Abstract
Abstract
Prevalence estimates of adjacent segment degeneration (ASD) following cervical spine surgery varied greatly in current studies. We conducted a systematic review and meta-analysis to summarize the point prevalence of ASD after cervical spine surgery.
Methods
Comprehensive electronic searches of PubMed, Embase, Web of Knowledge, and Cochrane Library databases were conducted to identify any study published from initial state to January 2016. Those reporting the prevalence of ASD after cervical surgery were included. A random-effects model was used to estimate the prevalence of radiographic ASD, symptomatic ASD, and reoperation ASD. Univariate meta-regression analyses were conducted to explore the potential associations between prevalence and length of follow-up. All analyses were performed using R version 3.2.3 (R Foundation for Statistical Computing).
Results
A total of 83 studies were included in the meta-analysis. The prevalence of radiographic ASD, symptomatic ASD, and reoperation ASD after cervical surgery was 28.28% (95% confidence interval [CI], 20.96–36.96), 13.34% (95% CI, 11.06–16.00), and 5.78% (95% CI, 4.99–6.69), respectively, in a general analysis. It was found 2.79%, 1.43%, and 0.24% additions per year of follow-up in the incidence of radiographic ASD, symptomatic ASD, and reoperation ASD, respectively.
Conclusion
This meta-analysis provides some details about the prevalence of radiographic ASD, symptomatic ASD, and reoperation ASD after cervical spine surgery. However, the results of this meta-analysis should be interpreted with caution because of the heterogeneity among the studies.
Keywords: adjacent segment disease, cervical surgery, meta-analysis, prevalence, review
1. Introduction
Cervical degenerative disease is a pathological condition that affects the adult spine and is a common cause of cervical radiculopathy and myelopathy in older patients. Cervical surgery can decompress the neural elements and stabilize the affected segments at the same time. However, adjacent segment degeneration (ASD) is often observed in patients who are followed for a long period.[1,2]
Postoperative degenerative changes include both radiographic ASD and symptomatic ASD. Radiographic ASD could develop into symptomatic ASD, which correlates with some clinical findings, and symptomatic ASD could lead to serious pain, dysfunction or need for additional surgery. Hilibrand et al[3] reported an incidence of 2.9% per year of the development of symptomatic ASD after single-level anterior cervical discectomy and fusion (ACDF) and estimated that about 25.6% of patients would have symptomatic ASD within 10 years after their index surgery. They also found that more than two-thirds of patients developing symptomatic ASD experienced failure of conservative treatment and required surgical procedures.
A reliable estimate of the prevalence of postoperative ASD is important for informing efforts to prevent, treat, and identify causes of ASD. Over the past few years, several meta-analyses have reported on ASD after lumbar or spinal surgery,[4–6] but to the best of our knowledge, no comprehensive meta-analysis of the epidemiological data on ASD following cervical surgery has been published. Thus, we conducted this systematic review and meta-analysis to obtain accurate figures on the prevalence of ASD after cervical surgery.
2. Methods
This study followed the systematic review methodology proposed in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[7] As all the analyses were performed by using data extracted from published trials, it is not necessary to obtain ethical approval for this study.
2.1. Literature search strategy
We searched PubMed, Embase, Web of Knowledge, and Cochrane Library databases for articles published up to January 2016, using the following search terms: (“adjacent level” or “adjacent segment”) and (“pathology” or “disease”) and (“cervical” or “spine” or “spinal”). The references of all publications were also retrieved to obtain further publications.
2.2. Inclusion and exclusion criteria
The following criteria were used for screening the literature. First, the study design included randomized controlled trials, cohort study, case–control study, and cross-sectional study. Second, sample size and point prevalence of ASD were provided or could be calculated. Third, the method of diagnosing ASD was described. Fourth, population was restricted to patients after cervical surgery.
Publications were excluded if they were review articles, case reports, editorials, or letters. Any biomechanical studies or clinical studies investigating cervical tumor, infection, or trauma were also excluded. The number of levels treated with total disc replacement (TDR) or ACDF was not a criterion for exclusion.
2.3. Data extraction and outcome measures
For each included study, the following information was extracted: first author, year of publication, country, sample size, surgical approach, study design, length of follow-up, and number of patients with ASD after surgery. The most comprehensive publication was used when several studies involved the same population.
2.4. Diagnosis of ASD and other criteria
Three main results were investigated in this study: radiographic ASD, symptomatic ASD, and reoperation ASD. Radiographic ASD was defined as radiographic changes at levels adjacent to a previous surgical segment. Symptomatic ASD is 1 type of radiographic ASD that leads to a new development of radiculopathy or myelopathy. Symptomatic ASD that required a further surgical intervention was considered reoperation ASD.
According to the different length of follow-up, <5 years was considered short-term follow-up; any period longer was considered long-term follow-up.
2.5. Assessment of methodological quality
Each included study was chosen independently by 2 authors using a published quality rating system designed especially for articles reporting on prevalence.[8] This 5-point scale system included whether the study design was appropriate for obtaining prevalence estimates; the sample was representative of the general population of patients after cervical surgery; the ASD diagnostic criteria were acceptable; diagnosis of ASD was performed on a consecutive or random sample of subjects; and the final diagnosis was known for 80% of eligible subjects.
2.6. Statistical analysis
We extracted data from each study, calculated the overall prevalence of ASD or risk ratios (RRs) with 95% confidence intervals (CIs), and obtained corresponding forest plots. Subgroup analysis was also conducted to discover the prevalence of different categories (number of operated level, approach, and type of surgery). In addition, we restricted analysis to randomized controlled trials that compared TDR and ACDF to investigate the different prevalence of reoperation ASD better between fusion and nonfusion techniques. Furthermore, univariate meta-regression analyses were conducted to explore the potential associations between prevalence and length of follow-up. Heterogeneity among studies was assessed by I2 and Q tests. If I2 value was <50% and P value was >0.10, it was considered significant heterogeneity. In this study, random-effects model was used to pool the results. The influence of individual studies on the overall prevalence estimate was explored by serially excluding each study in a sensitivity analysis. Begg test was used to test the publication bias.
All analyses were performed using R version 3.2.3 (R Foundation for Statistical Computing). P value of <0.05 was considered statistically significant.
3. Results
3.1. Literature search results
The first searches give a total of 849 records, and 295 records were duplicates. After review of the titles and abstracts, 438 were excluded. We retrieved full articles for further assessment, and 33 records were further excluded. Finally, 83 studies were included in the meta-analysis.[1–3,9–88]Figure 1 shows the details of the screening process.
Figure 1.
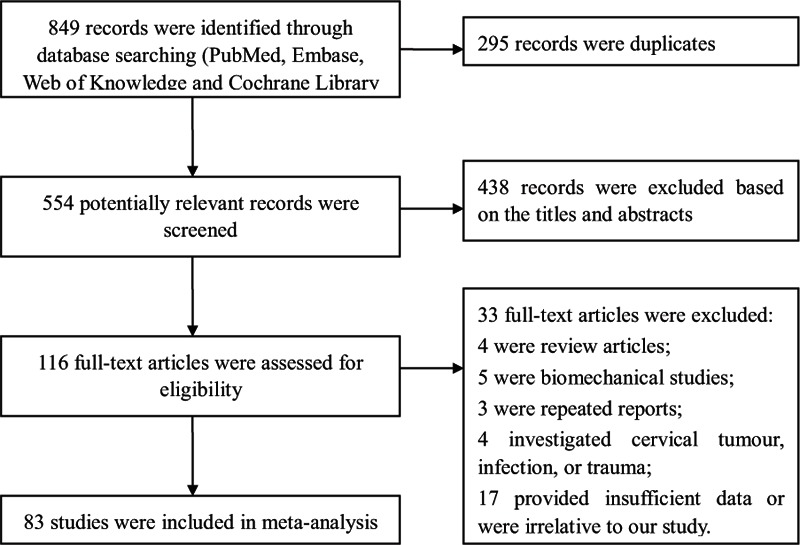
Flow diagram of the study selection process in the meta-analysis.
3.2. Study characteristics
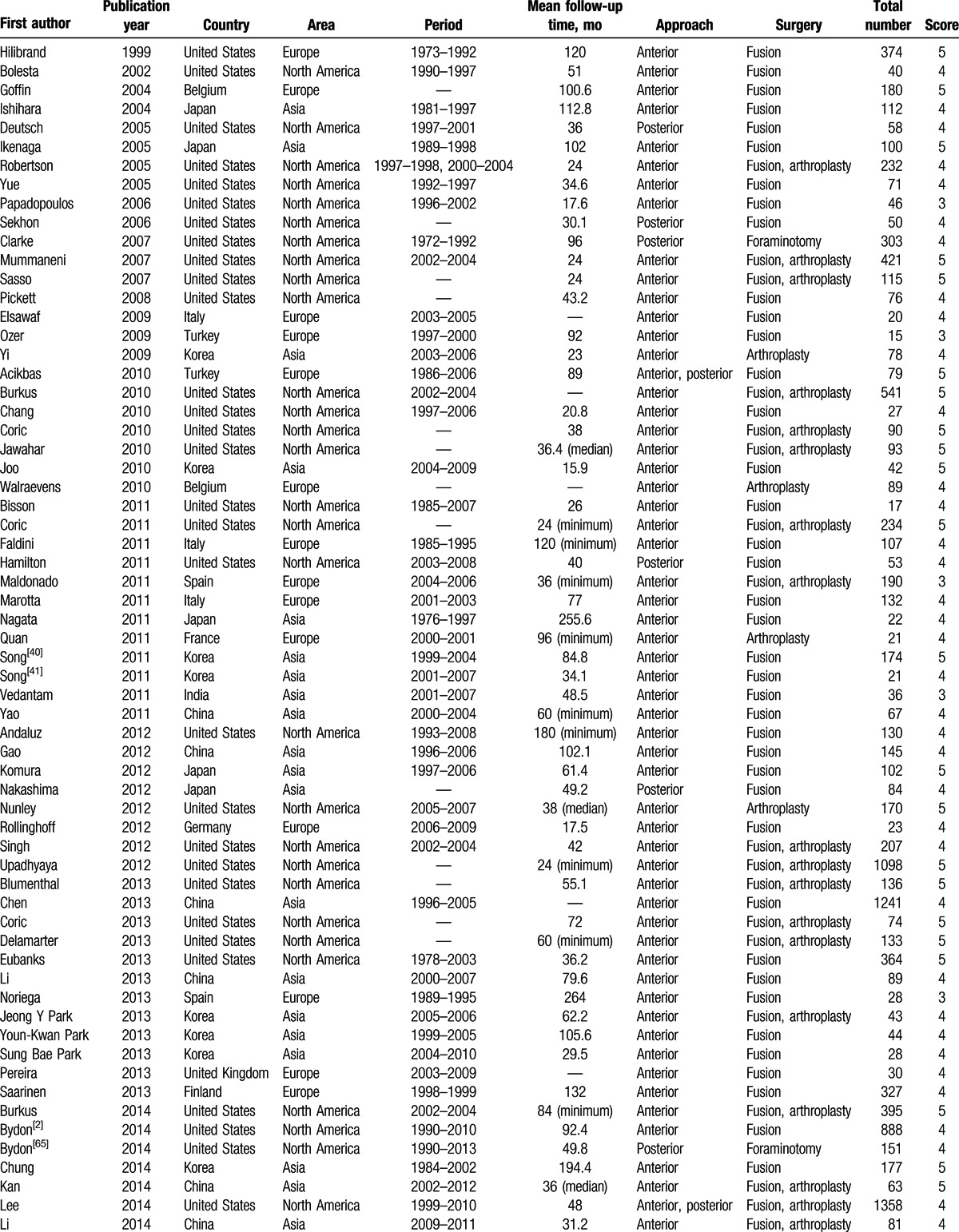
Of the 83 studies, 35 reported radiographic ASD, 24 reported symptomatic ASD, and 52 reported reoperation ASD. There were 4050, 4475, and 13,116 patients involved in the 3 types of ASD, respectively. The studies were from 14 countries. Among them, 36 took place in North America, 29 in Asia, 17 in Europe, and 1 in Oceania. The surgical procedures included ACDF, TDR, laminectomy, laminoplasty, posterior foraminotomy, and posterior cervical fusion. The quality score of the included studies ranged from 3 to 5 points. Detailed information on all included studies is shown in Table 1 .
Table 1.
Basic characteristics of included studies.
3.3. Prevalence of ASD
Thirty-five studies reported the prevalence of radiographic ASD after cervical surgery and revealed that the occurrence of radiographic ASD ranged from 4.74% to 92.22%; the pooled prevalence was 28.28% (95% CI, 20.96–36.96) (Fig. 2). There was significant heterogeneity for radiographic ASD (I2 = 95.90%; Q = 837.75; P < 0. 01).
Figure 2.
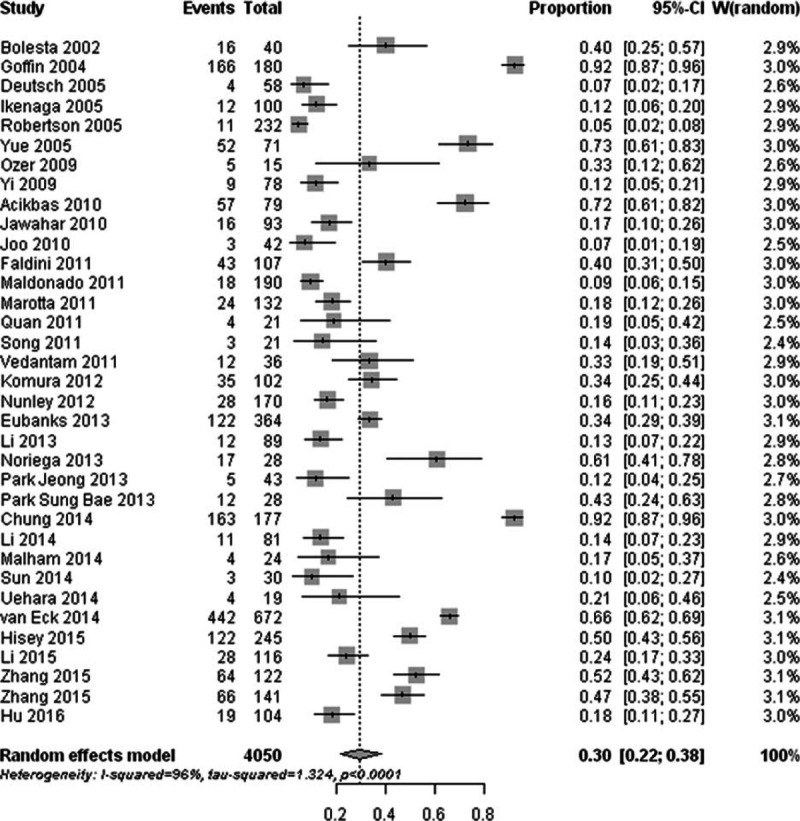
Forest plot of the prevalence of radiographic adjacent segment degeneration after cervical spine surgery.
The prevalence of symptomatic ASD ranged between 0% and 54.55% in 24 populations. The summary prevalence of symptomatic ASD was 13.34% (95% CI, 11.06–16.00) with significant heterogeneity (I2 = 76.90%; Q = 99.67; P < 0. 01) (Fig. 3).
Figure 3.
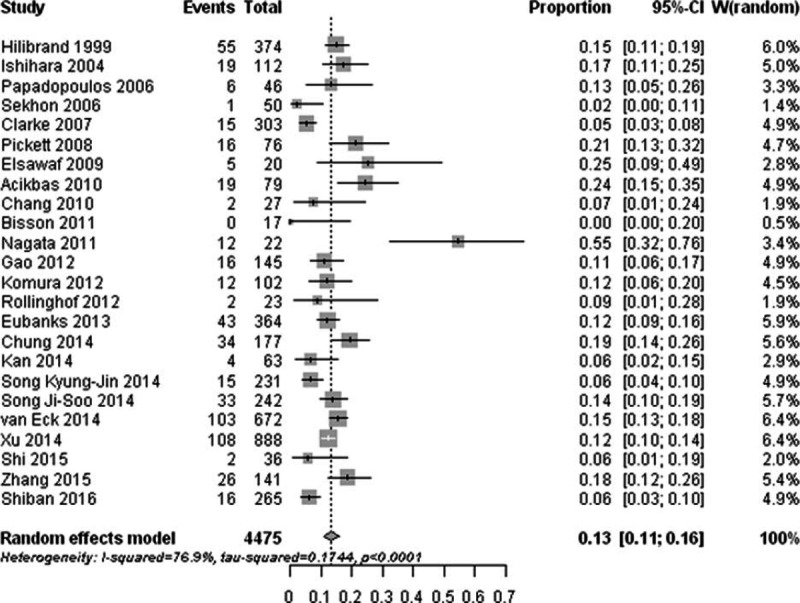
Forest plot of the prevalence of symptomatic adjacent segment degeneration after cervical spine surgery.
Reoperation ASD was reported in 52 studies, and the prevalence of reoperation ASD ranged from 0% to 16.90%; the pooled prevalence was 5.78% (95% CI, 4.99–6.69) (Fig. 4). There was significant heterogeneity (I2 = 69.60%; Q = 167.58; P < 0. 01) for the reoperation ASD.
Figure 4.
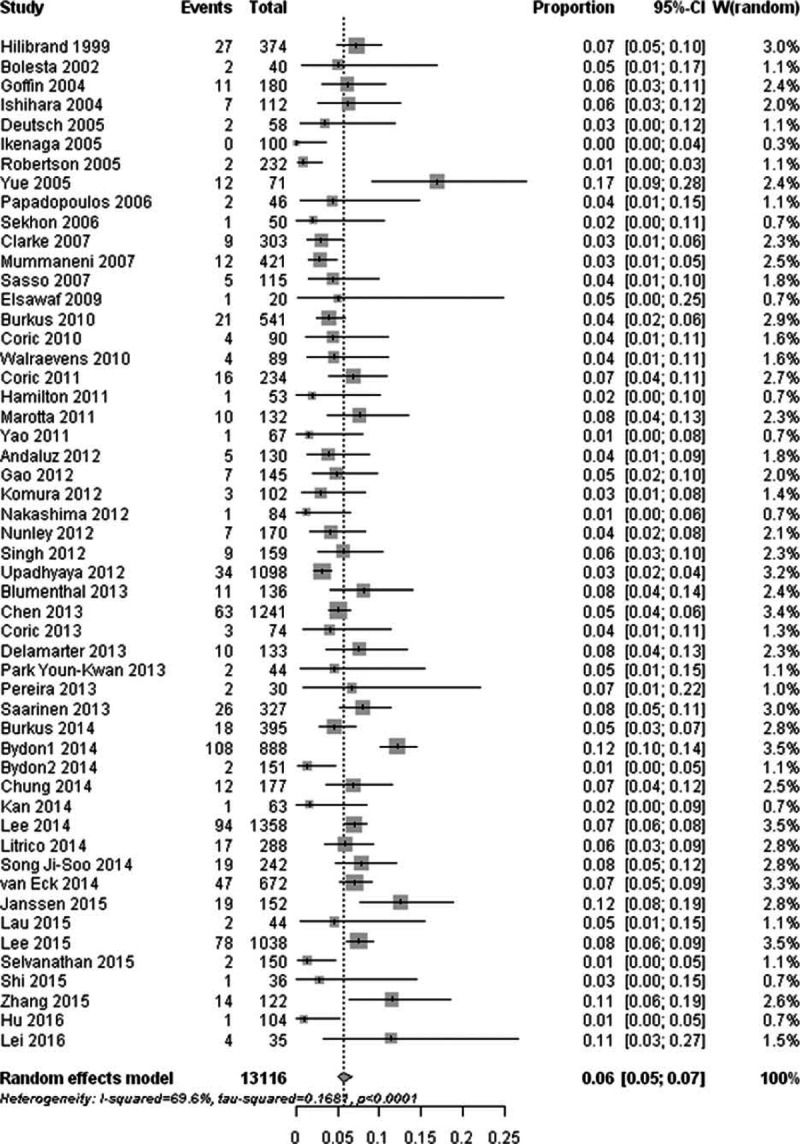
Forest plot of the prevalence of reoperation adjacent segment degeneration after cervical spine surgery.
3.4. Prevalence of ASD by study-level characteristics
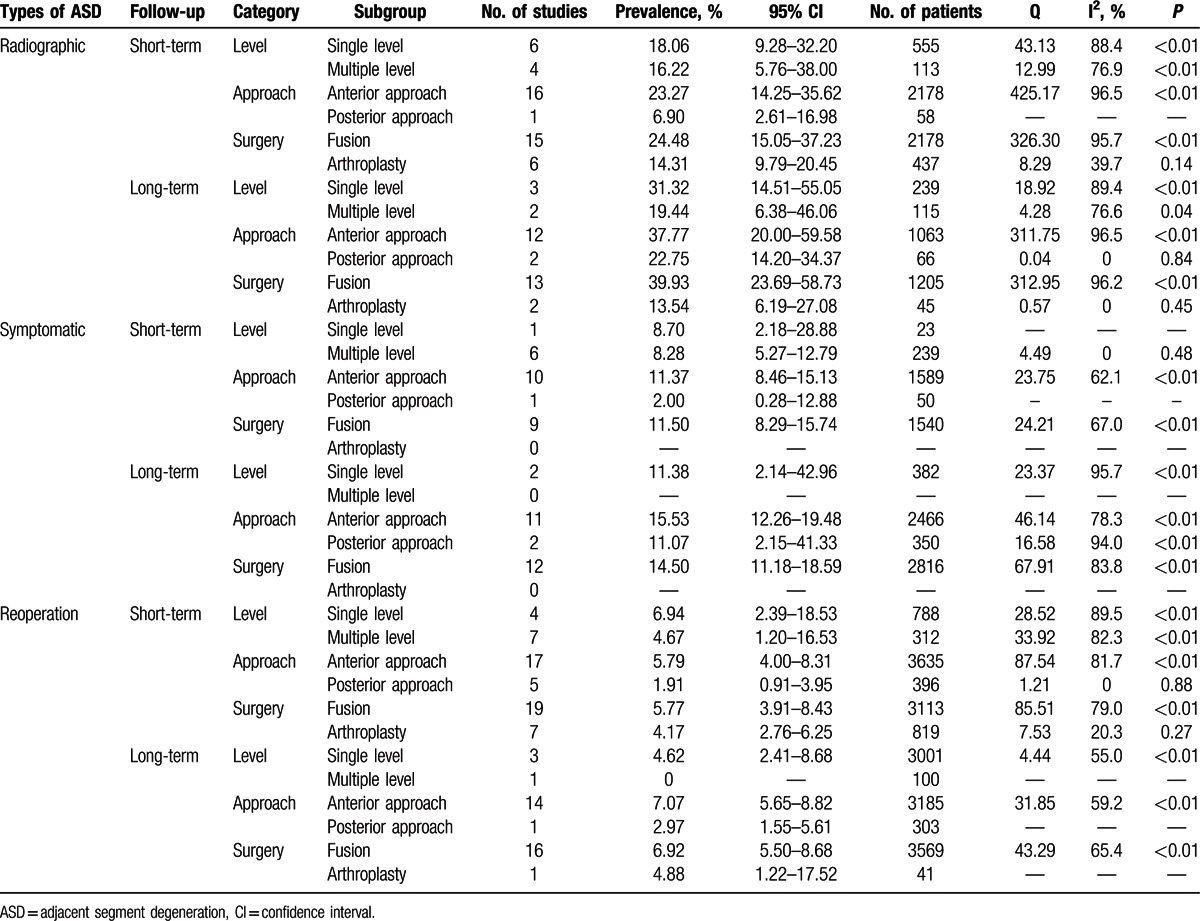
The studies were divided into short-term subgroup and long-term subgroup according to different length of follow-up, and then we summarized the stratified prevalence estimates based on number of level, approach, and surgery types. The pooled prevalence of ASD after single level, anterior approach and fusion surgery was higher than that after multiple level, posterior approach and arthroplasty. All main results of subgroup analyses are shown in Table 2.
Table 1 (Continued).
Basic characteristics of included studies.
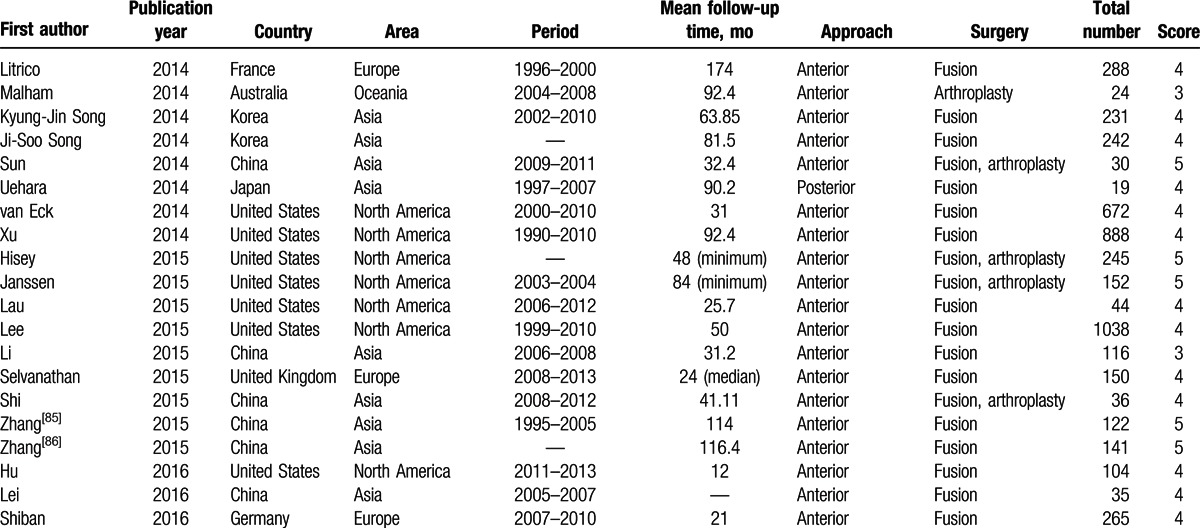
Table 2.
Main results of subgroup analyses.
3.5. Comparison of ASD between ACDF and TDR
Ten high-quality RCTs compared the prevalence of reoperation ASD after 1- or 2-level TDR with ACDF.[19,20,26,28,33,51,52,55,64,80] The test for heterogeneity was not significant (I2 = 31.3%; Q = 13.1; P = 0.16). The prevalence of reoperation ASD was significantly lower in the TDR group compared with the ACDF group (RR, 0.55; 95% CI, 0.35–0.85; P < 0.01) (Fig. 5).
Figure 5.
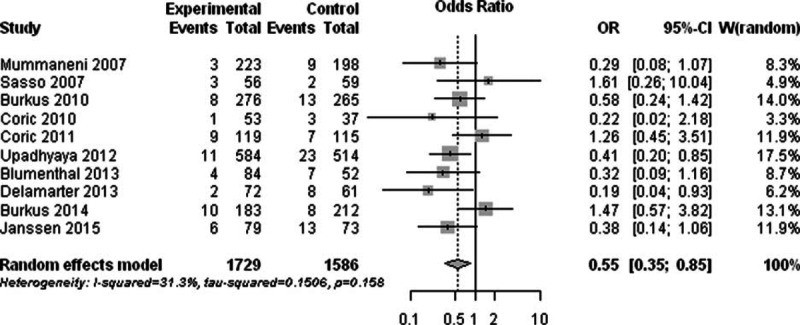
Forest plot showing the risk ratio of reoperation adjacent segment degeneration between the total disc replacement group and the anterior cervical discectomy and fusion group.
3.6. Length of follow-up and prevalence of ASD
Because the longitudinal studies reported different prevalence at different follow-up periods, we also investigated the association between length of follow-up and prevalence of ASD. The results of univariate meta-regression analysis found an addition of 2.79% (P < 0.01), 1.43% (P < 0.01), and 0.24% (P = 0.03) per year of follow-up in the development of radiographic ASD, symptomatic ASD, and reoperation ASD, respectively.
3.7. Sensitivity analysis and publication bias
Sensitivity analysis, in which the meta-analyses were serially repeated after exclusion of each study, demonstrated that no individual study affected the overall prevalence estimate of symptomatic ASD or reoperation ASD by more than 1%. Particular individual studies were affecting the overall prevalence estimate of radiographic ASD >1% but <2%.
The funnel plot found an apparent publication bias in the assessment of reoperation ASD (P = 0.02). No publication bias was found in the assessment of radiographic ASD (P = 0.49) or symptomatic ASD (P = 0.49).
4. Discussion
The pooled data of this meta-analysis showed that the prevalence of radiographic ASD, symptomatic ASD, and reoperation ASD after cervical surgery was 28.28%, 13.34%, and 5.78%, respectively. It showed that nearly half of the radiographic ASD patients would develop symptomatic ASD, and less than half of the symptomatic ASD would need additional cervical surgery.
The definition of radiographic ASD varied from 1 study to another, but the 1 proposed by Hilibrand et al[3] was used most: they divided ASD into 4 stages (from 1 to 4) according to plain radiography and magnetic resonance imaging, and stage 2 to stage 4 were considered indicative of radiographic ASD. However, in this meta-analysis, the number of studies using this definition was limited; thus, we cannot draw any convincing conclusions based on this definition. The unclear definition is the main cause of significant heterogeneity among studies in the assessment of radiography ASD. We think it could be better to assess and report radiographic ASD by using a unified classification of severity if possible. The definition of reoperation ASD, on the other hand, was more practical because it is much easier to be unified and thus, could provide more robust results and more precise information to us.
Because there was substantial clinical and statistical heterogeneity, subgroup analyses were performed to explore the sources of heterogeneity and clarify the prevalence of different categories. We found that the prevalence of ASD in multilevel subgroup was lower than that in single-level subgroups, and this result was consistent with the study conducted by Hilibrand et al.[3] This finding may be explained by analysis of the levels that underwent surgery. ASD was more likely to occur at the C5/6 and C6/7 levels than at other levels. In patients with a single-level procedure, the C5/6 or C6/7 was involved most, and the adjacent C6/7 or C5/6 was at high risk to degenerate.[46] ASD was less common after multilevel surgery because these procedures usually included the higher-risk levels and had an end adjacent to segments that were at lower risk for the development of new disease. Our result also showed that anterior-approach subgroups had a higher prevalence of ASD than posterior-approach subgroups. This result could be due to the same reason as already mentioned, because the posterior approach usually was performed on patients with multilevel disc degeneration.
Several biomechanical studies suggested that fusion causes increased stress and strain on neighboring motion segments, which potentially contribute to accelerated degeneration, whereas TDR not only maintains physiologic motion at the operated level but also minimizes changes at the adjacent segments.[89–91] In the clinical trials, ASD was also found in TDR patients after several years of follow-up.[92] In our meta-analysis, the prevalence of reoperation ASD after TDR was 44% lower than after ACDF. The prevalence of radiographic ASD and symptomatic ASD was not calculated because the relatively small number of studies reported corresponding data. However, this result gives us some clues that TDR may have the advantage of decreasing the incidence of postoperative ASD.
As we know, the prevalence of ASD increased with the extension of follow-up time.[11] This meta-analysis showed that with the addition of 1 follow-up year, the prevalence of radiographic ASD, symptomatic ASD, and reoperation ASD significantly increased by 2.79%, 1.43%, and 0.24%, respectively. Nevertheless, the cervical spine naturally undergoes degenerative changes with increasing age, and this fact poses a notable challenge when establishing ASD resulting from fusion[93] versus that occurring simply as a natural aging process. Herkowitz et al[94] studied patients with cervical radiculopathy after ACDF or posterior foraminotomy without fusion. After a mean of 4.2 follow-up years, 39% of patients developed ASD after fusion, but 50% of patients undergoing posterior foraminotomy also developed ASD at the operated and adjacent levels. Gore[95] studied the natural history of cervical spondylotic disease in 159 asymptomatic patients and found that about 12% developed symptomatic spondylotic disease over a 10-year period. These studies imply that fusion is not the only factor that influences the risk of ASD, and future studies could provide more convincing evidence on this topic.
Several limitations should be considered when interpreting the findings of this study. First, there was a substantial amount of heterogeneity among the studies. Although potential sources of heterogeneity were explored by subgroup analyses of number of level, approach, and surgery types, none of them could sufficiently explain the heterogeneity. We did not conduct the subgroup analysis by age, gender, study design, or other factors because these data vary greatly. Second, not all of the included studies were designed for the prevalence study. Some of them did not provide detailed characteristics of patients with ASD, and this may have led to the imprecision of the pooled data. Third, the diagnosis criteria of ASD were not uniform among the included studies. Radiographic ASD, for example, could be better assessed and reported using classifications of severity. A multicentre prospective study using a single validated definition of ASD could provide a more accurate estimate of the prevalence of ASD after cervical surgery.
This meta-analysis provides detailed information on the prevalence of radiographic ASD, symptomatic ASD, and reoperation ASD after cervical surgery. This information should be useful to surgeons and patients to gain a better understanding of ASD during follow-up. However, because of the limitations noted earlier, the results of this meta-analysis should be interpreted with caution.
Footnotes
Abbreviations: ACDF = anterior cervical discectomy and fusion, ASD = adjacent segment degeneration, CI = confidence interval, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR = risk ratio, TDR = total disc replacement.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Li J, Li Y, Kong F, et al. Adjacent segment degeneration after single-level anterior cervical decompression and fusion: disc space distraction and its impact on clinical outcomes. J Clin Neurosci 2015; 22:566–569. [DOI] [PubMed] [Google Scholar]
- 2.Bydon M, Xu R, De la Garza-Ramos R, et al. Adjacent segment disease after anterior cervical discectomy and fusion: incidence and clinical outcomes of patients requiring anterior versus posterior repeat cervical fusion. Surg Neurol Int 2014; 5 Suppl 3:S74–S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am 1999; 81:519–528. [DOI] [PubMed] [Google Scholar]
- 4.Xia XP, Chen HL, Cheng HB. Prevalence of adjacent segment degeneration after spine surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2013; 38:597–608. [DOI] [PubMed] [Google Scholar]
- 5.Ren C, Song Y, Liu L, et al. Adjacent segment degeneration and disease after lumbar fusion compared with motion-preserving procedures: a meta-analysis. Eur J Orthop Surg Traumatol 2014; 24 Suppl 1:S245–S253. [DOI] [PubMed] [Google Scholar]
- 6.Pan A, Hai Y, Yang J, et al. Adjacent segment degeneration after lumbar spinal fusion compared with motion-preservation procedures: a meta-analysis. Eur Spine J 2016; 25:1522–1532. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med 2009; 3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 8.Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 2008; 27:302–308. [DOI] [PubMed] [Google Scholar]
- 9.Bolesta MJ, Rechtine GRN, Chrin AM. One- and two-level anterior cervical discectomy and fusion: the effect of plate fixation. Spine J 2002; 2:197–203. [DOI] [PubMed] [Google Scholar]
- 10.Goffin J, Geusens E, Vantomme N, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech 2004; 17:79–85. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara H, Kanamori M, Kawaguchi Y, et al. Adjacent segment disease after anterior cervical interbody fusion. Spine J 2004; 4:624–628. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch H, Haid RW, Jr, Rodts GE, Jr, et al. Occipitocervical fixation: long-term results. Spine (Phila Pa 1976) 2005; 30:530–535. [DOI] [PubMed] [Google Scholar]
- 13.Ikenaga M, Shikata J, Tanaka C. Anterior corpectomy and fusion with fibular strut grafts for multilevel cervical myelopathy. J Neurosurg Spine 2005; 3:79–85. [DOI] [PubMed] [Google Scholar]
- 14.Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine 2005; 3:417–423. [DOI] [PubMed] [Google Scholar]
- 15.Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine (Phila Pa 1976) 2005; 30:2138–2144. [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulos EC, Huang RC, Girardi FP, et al. Three-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Spine (Phila Pa 1976) 2006; 31:897–902. [DOI] [PubMed] [Google Scholar]
- 17.Sekhon LH. Posterior cervical decompression and fusion for circumferential spondylotic cervical stenosis: review of 50 consecutive cases. J Clin Neurosci 2006; 13:23–30. [DOI] [PubMed] [Google Scholar]
- 18.Clarke MJ, Ecker RD, Krauss WE, et al. Same-segment and adjacent-segment disease following posterior cervical foraminotomy. J Neurosurg Spine 2007; 6:5–9. [DOI] [PubMed] [Google Scholar]
- 19.Mummaneni PV, Burkus JK, Haid RW, et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine 2007; 6:198–209. [DOI] [PubMed] [Google Scholar]
- 20.Sasso RC, Smucker JD, Hacker RJ, et al. Clinical outcomes of BRYAN cervical disc arthroplasty: a prospective, randomized, controlled, multicenter trial with 24-month follow-up. J Spinal Disord Tech 2007; 20:481–491. [DOI] [PubMed] [Google Scholar]
- 21.Pickett GE, Duggal N, Theodore N, et al. Anterior cervical corpectomy and fusion accelerates degenerative disease at adjacent vertebral segments. SAS J 2008; 2:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsawaf A, Mastronardi L, Roperto R, et al. Effect of cervical dynamics on adjacent segment degeneration after anterior cervical fusion with cages. Neurosurg Rev 2009; 32:215–224.discussion 224. [DOI] [PubMed] [Google Scholar]
- 23.Ozer AF, Oktenoglu T, Cosar M, et al. Long-term follow-up after open-window corpectomy in patients with advanced cervical spondylosis and/or ossification of the posterior longitudinal ligament. J Spinal Disord Tech 2009; 22:14–20. [DOI] [PubMed] [Google Scholar]
- 24.Yi S, Lee DY, Ahn PG, et al. Radiologically documented adjacent-segment degeneration after cervical arthroplasty: characteristics and review of cases. Surg Neurol 2009; 72:325–329.discussion 329. [DOI] [PubMed] [Google Scholar]
- 25.Acikbas SC, Ermol C, Akyuz M, et al. Assessment of adjacent segment degeneration in and between patients treated with anterior or posterior cervical simple discectomy. Turk Neurosurg 2010; 20:334–340. [DOI] [PubMed] [Google Scholar]
- 26.Burkus JK, Haid RW, Traynelis VC, et al. Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: results from a prospective randomized controlled clinical trial. J Neurosurg Spine 2010; 13:308–318. [DOI] [PubMed] [Google Scholar]
- 27.Chang SW, Kakarla UK, Maughan PH, et al. Four-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Neurosurgery 2010; 66:639–646.discussion 646–637. [DOI] [PubMed] [Google Scholar]
- 28.Coric D, Cassis J, Carew JD, et al. Prospective study of cervical arthroplasty in 98 patients involved in 1 of 3 separate investigational device exemption studies from a single investigational site with a minimum 2-year follow-up. Clinical article. J Neurosurg Spine 2010; 13:715–721. [DOI] [PubMed] [Google Scholar]
- 29.Jawahar A, Cavanaugh DA, Kerr EJR, et al. Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: results of 93 patients in three prospective randomized clinical trials. Spine J 2010; 10:1043–1048. [DOI] [PubMed] [Google Scholar]
- 30.Joo YH, Lee JW, Kwon KY, et al. Comparison of fusion with cage alone and plate instrumentation in two-level cervical degenerative disease. J Korean Neurosurg Soc 2010; 48:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walraevens J, Demaerel P, Suetens P, et al. Longitudinal prospective long-term radiographic follow-up after treatment of single-level cervical disk disease with the Bryan Cervical Disc. Neurosurgery 2010; 67:679–687.discussion 687. [DOI] [PubMed] [Google Scholar]
- 32.Bisson EF, Samuelson MM, Apfelbaum RI. Intermediate segment degeneration after noncontiguous anterior cervical fusion. Acta Neurochir (Wien) 2011; 153:123–127.discussion 128. [DOI] [PubMed] [Google Scholar]
- 33.Coric D, Nunley PD, Guyer RD, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine 2011; 15:348–358. [DOI] [PubMed] [Google Scholar]
- 34.Faldini C, Pagkrati S, Leonetti D, et al. Sagittal segmental alignment as predictor of adjacent-level degeneration after a Cloward procedure. Clin Orthop Relat Res 2011; 469:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton DK, Smith JS, Reames DL, et al. Safety, efficacy, and dosing of recombinant human bone morphogenetic protein-2 for posterior cervical and cervicothoracic instrumented fusion with a minimum 2-year follow-up. Neurosurgery 2011; 69:103–111.discussion 111. [DOI] [PubMed] [Google Scholar]
- 36.Maldonado CV, Paz RD, Martin CB. Adjacent-level degeneration after cervical disc arthroplasty versus fusion. Eur Spine J 2011; 20 Suppl 3:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marotta N, Landi A, Tarantino R, et al. Five-year outcome of stand-alone fusion using carbon cages in cervical disc arthrosis. Eur Spine J 2011; 20 Suppl 1:S8–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata T, Takami T, Yamagata T, et al. Significant relationship between local angle at fused segments and C2-7 angle: average duration of longer than 20 years after anterior cervical discectomy and fusion. J Craniovertebr Junction Spine 2011; 2:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quan GM, Vital JM, Hansen S, et al. Eight-year clinical and radiological follow-up of the Bryan cervical disc arthroplasty. Spine (Phila Pa 1976) 2011; 36:639–646. [DOI] [PubMed] [Google Scholar]
- 40.Song KJ, Choi BW, Jeon TS, et al. Adjacent segment degenerative disease: is it due to disease progression or a fusion-associated phenomenon? Comparison between segments adjacent to the fused and non-fused segments. Eur Spine J 2011; 20:1940–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song KJ, Kim GH, Choi BY. Efficacy of PEEK cages and plate augmentation in three-level anterior cervical fusion of elderly patients. Clin Orthop Surg 2011; 3:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vedantam A, Revanappa KK, Rajshekhar V. Changes in the range of motion of the cervical spine and adjacent segments at ≥ 24 months after uninstrumented corpectomy for cervical spondylotic myelopathy. Acta Neurochir (Wien) 2011; 153:995–1001. [DOI] [PubMed] [Google Scholar]
- 43.Yao N, Wang C, Wang W, et al. Full-endoscopic technique for anterior cervical discectomy and interbody fusion: 5-year follow-up results of 67 cases. Eur Spine J 2011; 20:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andaluz N, Zuccarello M, Kuntz C. Long-term follow-up of cervical radiographic sagittal spinal alignment after 1- and 2-level cervical corpectomy for the treatment of spondylosis of the subaxial cervical spine causing radiculomyelopathy or myelopathy: a retrospective study. J Neurosurg Spine 2012; 16:2–7. [DOI] [PubMed] [Google Scholar]
- 45.Gao R, Yang L, Chen H, et al. Long term results of anterior corpectomy and fusion for cervical spondylotic myelopathy. PLoS ONE 2012; 7:e34811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komura S, Miyamoto K, Hosoe H, et al. Lower incidence of adjacent segment degeneration after anterior cervical fusion found with those fusing C5-6 and C6-7 than those leaving C5-6 or C6-7 as an adjacent level. J Spinal Disord Tech 2012; 25:23–29. [DOI] [PubMed] [Google Scholar]
- 47.Nakashima H, Yukawa Y, Imagama S, et al. Complications of cervical pedicle screw fixation for nontraumatic lesions: a multicenter study of 84 patients. J Neurosurg Spine 2012; 16:238–247. [DOI] [PubMed] [Google Scholar]
- 48.Nunley PD, Jawahar A, Kerr EJR, et al. Factors affecting the incidence of symptomatic adjacent-level disease in cervical spine after total disc arthroplasty: 2- to 4-year follow-up of 3 prospective randomized trials. Spine (Phila Pa 1976) 2012; 37:445–451. [DOI] [PubMed] [Google Scholar]
- 49.Rollinghoff M, Zarghooni K, Hackenberg L, et al. Quality of life and radiological outcome after cervical cage fusion and cervical disc arthroplasty. Acta Orthop Belg 2012; 78:369–375. [PubMed] [Google Scholar]
- 50.Singh K, Phillips FM, Park DK, et al. Factors affecting reoperations after anterior cervical discectomy and fusion within and outside of a Federal Drug Administration investigational device exemption cervical disc replacement trial. Spine J 2012; 12:372–378. [DOI] [PubMed] [Google Scholar]
- 51.Upadhyaya CD, Wu JC, Trost G, et al. Analysis of the three United States Food and Drug Administration investigational device exemption cervical arthroplasty trials. J Neurosurg Spine 2012; 16:216–228. [DOI] [PubMed] [Google Scholar]
- 52.Blumenthal SL, Ohnmeiss DD, Guyer RD, et al. Reoperations in cervical total disc replacement compared with anterior cervical fusion: results compiled from multiple prospective food and drug administration investigational device exemption trials conducted at a single site. Spine (Phila Pa 1976) 2013; 38:1177–1182. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, He Z, Yang H, et al. Anterior cervical diskectomy and fusion for adjacent segment disease. Orthopedics 2013; 36:e501–e508. [DOI] [PubMed] [Google Scholar]
- 54.Coric D, Kim PK, Clemente JD, et al. Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: results in 74 patients from a single site. J Neurosurg Spine 2013; 18:36–42. [DOI] [PubMed] [Google Scholar]
- 55.Delamarter RB, Zigler J. Five-year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine (Phila Pa 1976) 2013; 38:711–717. [DOI] [PubMed] [Google Scholar]
- 56.Eubanks JD, Belding J, Schnaser E, et al. Congenital stenosis and adjacent segment disease in the cervical spine. Orthopedics 2013; 36:e1251–e1255. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Zheng Q, Guo X, et al. Anterior surgical options for the treatment of cervical spondylotic myelopathy in a long-term follow-up study. Arch Orthop Trauma Surg 2013; 133:745–751. [DOI] [PubMed] [Google Scholar]
- 58.Noriega DC, Kreuger A, Brotat M, et al. Long-term outcome of the Cloward procedure for single-level cervical degenerative spondylosis. Clinical and radiological assessment after a 22-year mean follow-up. Acta Neurochir (Wien) 2013; 155:2339–2344. [DOI] [PubMed] [Google Scholar]
- 59.Park JY, Kim KH, Kuh SU, et al. What are the associative factors of adjacent segment degeneration after anterior cervical spine surgery? Comparative study between anterior cervical fusion and arthroplasty with 5-year follow-up MRI and CT. Eur Spine J 2013; 22:1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park SB, Chung CK, Lee SH, et al. The impact of menopause on bone fusion after the single-level anterior cervical discectomy and fusion. J Korean Neurosurg Soc 2013; 54:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park YK, Moon HJ, Kwon TH, et al. Long-term outcomes following anterior foraminotomy for one- or two-level cervical radiculopathy. Eur Spine J 2013; 22:1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pereira EA, Chari A, Hempenstall J, et al. Anterior cervical discectomy plus intervertebral polyetheretherketone cage fusion over three and four levels without plating is safe and effective long-term. J Clin Neurosci 2013; 20:1250–1255. [DOI] [PubMed] [Google Scholar]
- 63.Saarinen T, Niemela M, Kivisaari R, et al. Early and late re-operations after anterior cervical decompression and fusion during an 11-year follow-up. Acta Neurochir (Wien) 2013; 155:285–291.discussion 291. [DOI] [PubMed] [Google Scholar]
- 64.Burkus JK, Traynelis VC, Haid RW, Jr, et al. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article. J Neurosurg Spine 2014; 21:516–528. [DOI] [PubMed] [Google Scholar]
- 65.Bydon M, Mathios D, Macki M, et al. Long-term patient outcomes after posterior cervical foraminotomy: an analysis of 151 cases. J Neurosurg Spine 2014; 21:727–731. [DOI] [PubMed] [Google Scholar]
- 66.Chung JY, Kim SK, Jung ST, et al. Clinical adjacent-segment pathology after anterior cervical discectomy and fusion: results after a minimum of 10-year follow-up. Spine J 2014; 14:2290–2298. [DOI] [PubMed] [Google Scholar]
- 67.Kan L, Kang J, Gao R, et al. Clinical and radiological results of two hybrid reconstructive techniques in noncontiguous 3-level cervical spondylosis. J Neurosurg Spine 2014; 21:944–950. [DOI] [PubMed] [Google Scholar]
- 68.Lee JC, Lee SH, Peters C, et al. Risk-factor analysis of adjacent-segment pathology requiring surgery following anterior, posterior, fusion, and nonfusion cervical spine operations: survivorship analysis of 1358 patients. J Bone Joint Surg Am 2014; 96:1761–1767. [DOI] [PubMed] [Google Scholar]
- 69.Li Z, Yu S, Zhao Y, et al. Clinical and radiologic comparison of dynamic cervical implant arthroplasty versus anterior cervical discectomy and fusion for the treatment of cervical degenerative disc disease. J Clin Neurosci 2014; 21:942–948. [DOI] [PubMed] [Google Scholar]
- 70.Litrico S, Lonjon N, Riouallon G, et al. Adjacent segment disease after anterior cervical interbody fusion: a multicenter retrospective study of 288 patients with long-term follow-up. Orthop Traumatol Surg Res 2014; 100 (suppl):S305–S309. [DOI] [PubMed] [Google Scholar]
- 71.Malham GM, Parker RM, Ellis NJ, et al. Cervical artificial disc replacement with ProDisc-C: clinical and radiographic outcomes with long-term follow-up. J Clin Neurosci 2014; 21:949–953. [DOI] [PubMed] [Google Scholar]
- 72.Qizhi S, Lei S, Peijia L, et al. A comparison of zero-profile devices and artificial cervical discs in patients with two noncontiguous levels of cervical spondylosis. Clin Spine Surg 2016; 29:E61–E66. [DOI] [PubMed] [Google Scholar]
- 73.Song JS, Choi BW, Song KJ. Risk factors for the development of adjacent segment disease following anterior cervical arthrodesis for degenerative cervical disease: comparison between fusion methods. J Clin Neurosci 2014; 21:794–798. [DOI] [PubMed] [Google Scholar]
- 74.Song KJ, Choi BW, Kim JK. Adjacent segment pathology following anterior decompression and fusion using cage and plate for the treatment of degenerative cervical spinal diseases. Asian Spine J 2014; 8:720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uehara M, Takahashi J, Mukaiyama K, et al. Mid-term results of computer-assisted cervical pedicle screw fixation. Asian Spine J 2014; 8:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Eck CF, Regan C, Donaldson WF, et al. The revision rate and occurrence of adjacent segment disease after anterior cervical discectomy and fusion: a study of 672 consecutive patients. Spine (Phila Pa 1976) 2014; 39:2143–2147. [DOI] [PubMed] [Google Scholar]
- 77.Xu R, Bydon M, Macki M, et al. Adjacent segment disease after anterior cervical discectomy and fusion: clinical outcomes after first repeat surgery versus second repeat surgery. Spine (Phila Pa 1976) 2014; 39:120–126. [DOI] [PubMed] [Google Scholar]
- 78.Hisey MS, Bae HW, Davis RJ, et al. Prospective, randomized comparison of cervical total disk replacement versus anterior cervical fusion: results at 48 months follow-up. J Spinal Disord Tech 2015; 28:E237–E243. [DOI] [PubMed] [Google Scholar]
- 79.Hu X, Ohnmeiss DD, Zigler JE, et al. Restoration of cervical alignment is associated with improved clinical outcome after one and two level anterior cervical discectomy and fusion. Int J Spine Surg 2015; 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janssen ME, Zigler JE, Spivak JM, et al. ProDisc-C total disc replacement versus anterior cervical discectomy and fusion for single-level symptomatic cervical disc disease: seven-year follow-up of the prospective randomized U.S. Food and Drug Administration investigational device exemption study. J Bone Joint Surg Am 2015; 97:1738–1747. [DOI] [PubMed] [Google Scholar]
- 81.Lau D, Chou D, Mummaneni PV. Two-level corpectomy versus three-level discectomy for cervical spondylotic myelopathy: a comparison of perioperative, radiographic, and clinical outcomes. J Neurosurg Spine 2015; 23:280–289. [DOI] [PubMed] [Google Scholar]
- 82.Lee JC, Lee SH, Peters C, et al. Adjacent segment pathology requiring reoperation after anterior cervical arthrodesis: the influence of smoking, sex, and number of operated levels. Spine (Phila Pa 1976) 2015; 40:E571–E577. [DOI] [PubMed] [Google Scholar]
- 83.Selvanathan SK, Beagrie C, Thomson S, et al. Anterior cervical discectomy and fusion versus posterior cervical foraminotomy in the treatment of brachialgia: the Leeds spinal unit experience (2008–2013). Acta Neurochir (Wien) 2015; 157:1595–1600. [DOI] [PubMed] [Google Scholar]
- 84.Shi JS, Lin B, Xue C, et al. Clinical and radiological outcomes following hybrid surgery in the treatment of multi-level cervical spondylosis: over a 2-year follow-up. J Orthop Surg Res 2015; 10:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang JT, Cao JM, Meng FT, et al. Cervical canal stenosis and adjacent segment degeneration after anterior cervical arthrodesis. Eur Spine J 2015; 24:1590–1596. [DOI] [PubMed] [Google Scholar]
- 86.Zhang JT, Wang LF, Liu YJ, et al. Relationship between developmental canal stenosis and surgical results of anterior decompression and fusion in patients with cervical spondylotic myelopathy. BMC Musculoskelet Disord 2015; 16:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lei T, Liu Y, Wang H, et al. Clinical and radiological analysis of Bryan cervical disc arthroplasty: eight-year follow-up results compared with anterior cervical discectomy and fusion. Int Orthop 2016; 40:1197–1203. [DOI] [PubMed] [Google Scholar]
- 88.Shiban E, Gapon K, Wostrack M, et al. Clinical and radiological outcome after anterior cervical discectomy and fusion with stand-alone empty polyetheretherketone (PEEK) cages. Acta Neurochir (Wien) 2016; 158:349–355. [DOI] [PubMed] [Google Scholar]
- 89.Chang UK, Kim DH, Lee MC, et al. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine 2007; 7:33–39. [DOI] [PubMed] [Google Scholar]
- 90.Dmitriev AE, Cunningham BW, Hu N, et al. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine (Phila Pa 1976) 2005; 30:1165–1172. [DOI] [PubMed] [Google Scholar]
- 91.McAfee PC, Cunningham BW, Hayes V, et al. Biomechanical analysis of rotational motions after disc arthroplasty: implications for patients with adult deformities. Spine (Phila Pa 1976) 2006; 31 (suppl):S152–S160. [DOI] [PubMed] [Google Scholar]
- 92.Nunley PD, Jawahar A, Cavanaugh DA, et al. Symptomatic adjacent segment disease after cervical total disc replacement: re-examining the clinical and radiological evidence with established criteria. Spine J 2013; 13:5–12. [DOI] [PubMed] [Google Scholar]
- 93.Matsunaga S, Kabayama S, Yamamoto T, et al. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine (Phila Pa 1976) 1999; 24:670–675. [DOI] [PubMed] [Google Scholar]
- 94.Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine (Phila Pa 1976) 1988; 13:774–780. [DOI] [PubMed] [Google Scholar]
- 95.Gore DR. Roentgenographic findings in the cervical spine in asymptomatic persons: a ten-year follow-up. Spine (Phila Pa 1976) 2001; 26:2463–2466. [DOI] [PubMed] [Google Scholar]


