Abstract
The aim of this study is to investigate the incidence and clinical outcomes of primary mediastinal large B-cell lymphoma (PMBL).
Here we did a retrospective analysis using the surveillance, epidemiology, and end results (SEER) database to analyze the incidences and survival of patients with PMBL diagnosed during 2001–2012 among major ethnic groups.
During 2001–2012, a total of 426 PMBL patients were identified, including 336 whites, 46 blacks, and 44 others. The incidence rates of female to male ratios in white, black, and other were 1.4938, 1.1202, and 1.7303 respectively, suggesting that the female-prominent disease occurrence was seen only in whites and others, but not in black population. Compared to white, the other had a worse 5-year overall survival (OS); however, factors including age, race, socioeconomic status, and stage associated with OS showed no significant difference among ethnic groups; thus, biology factors should be explored to explain the racial difference in OS.
In conclusion, our findings revealed diversities in demographic features and prognosis among different racial groups.
Keywords: end results program, epidemiology, incidence, primary mediastinal large B-cell lymphoma, race, surveillance, survival
1. Introduction
Primary mediastinal diffuse large B-cell lymphoma (PMBL) was first described in the 1980s[1] and is considered as a distinct clinicopathologic entity of diffuse large B-cell lymphoma (DLBCL). PMBL is characterized by a rapidly growing mediastinal mass, frequently accompanied by local invasiveness and occasionally with distant metastasis.[2,3] This subtype tends to occur in young adults in their third to fourth decade of age with a slightly female predominance.[3–5] Strikingly, not only clinical similarities exist between PMBL and Hodgkinlymphoma (HL), the gene expression profiles also demonstrate greater similarities between PMBL and HL than with DLBCL.[6,7] Due to the rarity of this disease, the optimal therapy has not been defined. However, recent studies have shown that regimens integrating rituximab into intensive chemotherapy might yield a better outcome.[8–11]
Studies on the molecular biology of PMBL have revealed interesting information. Gene expression profiling data showed that molecular pathways including REL, JAK-STAT, PDL1/PDL2, and nuclear factor κ-β(NF-κβ) are involved in the pathogenesis of PMBL.[12–16] In the therapeutic area, several retrospective studies have shown that integrated rituximab into chemotherapy have improved PMBL cure rates from 50% to 80%.[9–11] Rituximab is a monoclonal antibody against CD20 protein, which eleminates CD20-positive B lymphoma cells through a combination of immune-mediated effects including antibody-dependent cell-mediated cytotoxicity, complement-mediated lysis, and direct effects induced by CD20 ligation.[17] A prospective phase II study showed that dose-adjusted EPOCH in combination with rituximab could yield a 93% of 5-year event-free survival and 97% of OS.[8] Radiation is widely used in cancer therapy, which eliminates cancer cells by inducing DNA damage and generation of reactive oxygen species (ROS).[18–20] Considered the long-term toxicities of mediastinal radiation therapy in young PMBL patients, 1 approach currently being explored to minimize toxicity is to use chemoimmunotherapy without radiation consolidation. A recent study has shown that radiation yield no benefit for treatment of PBML in the era of rituximab.[21]
Disparities across races have been reported in large cohorts of other cancers including breast cancer, colon cancer, prostate cancer, lung cancer, multiple myeloma, and certain subtype of non-Hodgkin's lymphoma,[22–25] and such studies have yielded important information on the respective cancer occurrence and disease outcomes in various ethnic groups. Although several single-institution studies have described the disease clinical, immunophenotypic, and genotypic characteristics and clinical outcome of PMBL, there is a lack of study on the PMBL incidences among various ethnic groups and their respective patient survival. The objectives of this study were to examine the incident patterns and survival outcomes of PMBL in the major ethnic groups, including whites, blacks, American Indian, and Asian/Pacific Islanders using the SEER database.
2. Methods
2.1. Data source
Patients with PMBL were identified from the surveillance, epidemiology, and end results (SEER) program database of the National Cancer Institute in the United States. SEER is a program that collects and publishes cancer data from population-based cancer registries covering ∼28% of the US population.[26] The 18 registries in SEER-18 include ∼25% of White population, 26% of Black population, 38% of Hispanic population, 44% of American Indians and Alaska (A/PI) population, 50% of Asians, and 67% of Hawaiian/Pacific Islanders.[26] The 18 SEER registries including Atlanta, Detroit, Greater California, Greater Georgia, Hawaii, Iowa, Kentucky, Los Angeles, New Mexico, New Jersey, Rural Georgia, states of Connecticut, San Francisco-Oakland, Seattle-Puget Sound, San Jose-Monterey, the Alaska Native Tumor Registry, Louisiana, and Utah.
We analyzed the incidence and survival of PMBL diagnosed among residents of 18 SEER program registries during 2001–2012. The International Classification of Disease for Oncology (ICD-O)-3 code used for PMBL was 9679. Ethical approval of this study by Institutional review board (IRB) is not required as this study only involves analysis of publically available database (SEER) without linking to patient private information.
2.2. Study variables
SEER program database routinely contains variables including patients’ demographics (age, race/ethnicity, sex, and socioeconomic status), tumor histology, anatomical site, stage at diagnosis, radiation usage, survival and follow-up for vital status. Diagnosis of PMBL was based on the International Classification of Disease for Oncology (3rd edition, ICD-O-3) histology codes in the SEER data. Area socioeconomic status was determined by the county poverty rate,[26] which is the percentage of persons in the county living below the national poverty threshold in Census 2000. The county poverty rates in this study were categorized as reported previously.[26]
Our analyses were adjusted for age, sex, race (white, black, other), year of diagnosis, treatment with radiation, Lymphoma–Ann Arbor stage, and poverty. Incidence rates were calculated via the latest version of SEER∗Stat software version8.2.1 (http://www.seer.cancer.gov/seerstat), expressed as per 1,000,000 population, age-adjusted to the year 2000 US standard population. OS is typically measured from the date of diagnosis to the date of death or the last follow-up using the Kaplan–Meier method. The log-rank test was used to compare the survival difference. To analyze the clinical variables with OS, we used Cox proportional hazards models to calculate multivariable-adjusted hazard ratios (HR) and associated 95% confidence intervals (CI). A P value < 0.05 was considered as statistically significant. The statistical analysis was performed using the SPSS version 16.0 software (SPSS A, Inc, Chicage, IL).
3. Results
3.1. Incidence of PMBL according to race, gender, and age
Between 2001 and 2012, a total of 451 of PMBL patients were reported to the SEER 18 registries, 3 cases with unknown race, and 22 cases with age <18 years at diagnosis were excluded from the analysis. The final study population contained 426 cases (Fig. 1). The 426 cases of PMBL analyzed in this study included 336 whites, 46 blacks, and 44 others. Table 1 describes the clinical characteristics of PMBL based on ethnic groups. The median age of PMBL in white, black, and other was 37, 36, and 37 years, respectively. From 2001 to 2012, there was a trend toward increasing incidence rates in all subgroups by race and sex (Fig. 2). The age-adjusted PMBL incidence rates in white, black, and others were 0.4285, 0.3736, and 0.3793 per million person-years, respectively (Table 2). Females had higher incidence rate than males for PMBL. The female-to-male (F/M) incidence rate ratio (IRR) was 1.4635 for all races combined (P = 0.0001), 1.4938 for white (P = 0.0003), 1.1202 for black (P = 0.8304), and 1.7303 (P = 0.1076) for others (Table 2). Female predominant occurrence of PMBLwas observed in white and others, and this is consistent with previous published observation.[4,5] However, within the black cohort, female and male exhibited a similar incidence rate (P = 0.8304), which was unexpected and has not been reported previously. The age distribution of PMBL across race and sex is illustrated in Fig. 3. All races showed a unimodal pattern of age-distribution with incidence peak at age 30 to 39 years.
Figure 1.
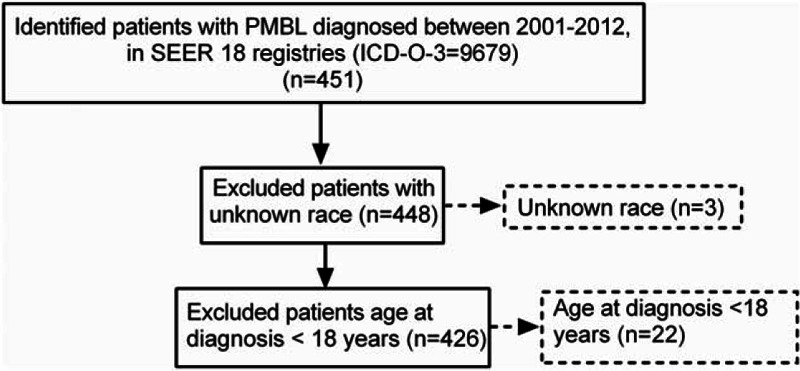
Selection of study cohort is shown. This figure provides an overview of the study cohort with reasons for inclusion/exclusion through the selection process. The numbers in boldface denote the cases included in the incidence and survival analyses. ICD-O-3 = International Classification of Diseases for Oncology, third edition; PMBL = primary mediastinal large B-cell lymphoma, SEER = surveillance, epidemiology, and end results.
Table 1.
Clinical features of patients with PMBL by race, SEER 18, 2001 to 2012.
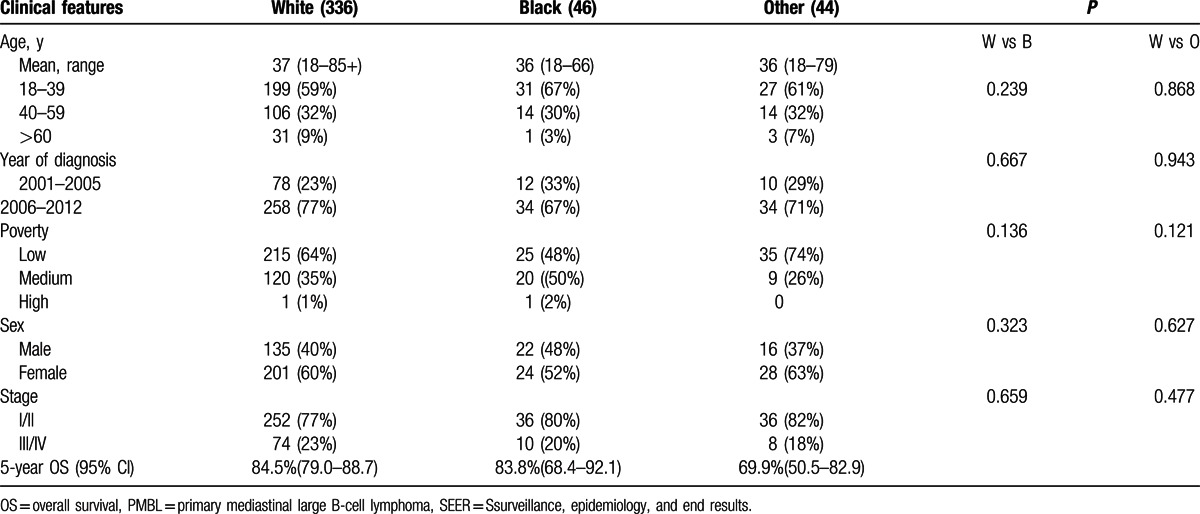
Figure 2.
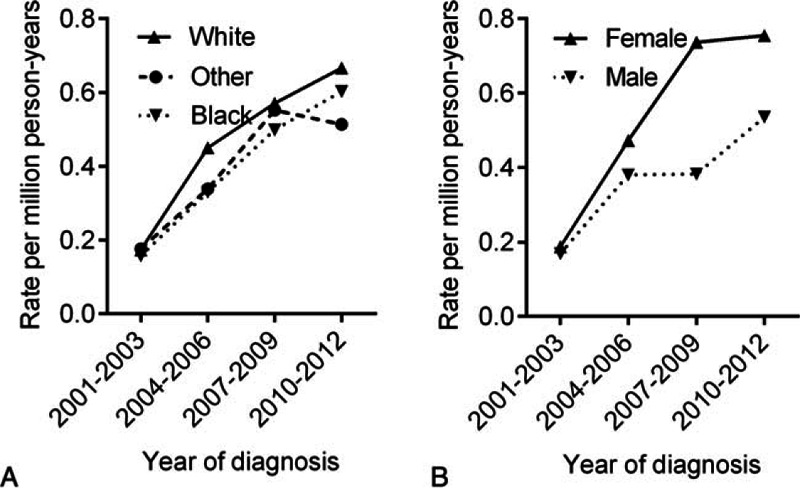
Trends in incidence of PMBL during the time period covered in this study according to race and sex. All incidence rates are age-adjusted to the 2000 US population and expressed as per 1000,000 population. PMBL = primary mediastinal large B-cell lymphoma.
Table 2.
Age-adjusted IRs and IRRs of PMBL based on race and sex, SEER 18, 2001–2012.
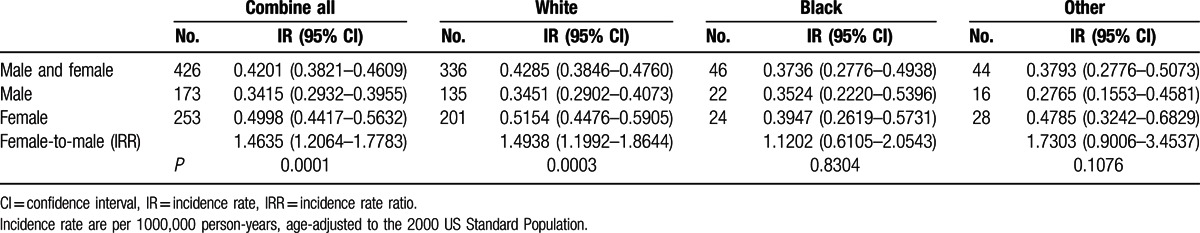
Figure 3.
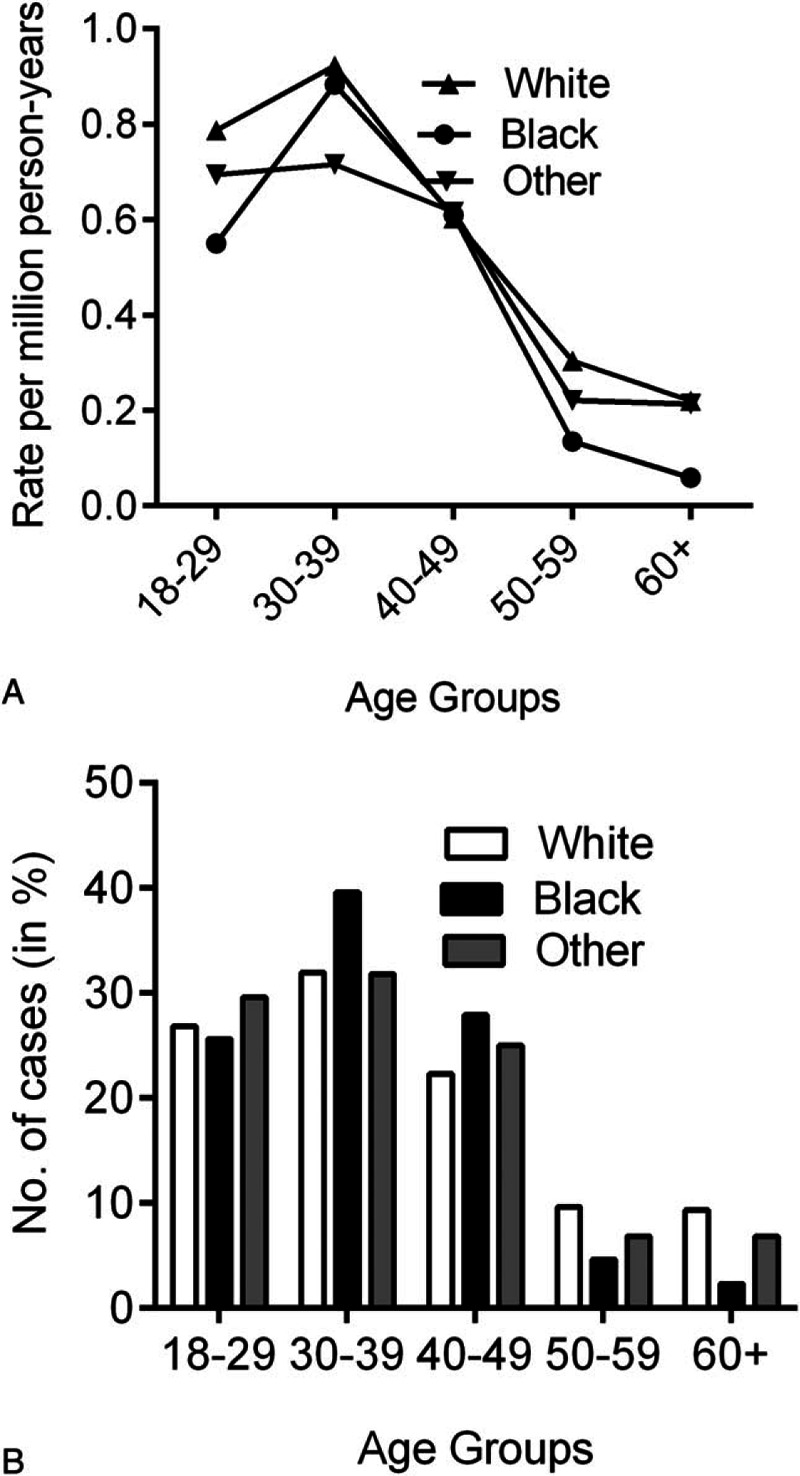
Age distribution of PMBL is shown by race. (A) Age-specific incidence rates by race, SEER-18, 2001 to 2012. (B) Horizontal axis represents the grouping of age at diagnosis. Vertical axis represents the proportion of patients in each age group of that particular race (white, black, and others). PMBL = primary mediastinal large B-cell lymphoma, SEER = surveillance, epidemiology, and end results.
3.2. Survival analysis
We analyzed overall survival (OS) according to race. The 5-year OS for whites, blacks, and others were 84.5%, 83.8% and 69.9% respectively (Table 1). Kaplan–Meier analysis showed that there was no significant difference in OS between blacks and whites, but the others group had a significantly lower OS compared to whites (Table 3). OS significantly decreased in patients >60 years with advanced stage, or with medium poverty (Fig. 4). We also analyzed radiation therapy on patient's survival. As shown in Fig. 5, radiation therapy significantly improved patients’ survival during period of 2001–2012. However, analysis of PMBL diagnosed in 2006–2012 showed that radiation did not influence OS, consistent with a recent publication.[21]
Table 3.
Univariate and multivariate analysis of prognostic factors in PMBL.
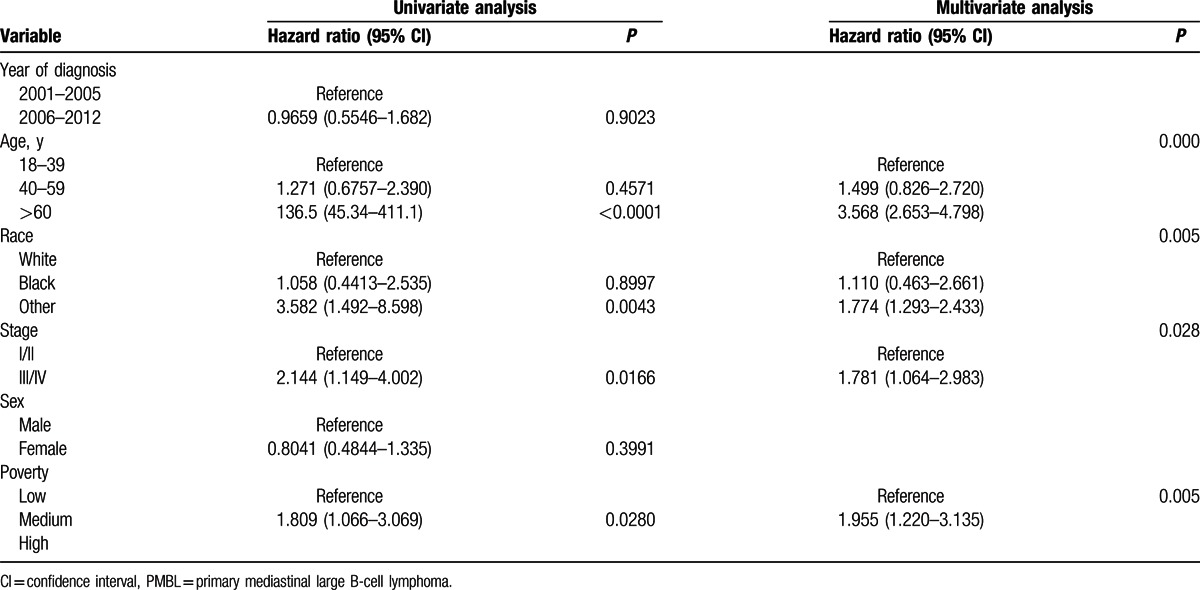
Figure 4.
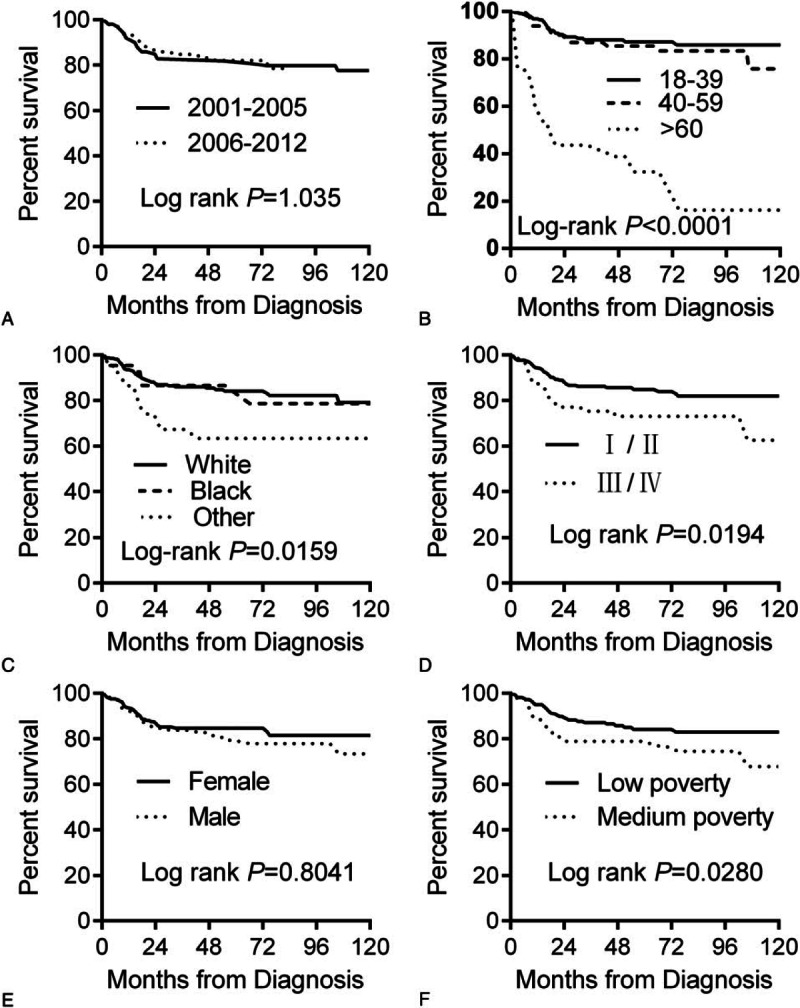
Kaplan–Meier curves for overall survival in patients with PMBL: (A) years diagnosed 2001 to 2005 vs 2006 to 2012; (B) by age; (C) by race; (D) by stage; (E) by sex; (F) by socioeconomic status. PMBL = primary mediastinal large B-cell lymphoma.
Figure 5.
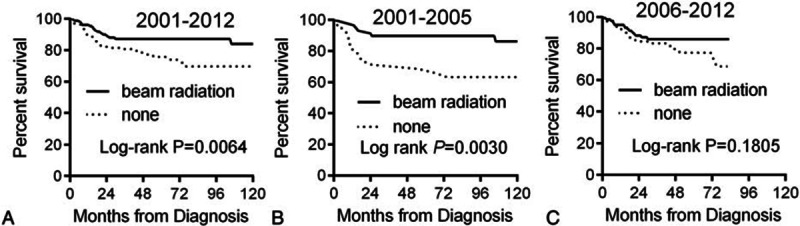
Kaplan–Meier curves for overall survival in patients received with or without radiation therapy: (A) years diagnosed 2001 to 2012; (B) years diagnosed 2001 to 2005; (C) years diagnosed 2006 to 2012.
On multivariate Cox regression (Table 3), patients >60 years had inferior OS compared with patients who were 18 to 39 year old (age 60+ vs age 18–39: HR = 3.568[2.653–4.798]); other had inferior OS compared with white (other vs white: HR = 1.774 [95% CI 1.293–2.433]); patients in the late stage also showed inferior OS compared with early stage: (III/IV vs I/II: HR = 1.781 [95% CI 1.064–2.983]); socioeconomic status was also an independent predictor of OS (medium poverty vs low poverty: HR = 1.955 [95% CI 1.220–3.135]).
4. Discussion
Currently there is very limited information reported in the literature regarding the demographics of primary mediastinal large B-cell lymphoma. In this study, we explored the disease incidence trends and survival of PMBL (defined by the WHO classification) among the major ethnic groups. Our analysis revealed a trend of increasing disease incidence rates in all racial and gender subgroups during 2001–2012 (Fig. 2). This increase may be partially attributed to increased risks of exposure to factors associated with lymphomagenesis, although the exact causes for this increase are still unclear. One plausible explanation for the higher incidence could be the increased recognition for this subtype of lymphoma. Previously, PMBL was considered as 1 subtype of DLBCL arising in the mediastinum. With the advances in recognition of the clinical characteristics and immunophenotypic features for this disease, PMBL was listed as a separate entity in WHO classification in 2001.[27] Recent progress in molecular characterization further helps the differentiation between PMBL and DLBCL.[6,7] As such, more PMBL are separated from other forms of DLBCL and classic Hodgkin lymphoma (cHL).
Our analysis revealed a unimodal distribution of age-specific incidence with peak at 30 to 39 years for whites, blacks, and other groups. Notably, blackwomen and men exhibited a similar incidence rate, whereas in whites the PMBL incidences were significantly higher in women. Although many factors including genetic susceptibility, environmental risk, dietary factors, occupational exposures, autoimmune diseases, infectious conditions, and socioeconomic status are known to affect cancer incidences,[28,29] the reasons for complex demographics of PMBL observed in different ethnic groups currently remain unclear. Further study in this area, especially investigation of the roles of genetic factors, gender factors, and gene–environment interactions in affecting development of PMBL is clearly needed.
We also found significant racial disparities in PMBL survival. Our analysis showed that white patients had a better overall survival (OS) compared with others. Interestingly, a single institution study in China showed that the 3-year OS for patients with PMBL was 70%,[30] which seems unfavorable compared to the OS in white and black population reported in the SEER database. There are several potential explanations of why survival may differ in different ethnic groups. The potential factors that contribute to the disparities in clinical outcomes include disease biology, host pharmacogenetics,[31] delay in therapy,[32] hospital factors,[33] access to health care,[34] and differences in socioeconomic status as noted previously.[35] In our study, there were no significant differences in disease stages, radiation (data not shown), and other factors that may impact the prognosis among different ethnic groups. Thus, biology factors should be further explored to explain the racial difference in OS.
We also evaluated the role of radiation for treatment of PMBL. Like Hodgkin's lymphoma, PMBL mainly affects young adults and shows the typical localized mediastinal tumor. The similar clinical problems associated with HL treatment, the long-term toxicities induced by mediastinal radiation therapy in young adult PMBL female patients are of particular concerns. The toxic side-effects include potential development of breast and lung cancers, hypothyroidism, and accelerated atherosclerotic heart disease. Whether radiation therapy is still essential for treatment of PMBL has been controversial. In the prerituximab era, retrospective analyses showed that dose-intensified regimens appeared more effective than CHOP, and the addition of radiation therapy was associated with improved long-term outcomes.[36–38] Since 2000, the anti-CD20 monoclonal antibody rituximab was incorporated into combinations with CHOP regimen for treatment of DLBCL.[39] The role of radiation in the treatment of PMBL should be re-evaluated in the era of rituximab-based chemotherapy. A recent prospective study showed that nonradiation containing dose-adjusted EPOCH-R regimen yield excellent outcome and obviate the need for RT in the large majority of patients.[8] In our study, we found radiation can significantly improve PMBL OS in patients diagnosed 2001 to 2005, when rituximab not widely used in USA, but have no influence on OS in patients diagnosed 2006 to 2012, a period of rituximab era; this observation is confirmed by a recent publication.[21]
The potential limitations of this study include the relatively small sample size and the lack of detail treatment information for the PMBL patients. Although the SEER database is the largest cancer registry in the United States, the sample size of PMBL in the current study is still relatively small. As no specific treatment regimens are available in the SEER databases, the impact of specific treatment regimens on patient survival remains unclear. As such, these data should be interpreted with caution.
In summary, our large population-based analysis of SEER database has led to a number of significant new findings. These novel findings include: (1) in contrast to the female-prominent disease occurrence, blacks have a similar female to male ratio incidence rate; (2) over the entire study period, OS was higher in whites than in others; (3) radiation therapy should be re-evaluated in the PMBL treatment in the rituximab era. More comprehensive studies are needed to further validate these observations.
Footnotes
Abbreviations: CI = confidence intervals, DLBCL = diffuse large B-cell lymphoma, HL = Hodgkin lymphoma, HR = hazard ratios, ICD-O = International Classification of Disease for Oncology, OS = overall survival, PMBL = primary mediastinal large B-cell lymphoma, ROS = reactive oxygen species, SEER = surveillance, epidemiology, and end results.
P-PL and K-FW contributed equally to this study.
Funding: this work was supported in part by the natural science foundation of Guangdong province (Grant number: 2014A030310421) and by grants 16ykpy20 from the Young Teacher Fund of Sun Yat-sen University.
The authors have no conflicts of interest to disclose.
References
- 1.Lichtenstein AK, Levine A, Taylor CR, et al. Primary mediastinal lymphoma in adults. Am J Med 1980; 68:509–514. [DOI] [PubMed] [Google Scholar]
- 2.van Besien K, Kelta M, Bahaguna P. Primary mediastinal B-cell lymphoma: a review of pathology and management. J Clin Oncol 2001; 19:1855–1864. [DOI] [PubMed] [Google Scholar]
- 3.Faris JE, LaCasce AS. Primary mediastinal large B-cell lymphoma. Clin Adv Hematol Oncol 2009; 7:125–133. [PubMed] [Google Scholar]
- 4.Boleti E, Johnson PW. Primary mediastinal B-cell lymphoma. Hematol Oncol 2007; 25:157–163. [DOI] [PubMed] [Google Scholar]
- 5.Cazals-Hatem D, Lepage E, Brice P, et al. Primary mediastinal large B-cell lymphoma. A clinicopathologic study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA (“Groupe d’Etude des Lymphomes de l’Adulte”) study. Am J Surg Pathol 1996; 20:877–888. [DOI] [PubMed] [Google Scholar]
- 6.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 2003; 198:851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood 2003; 102:3871–3879. [DOI] [PubMed] [Google Scholar]
- 8.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med 2013; 368:1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassilakopoulos TP, Pangalis GA, Katsigiannis A, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy in primary mediastinal large B-cell lymphoma: the emerging standard of care. Oncologist 2012; 17:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu LM, Fang H, Wang WH, et al. Prognostic significance of rituximab and radiotherapy for patients with primary mediastinal large B-cell lymphoma receiving doxorubicin-containing chemotherapy. Leuk Lymphoma 2013; 54:1684–1690. [DOI] [PubMed] [Google Scholar]
- 11.Zinzani PL, Stefoni V, Finolezzi E, et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: a retrospective study. Clin Lymphoma Myeloma 2009; 9:381–385. [DOI] [PubMed] [Google Scholar]
- 12.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116:3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joos S, Kupper M, Ohl S, et al. Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Res 2000; 60:549–552. [PubMed] [Google Scholar]
- 14.Schmitz R, Hansmann ML, Bohle V, et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J Exp Med 2009; 206:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood 2014; 123:2062–2065. [DOI] [PubMed] [Google Scholar]
- 16.Weniger MA, Gesk S, Ehrlich S, et al. Gains of REL in primary mediastinal B-cell lymphoma coincide with nuclear accumulation of REL protein. Genes Chromosomes Cancer 2007; 46:406–415. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Opin Immunol 2008; 20:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009; 458:780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell S, McMillan TJ. DNA damage and repair following treatment with ionizing radiation. Radiother Oncol 1990; 19:95–108. [DOI] [PubMed] [Google Scholar]
- 20.Ward JF. Biochemistry of DNA lesions. Radiat Res Suppl 1985; 8:S103–111. [PubMed] [Google Scholar]
- 21.Giri S, Bhatt VR, Pathak R, et al. Role of radiation therapy in primary mediastinal large B-cell lymphoma in rituximab era: a US population-based analysis. Am J Hematol 2015; 90:1052–1054. [DOI] [PubMed] [Google Scholar]
- 22.Evens AM, Antillon M, Aschebrook-Kilfoy B, et al. Racial disparities in Hodgkin's lymphoma: a comprehensive population-based analysis. Ann Oncol 2012; 23:2128–2137. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Wang Y, Wang Z, et al. Racial differences in three major NHL subtypes: descriptive epidemiology. Cancer Epidemiol 2015; 39:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Ma S. Racial differences in mantle cell lymphoma in the United States. BMC Cancer 2014; 14:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood 2010; 116:5501–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, Sun H, Sun X. Survival improvement by decade of patients aged 0-14 years with acute lymphoblastic leukemia: a SEER analysis. Sci Rep 2014; 4:4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffe ES, Harris NL, Stein H, et al. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 28.Koshiol J, Lam TK, Gridley G, et al. Racial differences in chronic immune stimulatory conditions and risk of non-Hodgkin's lymphoma in veterans from the United States. J Clin Oncol 2011; 29:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smedby KE, Hjalgrim H. Epidemiology and etiology of mantle cell lymphoma and other non-Hodgkin lymphoma subtypes. Semin Cancer Biol 2011; 21:293–298. [DOI] [PubMed] [Google Scholar]
- 30.Zhu YJ, Huang JJ, Xia Y, et al. Primary mediastinal large B-cell lymphoma (PMLBCL) in Chinese patients: clinical characteristics and prognostic factors. Int J Hematol 2011; 94:178–184. [DOI] [PubMed] [Google Scholar]
- 31.Sanoff HK, Sargent DJ, Green EM, et al. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol 2009; 27:4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedewa SA, Ward EM, Stewart AK, et al. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol 2010; 28:4135–4141. [DOI] [PubMed] [Google Scholar]
- 33.Breslin TM, Morris AM, Gu N, et al. Hospital factors and racial disparities in mortality after surgery for breast and colon cancer. J Clin Oncol 2009; 27:3945–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol 2006; 24:1357–1362. [DOI] [PubMed] [Google Scholar]
- 35.Kent EE, Morris RA, Largent JA, et al. Socioeconomic impacts on survival differ by race/ethnicity among adolescents and young adults with non-Hodgkin's lymphoma. J Cancer Epidemiol 2010; 2010:824691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Sanctis V, Finolezzi E, Osti MF, et al. MACOP-B and involved-field radiotherapy is an effective and safe therapy for primary mediastinal large B cell lymphoma. Int J Radiat Oncol Biol Phys 2008; 72:1154–1160. [DOI] [PubMed] [Google Scholar]
- 37.Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol 2006; 17:123–130.16236753 [Google Scholar]
- 38.Todeschini G, Secchi S, Morra E, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Brit J Cancer 2004; 90:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010; 116:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]


