Abstract
Post-traumatic stress disorder (PTSD) is suggested to be a structural and functional abnormality in the insula. The insula, which consists of distinct subregions with various patterns of connectivity, displays complex and diverse functions. However, whether these insular subregions have different patterns of connectivity in PTSD remains unclear. Investigating the abnormal functional connectivity of the insular subregions is crucial to reveal its potential effect on diseases specifically PTSD. This study uses a seed-based method to investigate the altered resting-state functional connectivity of insular subregions in PTSD. We found that patients with PTSD showed reduced functional connectivity compared with healthy controls (HCs) between the left ventral anterior insula and the anterior cingulate cortex. The patients with PTSD also exhibited decreased functional connectivity between the right posterior insula and left inferior parietal lobe, and the postcentral gyrus relative to HCs. These results suggest the involvement of altered functional connectivity of insular subregions in the abnormal regulation of emotion and processing of somatosensory information in patients with PTSD. Such impairments in functional connectivity patterns of the insular subregions may advance our understanding of the pathophysiological basis underlying PTSD.
Keywords: functional connectivity, insular subregions, post-traumatic stress disorder, resting-state
1. Introduction
Post-traumatic stress disorder (PTSD) is a prevalent and chronic anxiety disorder that frequently leads to significant distress and impairment in vital aspects of daily functioning.[1,2] One of the commonly believed cause of PTSD in the general population is motor vehicle accidents (MVAs).[3] Patients with PTSD often experience the following symptoms: hyperarousal, re-experiencing of the event, and avoidance of traumatic stimuli.[4] Current diagnosis of PTSD depends primarily on clinical signs and symptoms. The exact pathophysiological basis of PTSD remains largely unknown. Thus, improving our understanding of the pathophysiological mechanism of PTSD is essential in the clinical treatment and management of this disorder.[5]
Increased efforts have been dedicated for the neuroimaging studies of PTSD; the insular cortex is suggested to be implicated in the emotional cognitive processing and motor behavior in PTSD. Previous studies found that patients with PTSD exhibited increased activation in the insula during script-driven imagery task examination[6,7] and emotional memory retrieval task.[8] In addition, decreased activation of the insula in patients with PTSD has been found when performing emotional Stroop tasks, which suggests emotional-cognitive processing dysfunction.[9] A recent study reported an association between flashbacks in PTSD with insula and sensory area activation.[10] One recent study further showed that flashbacks may be involved in various types of motor behavior and imagery motor.[11]
Recent advances in resting-state functional magnetic resonance imaging (fMRI) have been made it possible to investigate spontaneous brain activities in vivo.[12,13] Compared to the traditional task-based approach, resting-state fMRI avoids potential performance confounds related to cognitive paradigms, and it is relatively easy to perform in clinical studies. Furthermore, resting-state functional connectivity (FC), which reflects the intrinsic relationships between brain areas,[14] has been widely used to effectively detect the pathophysiological mechanisms of psychiatric and neurological disorders.[15,16] Although the insula is emphasized as an integration center with structural and functional alterations in PTSD, limited resting-state FC studies have systematically explored insula connectivity in PTSD.
To date, none of the prior resting-state FC studies have taken insular subregions as regions of interest (ROIs) to explore the abnormal FC in PTSD. Consequently, whether more insular disruptions in FC are detected in PTSD remains unknown. In addition, insula is a functionally heterogeneous brain region that subserves a wide range of functions, including self-awareness, emotion regulation, visceral sensory, and sensorimotor processes.[17] A comprehensive resting-state FC analysis based on the subdivisions of the insula is critical to specifically detect the potential disease effect in PTSD. Various methodological approaches[18,19] have been developed recently to divide the insula into 3 subsystems with distinct FC patterns.[20] These subsystems are thought to be involved in emotional, sensorimotor, and higher cognitive processes. Several previous studies also suggested that the insular cortex plays a key role in linking emotion to cognitive processes and behavioral responses.[21,22]
The present study uses seed-based FC from 3 insular subregions to investigate the abnormal FC among the insular subregions in patients with PTSD.[20] We hypothesized the presence of aberrant functional connections in the insular subregions among patients with PTSD.
2. Methods
2.1. Participants
Twenty patients with PTSD who suffered from MVAs and 20 age, gender, and education-matched healthy individuals were recruited from the Southwest Hospital, Third Military Medical University. All patients suffered from serious MVAs that involved the death or near-death of other people. The PTSD diagnosis was made using the Clinician-Administered PTSD Scale for DSM-IV (CAPS-DX) by an attending psychiatrist and a trained interviewer.[23] Three main kinds of symptoms, including re-experiencing, avoidance, and increased arousal symptoms, were assessed in terms of frequency and intensity. The severity score of each symptom was computed by adding the frequency and intensity scores, which were summed up for all 17 symptom questions and/or the 3 symptoms. All patients in the present study met the DSM-IV (CAPS-DX) diagnostic criteria for PTSD. The clinical and demographic data of the participants are listed in Table 1. Moreover, both groups sustained neither a history of head injuries nor psychiatric and neurological disorders assessed using the Structured Clinical Interview for DSM-IV.[24] In addition, none of the patients with PTSD took psychotropic medication in the past 2 months. This study was approved by the Medical Research Ethics Committee of Southwest Hospital, Third Military Medical University. Written informed consents were signed by each participant.
Table 1.
Demographics and clinical characteristics of participants.
2.2. MRI data acquisition
All fMRI images were acquired on a 3.0 T Siemens MRI scanner (Trio; Siemens Medical, Erlangen, Germany). Participants were instructed to keep their eyes closed without falling asleep during the whole experiment. Foam pads were used to limit the participants’ head motion. The fMRI data were conducted using the echo-planar imaging (EPI) sequence. Sequence parameters are as follows: repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, matrix = 64 × 64, transverse slices = 36, slice thickness = 3 mm, and field of view = 220 mm × 220 mm. Scan lasted for 360 s for each participant.
2.3. Data preprocessing
Functional images were preprocessed using the Data Processing Assistant for Resting-State fMRI (DPARSF) toolbox.[25] The first 10 volumes were discarded because of unstable initial MRI signal. Acquisition time delay between slices and head motions were corrected for the remaining images. No participant indicated translational or rotational motion parameters >±3 mm or ±3°. The fMRI data were further spatially normalized to the Montreal Neurological Institute (MNI) EPI template image and resampled to 3 × 3 × 3 mm3. Afterward, the obtained fMRI data were spatially smoothed with an 8-mm full-width, half-maximum Gaussian kernel and detrended to abandon the linear trend.[26] Several spurious covariates were regressed, including the Friston 24 head motion parameters obtained from motion correction and white matter signal; cerebrospinal fluid was also regressed to reduce spurious variance. Finally, the resulting time series were band filtered (0.01–0.08 Hz) to reduce the effects of low-frequency drift and high-frequency noise.
2.4. Definition of insular subregions
Six seeds for the bilateral insular subregions were chosen for the participants. The insular lobe was divided into 3 insular subregions in each brain hemisphere by clustering the FC patterns.[20] The ROIs were anatomically defined by drawing an insular gray matter on the MNI 152 standard space.[20] Three insular subregions with distinct patterns of connectivity were identified (for bilateral brain hemispheres), including ventral anterior insula (vAI), dorsal anterior insula (dAI), and posterior insula (PI). All the voxels in the insular subregions were defined as ROIs for the following FC analysis of both patients with PTSD and healthy controls (HCs).
2.5. FC processing
Six voxel-wise FC maps based on predefined bilateral vAI, dAI, and PI were calculated by the Pearson correlation[27] for each participant. The ROI signal was obtained specifically by averaging the time series across the voxels of each ROI. The FC maps were obtained by calculating the correlation coefficients between each ROI signal and the time series from every other voxel across the whole brain. To improve normality, all correlation coefficients were converted to z-scores using Fisher r-to-z transformation.[28]
2.6. Statistical analysis
A 1-sample t test was performed for each ROI at each group to identify the connectivity pattern of the insluar subregions. The significant threshold was set at P < 0.001 (false discovery rate correction for multiple comparisons). Two-sample t tests were then performed to compare the FC maps between the patients with PTSD and HCs in order to determine the disrupted brain FC. Resulting statistical FC maps were thresholded at P < 0.05 (combined threshold of P < 0.05) by using the AlphaSim program in the REST software (http://resting-fmri.sourceforge.net). Between-group comparisons were confined to the voxels showed significant correlation maps for the 2 groups by applying a mask obtained from the combined 1-sample t test results. Furthermore, linear correlation analyses were conducted to explore the links between the FC values and the clinical variables in the PTSD group. Correlations between the mean z values within these significant regions from the between-group comparisons and the CAPS scores were performed. The threshold P < 0.05 was considered significant.
3. Results
We explored the differences in the resting-state FC between patients with PTSD and HCs based on the 6 ROIs of the insular subregions. Significant differences between the patients with PTSD and HCs were found in the left vAI and right PI network (P < 0.05, AlphaSim corrected). Results are shown in Figs. 1 and 2 (region details see in Table 2). Resting-state FC of the other 4 ROIs did not differ significantly between the 2 groups.
Figure 1.
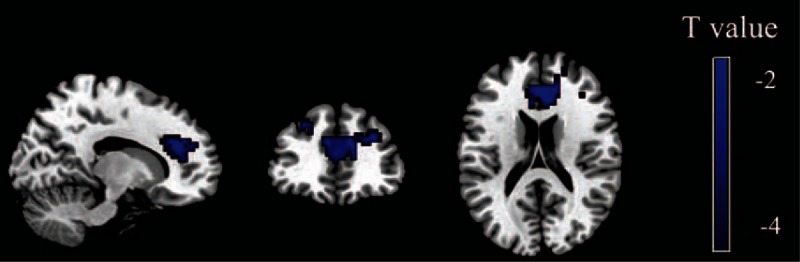
Brain regions with significant differences between patients with PTSD and HCs for the left vAI. The color bar represents the statistical T value, and blue color represents the decreased functional connectivity in patients with PTSD relative to HCs. In comparison with HCs, patients with PTSD exhibited decreased functional connectivity between the left vAI and ACC (P < 0.05, AlphaSim corrected, individual voxel threshold P < 0.05, and a minimum cluster size of 320 voxels). ACC = anterior cingulate cortex, HCs = healthy controls, PTSD = post-traumatic stress disorder, vAI = ventral anterior insula.
Figure 2.
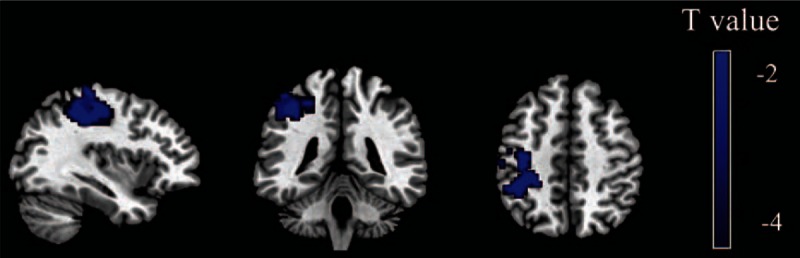
Brain regions with significant differences between patients with PTSD and HCs for the right PI. The color bar represents the statistical T value, and blue color represents the decreased functional connectivity in patients with PTSD relative to HCs. In comparison with HCs, patients with PTSD exhibited decreased functional connectivity between the right PI and left inferior parietal lobe and postcentral gyrus (P < 0.05, AlphaSim corrected, individual voxel threshold P < 0.05, and a minimum cluster size of 367 voxels). HCs = healthy controls, PI = posterior insula, PTSD = post-traumatic stress disorder.
Table 2.
The significantly differences of FC of insular subregions between PTSD and HCs.
The patients with PTSD showed decreased FC in the anterior cingulate cortex (ACC) in comparison with the HCs for the seed region of the left vAI. For the seed region of the right PI, we found that patients with PTSD exhibited decreased FC in the left inferior parietal lobe and postcentral gyrus. No significant correlation was detected between the FC values and CAPS scores.
4. Discussion
This study investigated the resting-state FC of 6 subregions of the insula to determine the differences between patients with PTSD and HCs. We found a wide range of abnormal FCs in PTSD based on the insular seeds during rest. Our study demonstrated that the FC between the insula and other brain regions was significantly altered among patients with PTSD. A significantly weaker FC was found between the left vAI and ACC. Furthermore, we observed a decreased FC between the right PI and the left inferior parietal lobe as well as postcentral gyrus among patients with PTSD. These findings add to the important evidence on altered FC of the insula and other brain regions, as well as provide new insights into the pathophysiological basis of PTSD.
4.1. Altered FC between the vAI and ACC
Compared to HCs, we observed reduced FC between the left vAI and ACC in patients with PTSD. The insula and ACC are believed to play essential roles in the integration of multimodal information vital in sensorimotor, homeostatic/allostatic, emotional, and cognitive functions[29,30] given their neuroanatomical features and connectivity.[31] In addition, these 2 regions are suggested as essential parts of the “salience network” involved in identifying internal and external stimuli, which guides motivated behavior.[32] Functional neuroimaging research has reported the involvement of the anterior insula in anticipating and responding to anxiety-provoking events,[33,34] especially in anxiety-prone or clinically anxious individuals,[35,36] including patients with PTSD.[37,38] Several previous studies reported the abnormal activation in ACC among patients with PTSD. For example, Lanius et al[39] found less ACC activation in patients with PTSD as they perform script-driven symptom provocation tasks in comparison with HCs. Decreased activation in ACC was also detected in patients with PTSD when viewing fearful faces.[40,41] The above-mentioned studies demonstrate that the ACC may play a key role in the development of PTSD. One study also found decreased FC between insula and ACC when patients with PSTD engaged in traumatic memory recall tasks compared with controls; this result suggests a hyperarousal response to traumatic stimulus in patients with PTSD.[42] Dysfunctions in these regions may be regarded as evidence of abnormal traumatic and negative stimulus detection, as well as disrupted somatic/physiological reactivity systems in PTSD. These finding may result in aberrant contextual processing of the traumatic stimuli. Similarly, the disruption in the FC between vAI and ACC observed in the present study may supply a neural mechanism of emotion dysregulation, which may be associated with the abnormal integration of interoceptive information with emotional salience to form a subjective bodily representation.[43] An early study showed that PTSD is related to the dysfunction in information processing and to the hypervigilence to salient and traumatic events.[44] In the present study, the decreased FC in the vAI-ACC system may suggest the dysfunction in the emotional salience monitoring involved in the hypersrousal/hypervigilance symptom in patients with PTSD.
4.2. Altered FC between PI and parietal system
Deceased FC between the right PI and the left inferior parietal lobe was observed in patients with PTSD. The PI was functionally connected to the somatosensory cortex, which was consistently considered to be associated to processing somatosensory information with affective or motivational significance.[17,20] The parietal lobe was also implicated in a hierarchy of sensory processing from primary sensory areas to unimodal and then polymodal association regions.[45] In addition, the parietal lobe was believed to be related to spatial orientation function,[46] which may subserve spatial and temporal information processing associated with a traumatic event.[47] The inferior parietal lobe and postcentral gyrus are 2 key regions in the parietal lobe[48] and both regions are known to be involved in multisensory interaction.[49,50] Previous studies found that PTSD exhibited abnormal activation of the inferior parietal lobe and postcentral gyrus, which suggests the aberrant sensory information processing in patients with PTSD.[51,52] Van der Kolk and Fisler observed that trauma is initially remembered in the form of somatosensory information, wherein trauma-related experiences show up in body memory sensations.[53] Flashbacks, or the sudden recall of traumatic experiences from the past, are identified as a re-experiencing symptom of PTSD that lead to great suffering for the people who experience them.[54] Flashbacks can happen in a wide range of modalities, including visual, auditory, olfactory, kinesthetic, and affective.[53] This symptom was often regarded as engaged in the intensity of sensory and the emotional re-experiencing of trauma.[55] In the present study, abnormal sensory information processing in the parietal lobe is hypothesized to be involved in the flashbacks of patients with PTSD through a pathway occurring within the PI-parietal circuit.
Collectively, the aberrant FCs of the insular subregions in our findings may advance our understanding of the pathophysiological mechanisms for PTSD, wherein the network associated with emotion regulations and sensorimotors exhibit disrupted FC. The disrupted FC in the salience network among patients with PTSD suggest that inefficient ACC regulation can result in overarching threat schemas that regulate over-estimations of threat and anxiety. The decreased FC between the PI and the parietal system may explain the abnormal somatosensory information processing and re-experience in patients with PTSD.
5. Limitations
The present study includes several limitations. First, our sample size is relatively small, and a larger sample size is required to confirm the current results in the future studies. Second, our study only explored patients with MAV. Thus, we urge caution when generalizing our findings to other traumatic events. Finally, we found no significant relationship between CAPS scores and FCs, which limit our findings to some extent. Therefore, more scales and tasks associated with PTSD symptoms are necessary in further investigations.
6. Conclusions
This is the first study to explore resting-state networks of the insular subregions in PTSD. Results showed that the vAI and PI networks differed between the patients with PTSD and HCs. In conclusion, the abnormal FCs in these regions may have important implications in understanding the pathophysiology of PTSD.
Footnotes
Abbreviations: ACC = anterior cingulate cortex, CAPS = Clinician-Administered PTSD Scale, dAI = dorsal anterior insula, DPARSF = Data Processing Assistant for Resting-State fMRI, EPI = echo-planar imaging, FA = flip angle, FC = functional connectivity, FDR = false discovery rate, fMRI = functional magnetic resonance imaging, FOV = field of view, HCs = healthy controls, MNI = Montreal Neurological Institute, MVAs = motor vehicle accidents, PI = posterior insula, PTSD = post-traumatic stress disorder, ROIs = regions of interest, TE = echo time , TR = repetition time, vAI = ventral anterior insula.
HuafuC and BX designed and collected the original imaging data. YZ, HengC, ML, XG, and HuafuC managed and analyzed the imaging data. YZ and HuafuC wrote the first draft of the manuscript. All authors have read and approved the final manuscript.
The work is supported by the 973 project (2012CB517901), 863 project (2015AA020505), the Natural Science Foundation of China (61533006), and the Fundamental Research Funds for the Central Universities (ZYGX2013Z004).
The authors have no conflicts of interest to disclose.
References
- 1.Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA 2006; 295:1023–1032. [DOI] [PubMed] [Google Scholar]
- 2.Van Ameringen M, Mancini C, Patterson B, et al. Post-traumatic stress disorder in Canada. CNS Neurosci Ther 2008; 14:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silove D, Brooks R, Steel Z, et al. Can structured interviews for posttraumatic stress disorder assist clinical decision-making after motor vehicle accidents? An exploratory analysis. Compr Psychiatry 2006; 47:194–200. [DOI] [PubMed] [Google Scholar]
- 4.Guze SB. Diagnostic and statistical manual of mental disorders (DSM-IV). Am J Psychiatry 1995; 152:1228. [Google Scholar]
- 5.Garfinkel SN, Liberzon I. Neurobiology of PTSD: a review of neuroimaging findings. Psychiatr Ann 2009; 39:370–381. [Google Scholar]
- 6.Lindauer R, Booij J, Habraken JB, et al. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: a randomized clinical trial. Psychol Med 2008; 38:543–554. [DOI] [PubMed] [Google Scholar]
- 7.Lanius RA, Frewen PA, Girotti M, et al. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res 2007; 155:45–56. [DOI] [PubMed] [Google Scholar]
- 8.Whalley MG, Rugg MD, Smith APR, et al. Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain Cogn 2008; 69:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin LM, Whalen PJ, Pitman R, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001; 50:932–942. [DOI] [PubMed] [Google Scholar]
- 10.Whalley MG, Kroes MC, Huntley Z, et al. An fMRI investigation of posttraumatic flashbacks. Brain Cogn 2013; 81:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran A, Bramham J, Collet C, et al. Motor imagery in clinical disorders: importance and implications. Front Psychiatry 2015; 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology 2014; 272:29–49. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Hu M, Wang S, et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39:326–331. [DOI] [PubMed] [Google Scholar]
- 14.van de Ven VG, Formisano E, Prvulovic D, et al. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp 2004; 22:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 2008; 21:424–430. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Xie B, Wang Y, et al. Characterization of post-traumatic stress disorder using resting-state fMRI with a multi-level parametric classification approach. Brain Topogr 2015; 28:221–237. [DOI] [PubMed] [Google Scholar]
- 17.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10:59–70. [DOI] [PubMed] [Google Scholar]
- 18.Kurth F, Zilles K, Fox PT, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Func 2010; 214:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cauda F, D’Agata F, Sacco K, et al. Functional connectivity of the insula in the resting brain. Neuroimage 2011; 55:8–23. [DOI] [PubMed] [Google Scholar]
- 20.Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex 2011; 21:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitschke JB, Sarinopoulos I, Mackiewicz KL, et al. Functional neuroanatomy of aversion and its anticipation. Neuroimage 2006; 29:106–116. [DOI] [PubMed] [Google Scholar]
- 22.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006; 60:383–387. [DOI] [PubMed] [Google Scholar]
- 23.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress 1995; 8:75–90. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, et al. The structured clinical interview for DSM-III-R personality disorders (SCID-II). part I: description. J Pers Disord 1995; 9:83–91. [Google Scholar]
- 25.Yan C, Zang Y. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 2010; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Guo W, Liu L, et al. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J affective disorders 2013; 146:401–406. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Guo W, Fouche JP, et al. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct Func 2015; 220:101–115. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Zhu C, Wang Y, et al. Disrupted cortical hubs in functional brain networks in social anxiety disorder. Clin Neurophysiol 2015; 126:1711–1716. [DOI] [PubMed] [Google Scholar]
- 29.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655–666. [DOI] [PubMed] [Google Scholar]
- 30.Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum Brain Mapp 2007; 28:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butti C, Hof PR. The insular cortex: a comparative perspective. Brain Struct Func 2010; 214:477–493. [DOI] [PubMed] [Google Scholar]
- 32.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlson JM, Greenberg T, Rubin D, et al. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc Cogn Affect Neurosci 2011; 6:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waugh CE, Wager TD, Fredrickson BL, et al. The neural correlates of trait resilience when anticipating and recovering from threat. Soc Cogn Affect Neurosci 2008; 3:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drabant EM, Kuo JR, Ramel W, et al. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage 2011; 55:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straube T, Mentzel H-J, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage 2007; 37:1427–1436. [DOI] [PubMed] [Google Scholar]
- 37.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simmons A, Strigo IA, Matthews SC, et al. Initial evidence of a failure to activate right anterior insula during affective set-shifting in PTSD. Psychosom Med 2009; 71:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry 2001; 158:1920–1922. [DOI] [PubMed] [Google Scholar]
- 40.Kim MJ, Chey J, Chung A, et al. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J Psychiatr Res 2008; 42:268–277. [DOI] [PubMed] [Google Scholar]
- 41.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62:273–281. [DOI] [PubMed] [Google Scholar]
- 42.Lanius RA, Williamson PC, Densmore M, et al. The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. Am J Psychiatry 2004; 161:36–44. [DOI] [PubMed] [Google Scholar]
- 43.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 2009; 30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clin Psychol Rev 2000; 20:1041–1065. [DOI] [PubMed] [Google Scholar]
- 45.Oxford University Press, Fuster JM. Cortex and Mind: Unifying Cognition. 2003. [Google Scholar]
- 46.Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol 2001; 11:157–163. [DOI] [PubMed] [Google Scholar]
- 47.Peres JF, Newberg AB, Mercante JP, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med 2007; 37:1481–1491. [DOI] [PubMed] [Google Scholar]
- 48.Springer Science & Business Media, Mast FW, Jäncke L. Spatial Processing in Navigation, Imagery and Perception. 2007. [Google Scholar]
- 49.Foxe JJ, Morocz IA, Murray MM, et al. Multisensory auditory-somatosensory interactions in early cortical processing revealed by high-density electrical mapping. Cogn Brain Res 2000; 10:77–83. [DOI] [PubMed] [Google Scholar]
- 50.Rizzolatti G, Ferrari PF, Rozzi S, et al. Novartis Foundation Symposium. 1999; Chichester, New York: John Wiley, 129. [Google Scholar]
- 51.Bryant RA, Felmingham KL, Kemp AH, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry 2005; 58:111–118. [DOI] [PubMed] [Google Scholar]
- 52.Falconer E, Bryant R, Felmingham KL, et al. The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci 2008; 33:413-422. [PMC free article] [PubMed] [Google Scholar]
- 53.Van der Kolk BA, Fisler R. Dissociation and the fragmentary nature of traumatic memories: overview and exploratory study. J Trauma Stress 1995; 8:505–525. [DOI] [PubMed] [Google Scholar]
- 54.Jones E, Vermaas RH, McCartney H, et al. Flashbacks and post-traumatic stress disorder: the genesis of a 20th-century diagnosis. Br J Psychiatry 2003; 182:158–163. [DOI] [PubMed] [Google Scholar]
- 55.Brewin CR. The nature and significance of memory disturbance in posttraumatic stress disorder. Ann Rev Clin Psychol 2011; 7:203–227. [DOI] [PubMed] [Google Scholar]


