antonioli DA, Goldman H. Changes in the location and type of gastric adenocarcinoma. Cancer 1982; 50:775–781. [DOI] [PubMed] [Google Scholar]
- 10.Henson DE, Dittus C, Younes M, et al. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med 2004; 128:765–770.doi:10.1043/1543-2165(2004)128<765:DTITIA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Fléjou J-F. WHO Classification of digestive tumors: the fourth edition. Ann Pathol 2011; 31 (suppl. 5):S27–S31.doi:10.1016/j.annpat.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Lauran P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt of a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64:31–49. [DOI] [PubMed] [Google Scholar]
- 13.Ming SC. Gastric carcinoma. A pathobiological classification. Cancer 1977; 39:2475–2485. [DOI] [PubMed] [Google Scholar]
- 14.Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn 1982; 32 (suppl. 2):329–347. [PubMed] [Google Scholar]
- 15.Kwon K-J, Shim K-N, Song E-M, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer 2013; 17:43–53.doi:10.1007/s10120-013-0234-1. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro MM, Sarmento JA, Sobrinho Simões MA, et al. Prognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinoma. Cancer 1981; 47:780–784. [DOI] [PubMed] [Google Scholar]
- 17.Taghavi S, Jayarajan SN, Davey A, et al. Prognostic significance of signet ring gastric cancer. J Clin Oncol 2012; 30:3493–3498.doi:10.1200/JCO.2012.42.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shridhar R, Almhanna K, Hoffe SE, et al. Increased survival associated with surgery and radiation therapy in metastatic gastric cancer: a Surveillance, Epidemiology, and End Results database analysis. Cancer 2013; 119:1636–1642.doi:10.1002/cncr.27927. [DOI] [PubMed] [Google Scholar]
- 19.Piessen G, Messager M, Leteurtre E, et al. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 2009; 250:878–887.doi:10.1097/SLA.0b013e3181b21c7b. [DOI] [PubMed] [Google Scholar]
- 20.Kim DY, Park YK, Joo JK, et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg 2004; 74:1060–1064.doi:10.1111/j.1445–1433.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C-G, Wang Z-N, Sun Z, et al. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: results from a chinese mono-institutional study. J Surg Oncol 2011; 103:700–703.doi:10.1002/jso.21878. [DOI] [PubMed] [Google Scholar]
- 22.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108.doi:10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 23.Bu Z, Zheng Z, Li Z, et al. Clinicopathological and prognostic differences between mucinous gastric carcinoma and signet-ring cell carcinoma. Chin J Cancer Res 2013; 25:32–38.doi:10.3978/j.issn.1000-9604.2013.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Zhang H, Tian L, et al. The difference in clinic-pathological features between signet ring cell carcinoma and gastric mucinous adenocarcinoma. Tumour Biol 2013; 34:2625–2631.doi:10.1007/s13277-013-0812-1. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Shi Y, Yuan J, et al. Evaluation of docetaxel- and oxaliplatin-based adjuvant chemotherapy in postgastrectomy gastric cancer patients reveals obvious survival benefits in docetaxel-treated mixed signet ring cell carcinoma patients. Med Oncol 2014; 31:1–11. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Zhu G, Zhang H, et al. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg 2009; 14:601–606.doi:10.1007/s11605-009-1127-9. [DOI] [PubMed] [Google Scholar]
- 27.Young J, Badgery-Parker T, Dobbins T, et al. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage 2015; 49:258–264.doi:10.1016/j.jpainsymman.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Edge S, Byrd D, Compton C, et al. AJCC Cancer Staging Manual. 7th ed.New York: Springer-Verlag; 2009. [Google Scholar]
- 29.Piessen G, Messager M, Robb WB, et al. Gastric signet ring cell carcinoma: how to investigate its impact on survival. J Clin Oncol 2013; 31:2059–2060.doi:10.1200/JCO.2012.47.4338. [DOI] [PubMed] [Google Scholar]
- 30.Yokota T, Kunii Y, Teshima S, et al. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med 1998; 186:121–130. [DOI] [PubMed] [Google Scholar]
- 31.Hass HG, Smith U, Jäger C, et al. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren's): single-center experience of 160 cases. Onkologie 2011; 34:682–686.doi:10.1159/000334545. [DOI] [PubMed] [Google Scholar]
- 32.Gurzu S, Kadar Z, Sugimura H, et al. Maspin-related orchestration of aggressiveness of gastric cancer. Appl Immunohistochem Mol Morphol 2016; 24:326–336. [DOI] [PubMed] [Google Scholar]
- 33.Wu H, Rusiecki JA, Zhu K, et al. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev 2009; 18:1945–1952.doi:10.1158/1055-9965.EPI-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui J, Liang H, Deng J, et al. Clinicopathological features and prognostic analysis of patients with signet ring cell gastric carcinoma. Zhonghua Zhong Liu Za Zhi 2015; 37:367–370. [PubMed] [Google Scholar]
- 35.Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol 1994; 3:221–227. [DOI] [PubMed] [Google Scholar]
- 36.Otsuji E, Yamaguchi T, Sawai K, et al. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol 1998; 67:216–220. [DOI] [PubMed] [Google Scholar]
- 37.Yao JC, Tseng JF, Worah S, et al. Clinicopathologic behavior of gastric adenocarcinoma in Hispanic patients: analysis of a single institution's experience over 15 years. J Clin Oncol 2005; 23:3094–3103.doi:10.1200/JCO.2005.08.987. [DOI] [PubMed] [Google Scholar]
- 38.Gill S, Shah A, Le N, et al. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a canadian cancer center. J Clin Oncol 2003; 21:2070–2076.doi:10.1200/JCO.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 39.Shim JH, Song KY, Kim H-H, et al. Signet ring cell histology is not an independent predictor of poor prognosis after curative resection for gastric cancer. Medicine (Baltimore) 2014; 93:e136.doi:10.1097/MD.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon HH, Khan M, Shi Q, et al. The prognostic value of clinical and pathologic factors in esophageal adenocarcinoma: a mayo cohort of 796 patients with extended follow-up after surgical resection. Mayo Clin Proc 2010; 85:1080–1089.doi:10.4065/mcp.2010.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dittmar Y, Schüle S, Koch A, et al. Predictive factors for survival and recurrence rate in patients with node-negative gastric cancer—a European single-centre experience. Langenbecks Arch Surg 2015; 400:27–35.doi:10.1007/s00423-014-1226-2. [DOI] [PubMed] [Google Scholar]
- 42.Bamboat ZM, Tang LH, Vinuela E, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol 2014; 21:1678–1685.doi:10.1245/s10434-013-3466-8. [DOI] [PubMed] [Google Scholar]
- 43.Heger U, Blank S, Wiecha C, et al. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol 2014; 21:1739–1748.doi:10.1245/s10434-013-3462-z. [DOI] [PubMed] [Google Scholar]
- 44.Kunisaki C, Shimada H, Nomura M, et al. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg 2004; 91:1319–1324.doi:10.1002/bjs.4637. [DOI] [PubMed] [Google Scholar]
- 45.Lee HH, Song KY, Park CH, et al. Undifferentiated-type gastric adenocarcinoma: prognostic impact of three histological types. World J Surg Oncol 2012; 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah MA, Khanin R, Tang L, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res 2011; 17:2693–2701.doi:10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan IB, Ivanova T, Lim KH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology 2011; 141:476–485.485.e1–485.e11. doi:10.1053/j.gastro.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24:2903–2909.doi:10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


