Abstract
The present study had 2 objectives, first, to investigate possible relationships between increased gestational weight gain and demographic, clinical, paraclinical, genetic, and bioimpedance (BIA) characteristics of Romanian mothers, and second, to identify the influence of predictors (maternal and newborns characteristics) on our outcome birth weight (BW).
We performed a cross-sectional study on 309 mothers and 309 newborns from Romania, divided into 2 groups: Group I—141 mothers with high gestational weight gain (GWG) and Group II—168 mothers with normal GWG, that is, control group.
The groups were evaluated regarding demographic, anthropometric (body mass index [BMI], middle upper arm circumference, tricipital skinfold thickness, weight, height [H]), clinical, paraclinical, genetic (interleukin 6 [IL-6]: IL-6 -174G>C and IL-6 -572C>G gene polymorphisms), and BIA parameters.
We noticed that fat mass (FM), muscle mass (MM), bone mass (BM), total body water (TBW), basal metabolism rate (BMR) and metabolic age (P < 0.001), anthropometric parameters (middle upper arm circumference, tricipital skinfold thickness; P < 0.001/P = 0.001) and hypertension (odds ratio = 4.65, 95% confidence interval: 1.27–17.03) were higher in mothers with high GWG. BW was positively correlated with mothers’ FM (P < 0.001), TBW (P = 0.001), BMR (P = 0.02), while smoking was negatively correlated with BW (P = 0.04). Variant genotype (GG+GC) of the IL-6 -572C>G polymorphism was higher in the control group (P = 0.042).
We observed that high GWG may be an important predictor factor for the afterward BW, being positively correlated with FM, TBW, BMR, metabolic age of the mothers, and negatively with the mother's smoking status. Variant genotype (GG+GC) of the IL-6 -572C>G gene polymorphism is a protector factor against obesity in mothers. All the variables considered explained 14.50% of the outcome variance.
Keywords: bioimpedance, gestational weight gain, IL-6 gene polymorphisms, mothers, newborns
1. Introduction
Birth weight (BW) is an important parameter of the newborn's health status.[1] Low BW is associated with prematurity and growth retardation, with serious consequences to the newborn such as a difficult adaptation to extrauterine life, severe postpartum complications, and even death.[1,2] Large BW is associated with obstetrical complications such as shoulder dystocia and imposes a cesarean section.[3,4] In addition, large BW is an important predictor factor for the afterward weight of the baby through the increase of adipose cells number, and can be associated or can determine diabetes mellitus and cardiovascular diseases later in life.[1,5]
Intrauterine malnutrition, especially the decreased intake of vitamin B12 and folic acid, can modify the functioning of some key genes. The not correspondingly fed fetus will be programmed to deposit all the available energy from the organism with the increase of obesity and metabolic diseases’ risk at maturity.[6,7] On the other hand, excessive energetic intake, especially of amino acids and glucose stimulates the secretion of Insulin-like growth factor 1 (IGF-1) and insulin determining obesity and diabetes mellitus in children.[8–10] BW is determined by a series of environmental, biological, social, and also genetic factors that exert their action during time and over generations.[1,11,12]
Maternal weight, and especially mother's obesity became epidemic and a flagella of the 21st century, being associated with adverse pregnancy and birth outcomes.[13] Maternal obesity increased lately; thus, in the United States 64% of the women at fertile age are overweight and 35% are obese, and in Great Britain 1 of 5 women is obese before becoming pregnant.[14] Excessive maternal weight gain can have different effects on the normal course of the delivery, problems associated with the normal evolution of the pregnancy, associated gestational diabetes, preeclampsia/eclampsia and dystocic deliveries, but also decisive influences on the newborn's weight and ulterior complications.[13,14] Studies have proven that mother's obesity is associated with macrosomia.[1] Therefore, the Institute of Medicine (IOM) revised the guides for gestational weight gain (GWG) for women in the different World Health Organisation BMI categories.[15,16] Thus, as the mother's BMI is higher, the recommended weight gain of the pregnant woman will be lower.[1,15,17] The IOM recommends a weight gain of 5 to 9 kg for obese pregnant women.[13,15] The body mass composition of the mother should own an important role in the determinism of the newborns’ weight. Thus, using bioimpedance (BIA) the manner in which the maternal body composition parameters (fat and fat-free mass [FFM]) influenced BW was evaluated. Although the studies of Abrams and Neufeld reported that maternal weight gain in the second trimester is associated with the fetal growth and BW,[18,19] the study of Farah underlined that gestational weight gain before the third trimester influences the BW in women with normal BMI and overweight, and in addition maternal FFM and weight gain during pregnancy influence BW.[1,20,21] There are also some studies performed on Danish women that reported a mild weight gain at birth, coupled with excessive weight gain of the mother during pregnancy.[22]
The studies pointed out the relationship between BW and mother's body composition, reporting a high incidence of macrosomia and neonatal adiposity,[23–25] in both nullipara and multipara women.[13]
In a review elaborated by Freeman[23] it was proved that the origin of obesity is very early. It discussed the interaction between genetic factors and in utero environment that have an impact on the development of fetal obesity and programs the future child's obesity. During pregnancy, the metabolism of pregnant women is impaired; thus, these women have an increased percentage of adipose tissue and they are insulin resistant and exposed to a higher risk of vascular and metabolic diseases. O’Reilly and Reynolds[14] also underlined the role of prenatal and postnatal factors as well as the degree of obesity, environmental factors, activity, diet, lifestyle factors as sports, and also genetic factors (that explain the descendants’ BMI in a percentage of 20%–90%) in determining obesity in the conception product.
A reduced degree of inflammation is correlated with obesity, insulin resistance, and metabolic diseases. In the pathogenesis of these diseases, an important role is owned by the proinflammatory cytokines. Interleukin 6 (IL-6) is a mediator of the immune and inflammatory response, especially regulating the response of the acute phase, which influences the function of adipose tissue as well as lipid and glucose metabolism.[26] In obesity, increased IL-6 serum levels were observed, especially in those with body FM excess.[27–29] The maternal composition suffers adaptive modifications during pregnancy. Thus, the FM, FFM, and total body water (TBW) are modified and have a special role in pregnancy and perinatal medicine.[29] Genetic studies underlined the fact that the IL-6 -174G>C gene polymorphism owns an important role and it is more frequently associated with obesity and type 2 diabetes mellitus, especially in a population with excess in body FM.[26,27,30–32] The studies of Vazarova and Illig[33,34] showed that the G allele is associated with the comorbidities of obesity, whereas other studies[35] showed that the variant C allele of the IL-6 -174G>C gene is associated with type 2 diabetes mellitus, hypertension, and cardiovascular diseases.[36,37] The IL-6 -174G>C gene polymorphism was associated with increased risk of coronary disease,[36,38] namely the C allele with an increased risk of arterial hypertension (AHT),[36] whereas the G allele of the IL-6 -572C>G gene polymorphism was associated in 95% of cases with obesity and type 2 diabetes mellitus,[39] correlations with the parameters of the lipid and protein metabolism being also established.[40] Another study[41] emphasized that the GG genotype of the IL-6 -572C>G gene polymorphism is associated with obesity in adults, whereas a different study observed that the CC genotype and the C allele of the IL-6 -572C>G gene polymorphism are highly associated with obesity in children.[42]
According to the 2008 WHO data in Romania, 51% of the adult population was overweight and 19.1% was obese[43]; in children obesity varies between 7.2% in Timişoara[44] and 29% in Cluj.[45] Another study showed that variant heterozygous genotype of IL-6 -174G>C single nucleotide polymorphism (SNP) is associated with obesity in children, whereas variant homozygous CC genotype of IL-6 -174G>C SNP is a protector factor for obesity in children.[42]
On the basis of the above mentioned facts, the objectives of our study were to investigate possible relationships between increased gestational weight gain and demographic, anthropometric (BMI, middle upper arm circumference [MUAC], tricipital skinfold thickness [TST], weight, height [H]), clinical, paraclinical (cholesterol [Chol], triglyceride, and transaminases levels), genetic (IL-6 -174G>C and IL-6 -572C>G gene polymorphisms), and BIA characteristics (FM, FFM, and TBW) of the mothers from a Caucasian population in Romania (southeastern Europe) and the influence of possible predictors (maternal and newborns genetic and BIA characteristics) on our outcome BW.
2. Material and methods
A cross-sectional study design was performed on a consecutive representative population of 407 mothers and their newborns, evaluated in an Obstetrics Gynecology Tertiary Hospital from Romania, between April 2015 and December 2015. The studied groups cases and controls were defined “a posteriori,” during the analysis phase and not at the design phase. Selection of subjects was independent of explicative variables or outcome. Because the sample size was subdivided into 2 groups (controls and cases), to assure their comparability, we chose the mothers with similar age and weight before pregnancy (n = 309). The criteria for inclusion in our study were age of the mother above 18 years and singleton pregnancy. We excluded from our study mothers and newborns with chronic diseases, patients showing presence of an infectious process (clinical signs and C-reactive protein >5 mg/L); parity >6; cases diagnosed with intrauterine growth retardation due to congenital malformations of the fetuses, patients without complete clinical, anthropometrical, laboratory, and genetic evaluation as well as cases who did not sign the informed consent.
According to the IOM[15] we used the following classification for GWG: underweight BMI < 18.5 kg/m2, recommended GWG 12.5 to 18 kg; normal weight BMI = 18.5 to 24.9 kg/m2, recommended GWG 11.5 to 16 kg; overweight BMI = 25 to 29.9 kg/m2, recommended GWG 7.00 to 11.5 kg; obese BMI >30 kg/m2, recommended GWG 5 to 9 kg.[15] Then, we classified the mothers with their newborns into 2 groups: the study group comprising women who had a weight gain higher than the superior limit of the reference interval (increased weight gain) and the control group containing women with a weight gain in the reference interval (normal weight gain). Group I comprised 141 mothers with high GWG and Group II included 168 mothers with normal GWG, that is, control group.
All mothers gave written informed consent for them and their child prior to inclusion in the study and research was performed in compliance with the principles of the Helsinki Declaration, and was approved by the ethics committee of the University of Medicine and Pharmacy of Tîrgu Mureţ (No 32/March 16, 2015).
2.1. Measurement characteristics
2.1.1. Anthropometric characteristics
All mother and newborn measurements were performed by a single trained person and included the following: weight (kg), height (cm), MUAC, and TST. Body weight was measured with a daily calibrated scale, with ±10 g error. Height was measured with a pedometer, calibrated daily, and was evaluated by standard deviation (SD) (0.1-cm error). MUAC was evaluated at the midpoint between shoulder and elbow tips, with the use of a tape measure calibrated in centimeters, whereas TST was measured in the posterior upper arm using a thickness caliper. BMI was computed by dividing weight (kg) by standing height squared (m2).
Bioelectrical impedance analysis (BIA) was performed using the Tanita BC-420 MA body composition analyzer (Tanita Corp, Tokyo, Japan). BIA measurements were done according to the manufacturer's guidelines at a frequency of 50 kHz. Participants were asked to void their bladder prior to the measurement. Height, sex, and age were entered manually; weight was recorded automatically with a 0.5-kg adjustment for the weight of clothes. The measurement procedure required the subject to stand barefooted on the analyzer. BIA assesses the difference in impedance caused by the fact that fat and lean tissues have different electrical properties. The Tanita Analyzer estimates FM, FFM, muscle mass (MM), and TBW.
2.1.2. Laboratory parameters
Chol, triglyceride, alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT) levels were measured by spectrophotometry on a Cobas Integra 400 plus automated analyzer for all surveyed mothers and newborns. Chol level was considered normal at <170 mg/dL and triglyceride level at <130 mg/dL.
2.1.3. Genotyping
Genomic DNA was isolated from fresh blood samples collected on EDTA (Acide Éthylènediaminetétracétique) from newborns and their mothers who met the inclusion criteria. IL-6 -174G>C gene polymorphism was investigated by amplification refractory mutation system—polymerase chain reaction (ARMS-PCR) as previously described by Daneshmandi et al.[46] The polymerase chain reaction—restriction fragment length polymorphism (PCR-RFLP) method previously reported[47] was used to investigate the IL-6 -572C>G gene polymorphism.
2.2. Statistical analysis
2.2.1. Sample size
In order to assure the reliability (calibration) of the regression model, we considered the following requirement[48]: the average number of predictors (p) had to be less than q/15, where q represented “limiting sample size.” For example, in the case of linear regression for 20 predictors it was necessary to have a minimum of 300 subjects.
Post-hoc sample size calculation using G∗Power (v.3.1.9.2) software showed that a sample size of 309 subjects achieved a 99% power with a medium effect size (f2) of 0.18 and 23 degrees of freedom for linear multiple regression and an alpha level of 0.05.
Descriptive statistics used to describe the studied variables were both mean ± SD or median (25th percentile–75th percentile) for quantitative variables and frequencies for qualitative variables. Unpaired Student t tests for parametric data and Mann–Whitney U tests for nonparametric data were done to identify differences between compared groups regarding each quantitative characteristic. A χ2 test was used to evaluate the Hardy–Weinberg equilibrium and χ2 or Fisher's exact test were done to analyze the frequency distribution. The univariate and multivariate linear regression were done to evaluate the crude and covariate-adjusted individual impact of interest variables on GWG and BW, respectively. To quantify the magnitude of dependence between predictor and outcome variable, we calculated the unadjusted and adjusted estimates of regression coefficients with their associated 95% confidence interval (CI). Statistical significance was achieved when the estimated level of significance for all 2-sided tests (P < 0.05). Statistical analysis was performed using R software version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and Statistica version 7.0 (StatSoft Inc, Tulsa, OK).
3. Results
3.1. Demographic, anthropometric, clinical, paraclinical, and BIA characteristics of the mothers and their newborns
From all mothers who presented at the Obstetrics Gynecology Tertiary Hospital from Romania, the eligibility criteria for participating in our study were fulfilled by 407 mothers with their 407 newborns. After a closer selection of the cases, only 309 mothers and 309 newborns were included in the present study.
The mean age for all the “mothers” included in the study was 29.20 ± 5.44 years (range between 18 and 43 years) and the height was 163.70 ± 5.89 cm with a range between 145 and 180 cm. Regarding the educational degree, 47.20% had superior studies, 39.50% had <12 grades and 9.40% had between 13 and 14 grades; 67.30% of them were employed, 16.50% were smokers, 0.60% were diabetics, and only 4.50% had gestational AHT (Table 1).
Table 1.
Demographic, anthropometric, clinical, paraclinical, and BIA characteristics in mothers and their newborns.
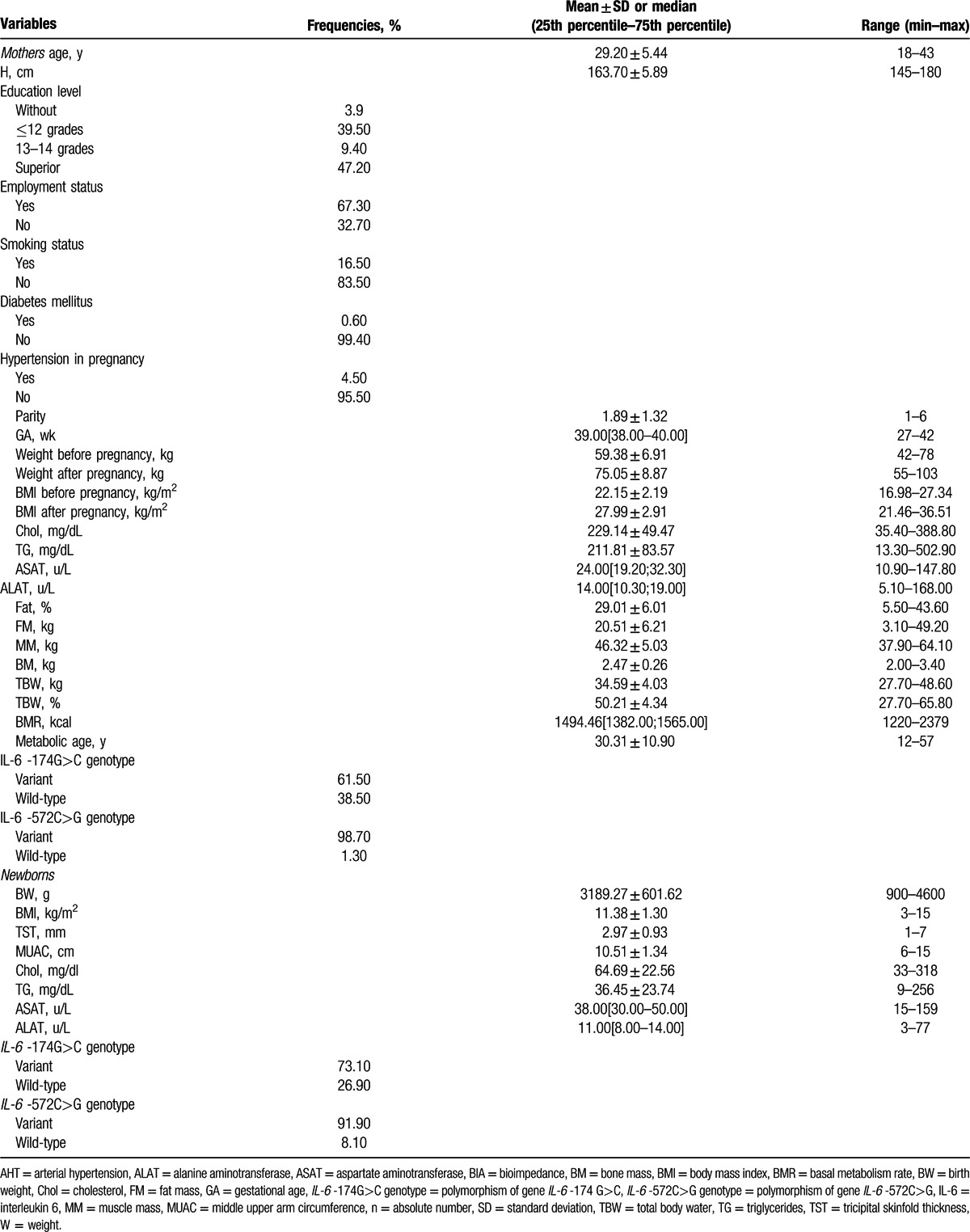
The number of mothers’ pregnancies was 1.89 ± 1.32, and the medium length of gestation was 39.00 weeks. At the onset of pregnancy, the medium weight of the women was 59.38 ± 6.91 kg and the BMI was 22.15 ± 2.19 kg/m2. At the end of the gestational period, mothers’ weight was 75.05 ± 8.87 kg with a BMI of 27.99 ± 2.91 kg/m2. Among the laboratory parameters, Chol and triglycerides evaluated at the end of the gestational period were above the reference range (229.14 ± 49.47 mg/dL and 211.81 ± 83.57 mg/dL, respectively), whereas transaminases had normal levels (19.20–32.30 U/L for ASAT and 10.30–19.00 U/L for ALAT) (Table 1). BIA parameters obtained from the mothers underlined an FM of 20.51 ± 6.21 kg, MM of 46.32 ± 5.03 kg, bone mass (BM) 2.47 ± 0.26 kg, TBW 34.59 ± 4.03 kg, a BMR of 1494.46 (1382.00; 1565.00), and a metabolic age of 30.31 ± 10.90 years. Regarding the IL-6 -174G>C genotype, we found the variant genotype in 61.50% of mothers and for IL-6 -572C>G we observed the presence of variant genotype in 98.70% of the cases (Table 1).
The newborn medium weight among the entire group was 3189.27 ± 601.62 g, with a BMI of 11.38 ± 1.30 kg/m2, TST 2.97 ± 0.93 mm, and a MUAC of 10.51 ± 1.34 cm. Chol, triglycerides, and transaminases in newborns had normal levels. For the IL-6 -174G>C gene polymorphism in newborns, we found the variant genotype in 73.10% of cases, whereas for the IL-6 -572C>G gene polymorphism the variant genotype was observed in 91.90% of cases (Table 1).
3.2. Maternal characteristics and GWG
GWG represented division criteria for the pregnant women: Group I (the study group) consisted of 141 pregnant women with high GWG according IOM and Group II consisted of 168 pregnant women with normal GWG.
In Table 2, we showed the descriptive statistics of anthropometric, biochemical, and BIA parameters of the 2 groups of mothers. Mothers’ age in the 2 groups were similar (Student t test assuming equal variances, statistics t(307) = 1.10, P = 0.27). We did not identify any significant differences in the 2 studied groups regarding the values of gestational age (GA) (Mann–Whitney test, statistics U = 10363.50, P = 0.05), and weight before pregnancy (Student t test assuming unequal variances, statistics t(273.29) = 1.92, P = 0.056).
Table 2.
Demographic, anthropometric, clinical, paraclinical, and BIA characteristics in mothers from the 2 groups.
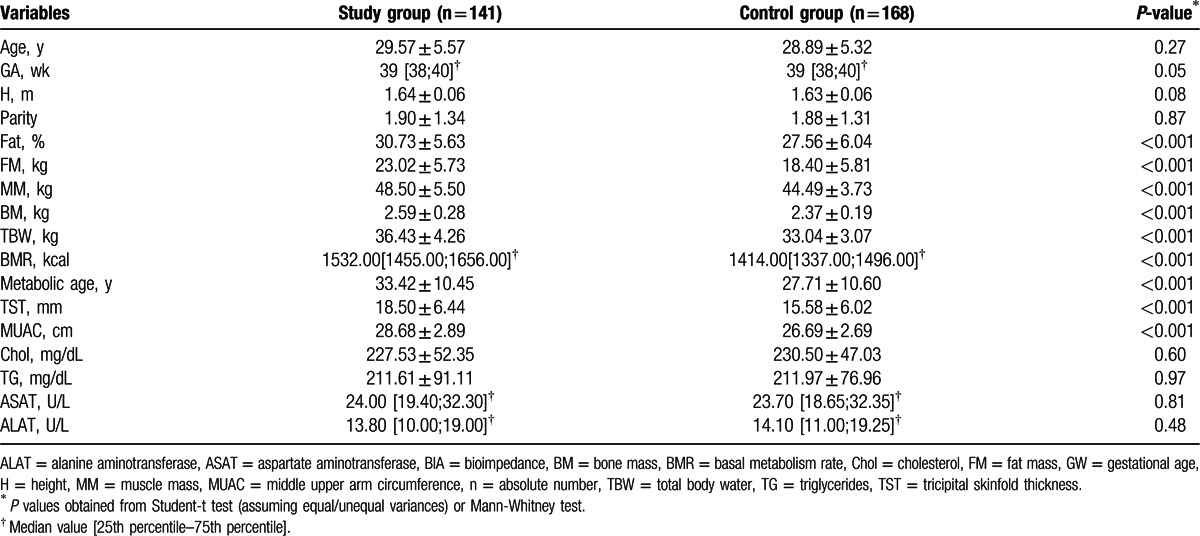
There was a significant (P < 0.001) increase in weight at the end of pregnancy in the study group (80.56 ± 7.90 kg) versus the control group (70.42 ± 6.74 kg), and a significantly higher BMI (P < 0.001) at the end of pregnancy in the study group (29.84 ± 2.63 kg/m2) versus the control group (26.44 ± 2.12 kg/m2).
Regarding the BIA parameters, we observed that FM (Fig. 1), MM, BM, TBW (Fig. 2), BMR, and metabolic age were significantly higher in mothers with high GWG in comparison with those from the control group, P values proving statistical significance for every parameter (P < 0.001). We also emphasize that in the high GWG group, TST, and MUAC were significantly higher than in the control group (P < 0.001/P < 0.001). We did not obtain any correlations with paraclinical indicators, such as Chol, triglycerides, ASAT, ALAT.
Figure 1.
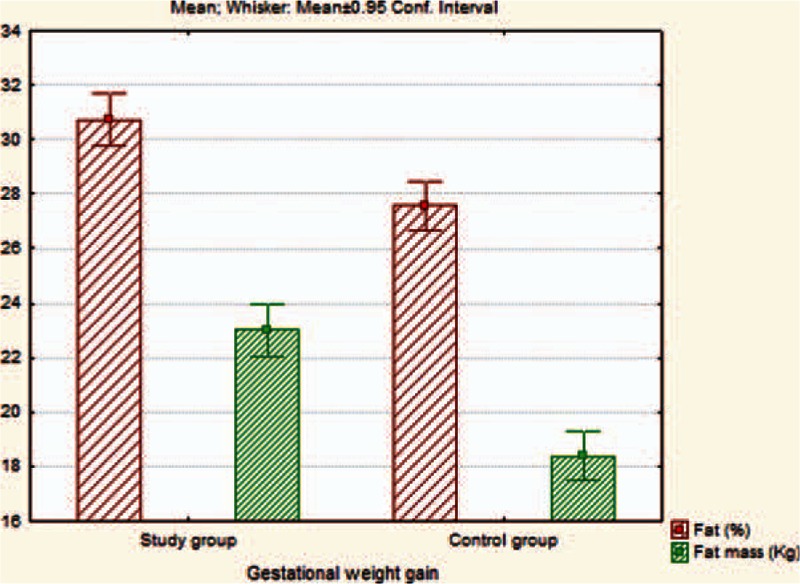
The assessment of differences regarding fat (%) and FM (kg) on 2 studied groups. FM = fat mass.
Figure 2.
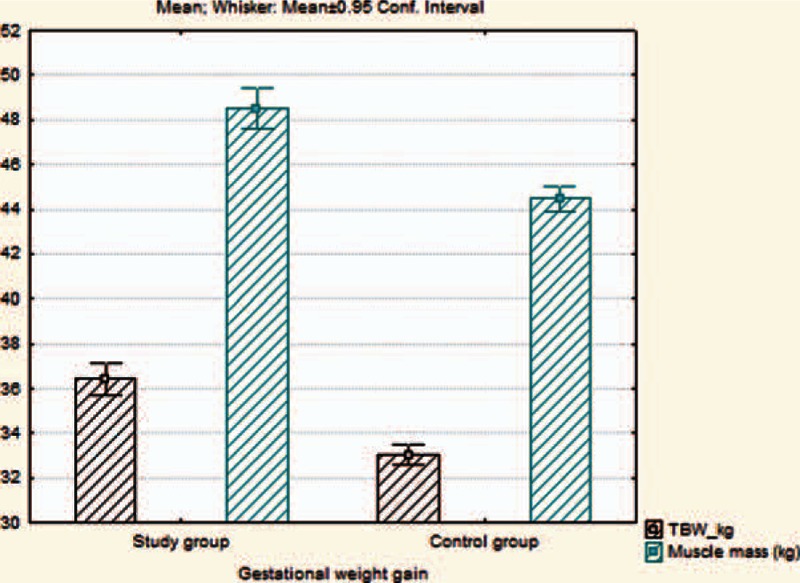
The evaluation of differences regarding TBW and MM on 2 studied groups. MM = muscle mass, TBW = total body water.
Our study also included the comparative evaluation of multiple social, environmental, and clinical factors. Regarding the association between the level of education and GWG during pregnancy, we did not observe a statistical significance (Fisher test P = 0.11), although a higher percentage of GWG in pregnant women with 13 to 14 grades or a university degree (55.2%; 48.6%) was observed.
We did not find any significant statistical association between GWG and vaginal versus cesarean section (χ2 = 0.908, P = 0.341), smoking (χ2 = 1.01, P = 0.40), diabetes mellitus (P = 0.21), and the number of pregnancies (χ2 = 0.98, P = 0.61).
The presence of increased GWG was a risk factor for hypertension in pregnancy (P = 0.01, odds ratio = 4.65, 95% CI: 1.27–17.03), the risk of developing AHT in pregnant in women with high GWG being 4.65 times higher in comparison to those in the group with normal GWG.
We also evaluated the manner in which IL-6 -174G>C and IL-6 -572C>G gene polymorphisms were associated with GWG. For the combined variant genotype [CC+CG] IL-6 -174G>C, we did not observe a statistical significance between the 2 groups (P = 0.06). In addition, we noticed that 44.2% of mothers with variant genotype had high GWG versus 55.8% of mothers with normal genotype.
Regarding the combined variant genotype [GG+GC] of the IL-6 -572C>G gene polymorphism, we observed a significantly higher frequency (P = 0.04) in the control group (55.1%) versus the study group (44.9%), which means that the variant genotype is a protective factor for GWG, and therefore against obesity.
Regarding the association between the level of education and GWG, we did not find a statistical significance (Fisher test P = 0.09), but nonetheless we found a higher percentage of GWG in pregnant women with high-school, college, or university degree (63.6%, 55.2%, or 48.6%, respectively).
3.2.1. BW depending on maternal and newborn characteristics
The results of simple linear regression showed that mother's height (P = 0.04), GWG (P = 0.01), GA (P < 0.001), FM (%) (P < 0.001), FM (kg) (0.002), TBW (P = 0.001), BMR (kcal) (P = 0.02), and metabolic age (P = 0.001) positively influenced BW, whereas smoking status was negatively correlated with BW (P = 0.04), meaning that mothers who smoked had newborns with lower BW compared with nonsmoker ones (Table 3).
Table 3.
Crude (unadjusted) impact of maternal and newborn characteristics on BW evaluated by simple linear regression.
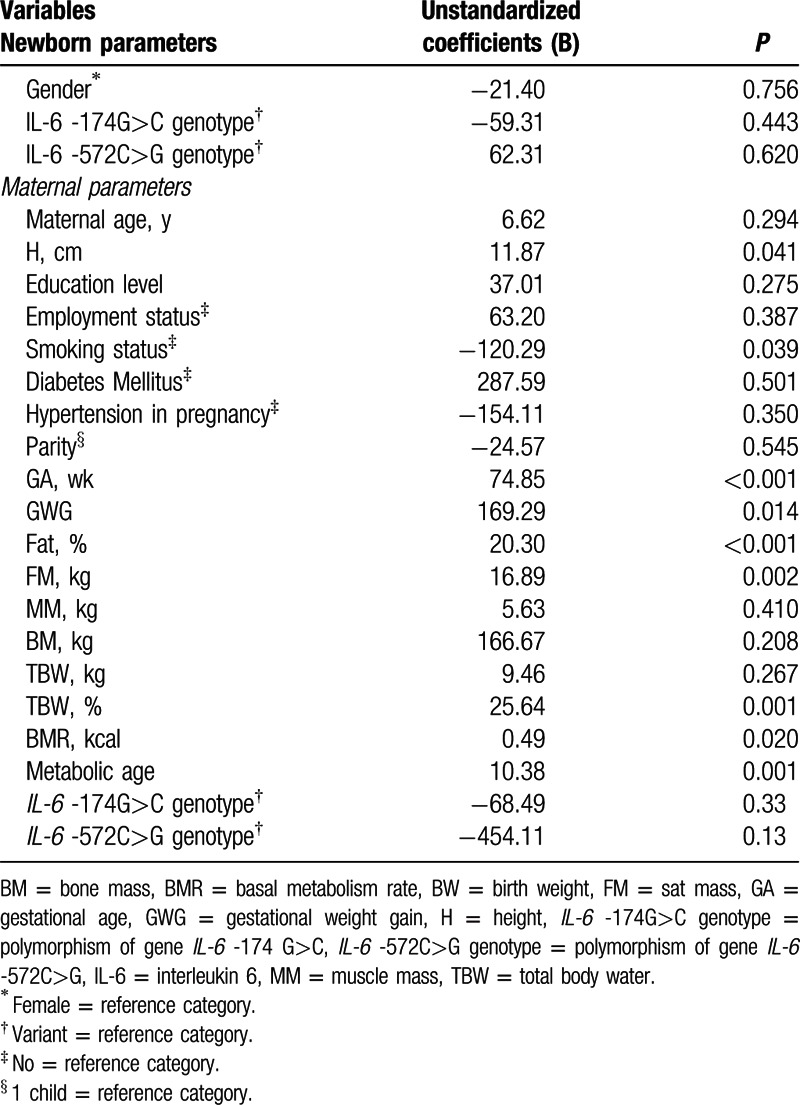
To establish which of the variables were independent predictors of BW after adjusting for other exogenous variables, we performed a multivariate linear regression analysis containing all variables whose P < 0.25 in univariate regression. In addition, we omitted from the regression the highly correlated variables: maternal TBW (%), maternal TBW (kg), BMR, and metabolic age. The tested multivariable model contained the following predictors: height (cm), smoking status, GA, GWG, FM (%), and mothers’ IL-6 -572G>C variant (GC, CC) genotype. In the regression results, only GW (unstandardized regression coefficient = 66.81, P < 0.001) and FM% (unstandardized regression coefficient = 13.26, P = 0.023) remained independent predictors of BW, whereas height, smoking status and mothers’ IL-6 -572G>C variant genotype were not predictive for BW. All variables considered in the final model explained 14.50% of the outcome variance. No statistical correlations were found between GWG and other different maternal risk factors such as maternal age, employment status, diabetes mellitus, gestational AHT, and parity.
4. Discussions
In the specialty literature numerous published studies tried to establish correlations between mother's weight at the beginning of the pregnancy, weight gain during the gestational period and the newborn's nutritional status at birth, and to establish possible risk factors for the child's evolution toward overweight or malnutrition. Thus in the review of Lau et al[49], it is noticed that GWG is a potential risk factor for child's obesity and that the results must be interpreted also in association with some familial characteristics, such as genetic, maternal factors, and the child's life style. Lau underlined that a GWG above the IOM[15] admitted limits increases the risk of overweight/obesity in the child with the age between 5 and 8 years with a percentage between 27% and 73%. It is also discussed the fact that there are 2 possible mechanisms regarding the influence of GWG in the accumulation of fetal adipose tissue: direct transfusion of free fatty acids from the mother to the fetus which is increased if the GWG is more expressed in the second trimester of pregnancy,[15,50] or through the synthesis of free fatty acids from the glucose provided by the mother, who exposes the fetus to a supplementary intake of glucose.[15] Branum et al[51] pointed out that the association between GWG and the child's BMI is no longer present if we also take under consideration familial factors. In addition, Lawlor et al[52] underlined that genetic and environmental factors have a more important role in determining the child's weight, compared with intrauterine ones. Another study performed on 42,133 women and their children[53] minimized the role of familial factors stating that a GWG over the accepted limit significantly increased the BMI in children with the age of 11.9 years, increasing by 8% the risk of obesity in children whose mothers gained over 18 kg during the pregnancy. These parameters were independent of GA, maternal smoking status, parity, children's age, and BMI.[53]
In the study of Farah et al,[1] by the use of a multivariate analysis, the evaluated factors influenced GWG in 5% and the study of Ferrari et al[25] using a linear regression showed that GWG has a great impact on BW, having a total outcome variance of 8.4%. In our study the mother's weight gain (expressed especially by the FM and GA) had a total variance of 14.5%.
Ferrari et al[25] also underlined that the mother's excessive weight gain determines an increase in BW, a lower Apgar score at birth and a lower pH in the blood from the umbilical cord, in comparison to the group with adequate weight gain during pregnancy. Macrosomic newborns are more exposed to further development of overweight and obesity during childhood and have an increased risk of cardiovascular and metabolic disorders.[54,55] In addition, it was noticed that infants with normal weight mothers had a BW of 3261.0 g in comparison to infants with obese mothers who had a BW of 3336.0 g, with a P < 0.05; also, women with higher than admitted GWG values had 54.5% higher chances of having macrosomic children than women with normal GWG.[25]
The review of Faucher and Barger[16] on 740,000 obese women pointed out that obese women have a risk for increased GA and higher newborns’ BW. Similarly, in our study we noticed that the increase in GA positively influenced the newborns’ BW (P < 0.001) (an increase with 1 week in GA determined an increment in the newborns’ BW by 74.85 g).
In another retrospective study performed by Farah on 262 cases, no correlation was identified between BW and BMI from early pregnancy, the mother's body composition being also evaluated through BIA techniques, like in our study. On the other hand, they noticed that BW was correlated with parity, smoking, and GA at delivery.[1] In our study we also noticed a direct proportional influence of GWG on BW and GA at delivery (P < 0.001), together with a reverse proportional relationship to the mothers’ smoking status (P = 0.039, B coefficient = −120.29). BW was correlated with GWG before the first trimester (P = 0.027), and not in the third trimester. Unlike this study[1] and an American one,[56] which established a correlation between FFM (P = 0.027) and FM at 28 to 37 gestational weeks and predicted BW, we obtained significant correlations between TBW (%) (P = 0.001), Fat (%), FM, BMR, metabolic age in mothers at delivery, and BW (P < 0.001/0.002/0.020/0.001).
The study of Ghezzi et al[57] noticed that BW is influenced by the TBW and extracellular water in the second trimester of pregnancy, fact also noticed by us through the correlation between the TBW and BW (P = 0.001). The study of Sanin Aguirre et al[58] performed in Mexico on 196 mothers and their fetuses, and that of Kent et al[20] performed on 2618 women from Ireland also established obvious correlations between BW and TBW, and between BW and FFM, respectively, but not FM. Anyway, recent studies established a connection between the risk of fetal macrosomia and maternal obesity,[4] and correlations with the child's overweight/obesity at the age of 11.9 years.[53] Kent et al also underlined that the recommendations regarding gestational weight gain of pregnant woman approved by the IOM should be revised, and that BMI is not a faithful factor for the evaluation of adipose tissue since BW and GWG are especially correlated with FFM and TBW.[20]
Sanin Aguirre et al[58] in a cross-sectional study on 196 pairs of mothers and newborns noticed that FM was a faithful predictor for BW in the group of mothers with low BMI. This study also pointed out that the bivariate model that included TBW and GA, mother's age, gender, and weight of the placenta established an obvious relationship with the mother's weight.[58] On the other hand, Larciprete et al[29] in a study performed on 198 pregnant patients noticed normal TBW values in relation with GWG. TBW and intracellular water increased slightly during gestation. In addition, they observed that FM deposition and reactance (another indirect indicator of the FM), not just TBW were responsible for GWG and correlated with BW.[29] In 2 previous studies by the same authors a reduction of TBW in the third trimester of pregnancy in women with gestational AHT [59] and an increase of TBW in women with gestational AHT treated with nifedipine[60] were noticed. In our study, we noticed that AHT was 4.65 times higher in women with increased GWG in comparison to those with normal weight gain (P = 0.013, odds ratio = 4.65, 95% CI: 1.27–17.03). The mother's smoking status was associated with a smaller BW. We observed that 16.5% of mothers were smokers, percentage similar with that from other studies, namely 17.7% in the study of Farah.[1] IL-6 is an important mediator of the immune and inflammatory response, which is involved in the hepatic function and lipid metabolism, being increased in the adipose tissue, and especially in body FM excess.[26,27,29,31] Several published studies pointed out that the G allele of the IL-6 -174G>C gene polymorphism is associated in general population with obesity and hypertension,[33,34] whereas other studies established correlations between the C allele and complications of obesity (hypertension and cardiovascular diseases).[36,37] In a previously published study, we noticed that the heterozygous variant CG genotype of the IL-6 -174G>C gene polymorphism was associated with obesity, whereas the CC genotype of the same gene was a protector factor for obesity in children.[42] In our study, we did not find any correlation regarding the G or C alleles of the IL-6 174 G>C gene polymorphism and the biochemical or anthropometric parameters, and neither between this polymorphism in mothers and their newborns. Regarding the IL-6 -572C>G gene polymorphism, a study performed in China pointed out the fact that the GG genotype is more frequently associated with obesity,[41] whereas other studies underlined that the CC genotype, and the C allele, respectively, are associated in a higher percentage with obesity.[42]
In our study we observed that the combined variant genotype (GG + GC) of the IL-6 -572C>G SNP was more frequently encountered in the control group (55.1%) in comparison with the study group (P = 0.042), which means that the variant genotype was a protector against weight gain in pregnant women. Nonetheless, there is a reduced number of studies that pointed out the association between the IL-6 -572C>G gene polymorphism and anthropometric and laboratory parameters.[26,61,62]
4.1. Limitations of this study
The present study had certain limitations, such as the relatively small number of cases included in the study, which diminished the statistical power of the study, even though initially there were 407 cases the number decreased after eliminating the extreme weight values (values more than 3.5 SD); therefore, we included in our final model the group with increased GWG (n = 141) and the control group with normal GWG (n = 168) together with their newborns. Another limitation was the impossibility to perform BIA measurements in newborns due to the limitations of the Tanita device used in this purpose (it allows the measurements only for children with the age above 5 years). Thirdly, we only evaluated the population from a single area of Romania, the center of the country, thus a single area of Europe. We did not evaluate the complications of obesity, such as metabolic syndrome, insulin resistance, and we did not take under consideration the ingestion of alimentary principles (glucids, lipids, proteins), as well as the geographic and environmental factors (even though some of the factors such as smoking, gestational AHT, the educational degree were included in the risk factors), factors that can be important in determining GWG, and BW. Due to the fact that obesity is a multifactorial disease, we evaluated the gene polymorphisms in mothers and their children, but we did not investigate the IL-6 -174G>C and -572C>G SNPs in their respective fathers, which is our future goal. In addition, it would be very useful for this study to be extended on an even bigger geographic area of Europe.
4.2. Strengths of this study
The accuracy of collecting data at the moment of birth for all the women admitted in a tertiary obstetrics gynecology center and for their newborns; mothers and their newborns being consecutively included in the study, without being chosen by convenience; use of BIA techniques for appreciating the FFM, TBW; improved knowledge on the role of the 2 studies SNPs in the development of obesity and the prediction of the fetuses’ BW. For all patients we calculated the BMI according to their weight and height, not using a self-reported weight and height. All clinical, laboratory, anthropometric and genetic parameters, except the BIA ones, were calculated both in the mothers and in their newborns. We must underline that this is the first cross-sectional study of this type in Romania on mothers and their newborns, and which for the first time established correlations between GWG evaluated through clinical, anthropometric, BIA, laboratory parameters, 2 gene polymorphisms, and its influence on the newborns’ BW. Due to this fact and because we did not find in the literature correlations between the IL-6 -174G>C and IL-6 -572C>G gene polymorphisms, we consider this study to be a pilot one, that must be extended on a bigger population, as well as transversally on a longer period, and to evaluate the newborns’ nutritional status further in life (at 6 months, 1 year, 2 years, 3 years); in this way, we would be able to identify more clearly the manner in which these factors influence the long-term nutritional status in these children.
GWG can influence the nutritional status in BW, fact proven by the evaluation of anthropometric and BIA factors. The BIA parameters performed in mothers such as FM, MM, BM, TBW, BMR, and metabolic age were higher in mothers with high GWG in comparison with those from the control group (P < 0.001), and also anthropometric parameters like MUAC, TST were higher in the study group (P < 0.001). The incidence of AHT was 4.65 times higher in women with gestational weight over the IOM accepted limits, high GWG being considered a risk factor for AHT (P = 0.013). Linear regression showed that BW was positively correlated with FM (P < 0.001), TBW (P = 0.001), BMR (P = 0.02), and metabolic age (P = 0.001) of the mothers; in addition, mothers’ smoking status was negatively correlated with BW (P = 0.039). The variant genotype [GG+GC] of IL-6 -572C>G was more frequently encountered in the control group versus the study group with high GWG (P = 0.042), while IL-6 -174G>C did not influence the mothers’ and their children's nutritional status.
5. Conclusions
High GWG may be an important predicting factor for the afterward weight of the fetus. We may consider that BIA parameters such as FFM (TBW, MM, BM) and also FM in mothers are associated with high GWG, characterizing in the best way the mother's nutritional status. Also, MUAC, TST, and gestational AHT are correlated with high GWG in mothers. BW is positively correlated with maternal FM, TBW, BMR, metabolic age, and negatively correlated to the smoking status. In our study on Caucasian mothers from Romania, we observed that the variant genotype of the IL-6 -572C>G polymorphism was a protection factors against the development of mother obesity, whereas the IL-6 -174G>C polymorphism did not influence the mothers’ and their children's nutritional status. All variables considered in the final model explained 14.50% of the outcome variance. Our study pointed out the most important factors that influence BW, further studies on bigger groups and more extended area being necessary.
Footnotes
Abbreviations: AHT = arterial hypertension, ALAT = alanine aminotransferase, ASAT = aspartate aminotransferase, BIA = bioimpedance, BM = bone mass, BMI = body mass index, BMR = basal metabolism rate, BW = birth weight, Chol = cholesterol, FM = fat mass, GA = gestational age, GWG = gestational weigh gain, H = height, IL-6 = interleukin 6, IOM = Institute of Medicine, MM = muscle mass, MUAC = middle upper arm circumference, SD = standard deviation, TBW = total body water, TST = tricipital skinfold thickness, W = weight.
CM and COM equally contributed to the writing of this article.
The authors have no conflicts of interest to disclose.
Cristina Oana Mărginean and Claudiu Mărginean contributed equally to this article.
References
- 1.Farah N, Stuart B, Donnelly V, et al. The influence of maternal body composition on birth weight. Eur J Obstet Gynecol Reprod Biol 2011; 157:14–17. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein IM, Horbar JD, Badger GJ, et al. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 2000; 182 (1 pt 1):198–206. [DOI] [PubMed] [Google Scholar]
- 3.Mocanu EV, Greene RA, Byrne BM, et al. Obstetric and neonatal outcome of babies weighing more than 4.5 kg: an analysis by parity. Eur J Obstet Gynecol Reprod Biol 2000; 92:229–233. [DOI] [PubMed] [Google Scholar]
- 4.Heslehurst N, Simpson H, Ells LJ, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev Off J Int Assoc Study Obes 2008; 9:635–683. [DOI] [PubMed] [Google Scholar]
- 5.Plagemann A, Harder T, Kohlhoff R, et al. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord J Int Assoc Study Obes 1997; 21:451–456. [DOI] [PubMed] [Google Scholar]
- 6.Haschke F, Ziegler EE, Grathwohl D. Fast growth of infants of overweight mothers: can it be slowed down? Ann Nutr Metab 2014; 64 Suppl 1:19–24. [DOI] [PubMed] [Google Scholar]
- 7.Haschke F, Steenhout P, Grathwohl D, et al. Evaluation of growth and early infant feeding: a challenge for scientists, industry and regulatory bodies. World Rev Nutr Diet 2013; 106:33–38. [DOI] [PubMed] [Google Scholar]
- 8.Koletzko B, Beyer J, Brands B, et al. Early influences of nutrition on postnatal growth. Nestlé Nutr Inst Workshop Ser 2013; 71:11–27. [DOI] [PubMed] [Google Scholar]
- 9.Dörner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm Metab Res Horm Stoffwechselforschung Horm Métabolisme 1994; 26:213–221. [DOI] [PubMed] [Google Scholar]
- 10.Plagemann A. “Fetal programming” and “functional teratogenesis”: on epigenetic mechanisms and prevention of perinatally acquired lasting health risks. J Perinat Med 2004; 32:297–305. [DOI] [PubMed] [Google Scholar]
- 11.Koletzko B. Metabolism and nutrition before and during pregnancy and after birth exert lasting effects on physiology, function, health, and performance well into adulthood and old age. Preface. Am J Clin Nutr 2011; 94 (6 suppl):1747S. [DOI] [PubMed] [Google Scholar]
- 12.Lucas A. Growth and later health: a general perspective. Nestlé Nutr Workshop Ser Paediatr Programme 2010; 65:1–9. [DOI] [PubMed] [Google Scholar]
- 13.Haugen M, Brantsæter AL, Winkvist A, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth 2014; 14:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf) 2013; 78:9–16. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen KM, Yaktine AL. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (US); 2009; http://www.ncbi.nlm.nih.gov/books/NBK32813/http://www.ncbi.nlm.nih.gov/books/NBK32813/. Accessed February 17, 2016. [PubMed] [Google Scholar]
- 16.Faucher MA, Barger MK. Gestational weight gain in obese women by class of obesity and select maternal/newborn outcomes: A systematic review. Women Birth J Aust Coll Midwives 2015; 28:e70–e79. [DOI] [PubMed] [Google Scholar]
- 17.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev Off J Int Assoc Study Obes 2001; 2:141–147. [DOI] [PubMed] [Google Scholar]
- 18.Abrams B, Selvin S. Maternal weight gain pattern and birth weight. Obstet Gynecol 1995; 86:163–169. [DOI] [PubMed] [Google Scholar]
- 19.Neufeld LM, Haas JD, Grajéda R, et al. Changes in maternal weight from the first to second trimester of pregnancy are associated with fetal growth and infant length at birth. Am J Clin Nutr 2004; 79:646–652. [DOI] [PubMed] [Google Scholar]
- 20.Kent E, O’Dwyer V, Fattah C, et al. Correlation between birth weight and maternal body composition. Obstet Gynecol 2013; 121:46–50. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evid Report Technology Assess 2008; 168:1–223. [PMC free article] [PubMed] [Google Scholar]
- 22.Nohr EA, Vaeth M, Baker JL, et al. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008; 87:1750–1759. [DOI] [PubMed] [Google Scholar]
- 23.Freeman DJ. Effects of maternal obesity on fetal growth and body composition: implications for programming and future health. Semin Fetal Neonatal Med 2010; 15:113–118. [DOI] [PubMed] [Google Scholar]
- 24.Guelinckx I, Devlieger R, Beckers K, et al. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev Off J Int Assoc Study Obes 2008; 9:140–150. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari N, Mallmann P, Brockmeier K, et al. Secular trends in pregnancy weight gain in German women and their influences on foetal outcome: a hospital-based study. BMC Pregnancy Childbirth 2014; 14:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henningsson S, Håkansson A, Westberg L, et al. Interleukin-6 gene polymorphism -174G/C influences plasma lipid levels in women. Obesity (Silver Spring, MD) 2006; 14:1868–1873. [DOI] [PubMed] [Google Scholar]
- 27.Goyenechea E, Parra D, Martínez JA. Impact of interleukin 6-174G>C polymorphism on obesity-related metabolic disorders in people with excess in body weight. Metabolism 2007; 56:1643–1648. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Real J-M. Genetic predispositions to low-grade inflammation and type 2 diabetes. Diabetes Technol Ther 2006; 8:55–66. [DOI] [PubMed] [Google Scholar]
- 29.Larciprete G, Valensise H, Vasapollo B, et al. Maternal body composition at term gestation and birth weight: is there a link? Acta Diabetol 2003; 40 Suppl 1:S222–224. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Jansson P-A, Pellmé F, et al. Effect of the interleukin-6 (-174) g/c promoter polymorphism on adiponectin and insulin sensitivity. Obes Res 2005; 13:813–817. [DOI] [PubMed] [Google Scholar]
- 31.Goyenechea E, Parra MD, Martínez Hernández JA. Role of IL-6 and its -174G>C polymorphism in weight management and in the metabolic comorbidities associated with obesity. An Sist Sanit Navar 2005; 28:357–366. [DOI] [PubMed] [Google Scholar]
- 32.Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun 2003; 17:350–364. [DOI] [PubMed] [Google Scholar]
- 33.Vozarova B, Fernández-Real J-M, Knowler WC, et al. The interleukin-6 (-174) G/C promoter polymorphism is associated with type-2 diabetes mellitus in Native Americans and Caucasians. Hum Genet 2003; 112:409–413. [DOI] [PubMed] [Google Scholar]
- 34.Illig T, Bongardt F, Schöpfer A, et al. Significant association of the interleukin-6 gene polymorphisms C-174G and A-598G with type 2 diabetes. J Clin Endocrinol Metab 2004; 89:5053–5058. [DOI] [PubMed] [Google Scholar]
- 35.Möhlig M, Boeing H, Spranger J, et al. Body mass index and C-174G interleukin-6 promoter polymorphism interact in predicting type 2 diabetes. J Clin Endocrinol Metab 2004; 89:1885–1890. [DOI] [PubMed] [Google Scholar]
- 36.Humphries SE, Luong LA, Ogg MS, et al. The interleukin-6-174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J 2001; 22:2243–2252. [DOI] [PubMed] [Google Scholar]
- 37.Berthier M-T, Paradis A-M, Tchernof A, et al. The interleukin 6-174G/C polymorphism is associated with indices of obesity in men. J Hum Genet 2003; 48:14–19. [DOI] [PubMed] [Google Scholar]
- 38.Stephens JW, Hurel SJ, Cooper JA, et al. A common functional variant in the interleukin-6 gene is associated with increased body mass index in subjects with type 2 diabetes mellitus. Mol Genet Metab 2004; 82:180–186. [DOI] [PubMed] [Google Scholar]
- 39.Grallert H, Huth C, Kolz M, et al. IL-6 promoter polymorphisms and quantitative traits related to the metabolic syndrome in KORA S4. Exp Gerontol 2006; 41:737–745. [DOI] [PubMed] [Google Scholar]
- 40.Saxena M, Srivastava N, Banerjee M. Association of IL-6, TNF-( and IL-10 gene polymorphisms with type 2 diabetes mellitus. Mol Biol Rep 2013; 40:6271–6279. [DOI] [PubMed] [Google Scholar]
- 41.Yang X, Feng L, Li C, et al. Association of IL-6-174G > C and -572C > G polymorphisms with risk of young ischemic stroke patients. Gene 2014; 539:258–262. [DOI] [PubMed] [Google Scholar]
- 42.Mărginean CO, Bănescu C, Duicu C, et al. The role of IL-6 572 C/G, 190 C/T, and 174 G/C gene polymorphisms in children's obesity. Eur J Pediatr 2014; 173:1285–1296. [DOI] [PubMed] [Google Scholar]
- 43.Romania—WHO Country Profile—Romania-WHO-Country-Profile.pdf. http://www.euro.who.int/__data/assets/pdf_file/0014/243320/Romania-WHO-Country-Profile.pdf?ua=1 Accessed February 18, 2016. [Google Scholar]
- 44.Chiriţă E, Puiu M, Gafencu M, et al. Growth References for School aged Children in Western Romania. Acta Endocrinol (Copenh) 2012; 8:133–152. [Google Scholar]
- 45.Valean C, Tatar S, Nanulescu M, et al. Prevalence of obesity and overweight among school children in Cluj-Napoca. Acta Endocrinol (Copenh) 2009; 5:213–219. [Google Scholar]
- 46.Daneshmandi S, Pourfathollah AA, Pourpak Z, et al. Cytokine gene polymorphism and asthma susceptibility, progress and control level. Mol Biol Rep 2012; 39:1845–1853. [DOI] [PubMed] [Google Scholar]
- 47.Zhang D, Zhou Y, Wu L, et al. Association of IL-6 gene polymorphisms with cachexia susceptibility and survival time of patients with pancreatic cancer. Ann Clin Lab Sci 2008; 38:113–119. [PubMed] [Google Scholar]
- 48.Frank E, Harrell J. Regression modeling strategies with applications to linear models, logistic and ordinal regression and survival analysis. Multivariable Modeling Strategies. 2nd edCham: Springer-Verlag; 2015. [Google Scholar]
- 49.Lau EY, Liu J, Archer E, et al. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J Obes 2014; 2014:524939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarr O, Yang K, Regnault TRH. In utero programming of later adiposity: the role of fetal growth restriction. J Pregnancy 2012; 2012:134758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Branum AM, Parker JD, Keim SA, et al. Prepregnancy body mass index and gestational weight gain in relation to child body mass index among siblings. Am J Epidemiol 2011; 174:1159–1165. [DOI] [PubMed] [Google Scholar]
- 52.Lawlor DA, Lichtenstein P, Fraser A, et al. Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? A sibling study in a prospective cohort of 146,894 men from 136,050 families. Am J Clin Nutr 2011; 94:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludwig DS, Rouse HL, Currie J. Pregnancy weight gain and childhood body weight: a within-family comparison. PLoS Med 2013; 10:e1001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hermann GM, Dallas LM, Haskell SE, et al. Neonatal macrosomia is an independent risk factor for adult metabolic syndrome. Neonatology 2010; 98:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rooney BL, Mathiason MA, Schauberger CW. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern Child Health J 2011; 15:1166–1175. [DOI] [PubMed] [Google Scholar]
- 56.Butte NF, Ellis KJ, Wong WW, et al. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol 2003; 189:1423–1432. [DOI] [PubMed] [Google Scholar]
- 57.Ghezzi F, Franchi M, Balestreri D, et al. Bioelectrical impedance analysis during pregnancy and neonatal birth weight. Eur J Obstet Gynecol Reprod Biol 2001; 98:171–176. [DOI] [PubMed] [Google Scholar]
- 58.Sanin Aguirre LH, Reza-López S, Levario-Carrillo M. Relation between maternal body composition and birth weight. Biol Neonate 2004; 86:55–62. [DOI] [PubMed] [Google Scholar]
- 59.Valensise H, Andreoli A, Lello S, et al. Multifrequency bioelectrical impedance analysis in women with a normal and hypertensive pregnancy. Am J Clin Nutr 2000; 72:780–783. [DOI] [PubMed] [Google Scholar]
- 60.Valensise H, Larciprete G, Vasapollo B, et al. Nifedipine-induced changes in body composition in hypertensive patients at term. Eur J Obstet Gynecol Reprod Biol 2003; 106:139–143. [DOI] [PubMed] [Google Scholar]
- 61.Ramírez-López G, Portilla-de Buen E, Sánchez-Corona J, et al. Interleukin-6 polymorphisms are associated with obesity and hyperglycemia in Mexican adolescents. Arch Med Res 2013; 44:62–68. [DOI] [PubMed] [Google Scholar]
- 62.Hamid YH, Rose CS, Urhammer SA, et al. Variations of the interleukin-6 promoter are associated with features of the metabolic syndrome in Caucasian Danes. Diabetologia 2005; 48:251–260. [DOI] [PubMed] [Google Scholar]


