Supplemental Digital Content is available in the text
Keywords: mortality, pay-for-performance, type 2 diabetes
Abstract
Background:
The impact of pay-for-performance (P4P) programs on long-term mortality for chronic illnesses, especially diabetes mellitus, has been rarely reported. Several studies described the favorable impact of P4P for diabetes mellitus on medical utilizations or intermediate outcomes. Therefore, this study aimed to investigate the impact of a P4P program on mortality in patients with type 2 diabetes.
Methods:
The P4P group in this population-based cohort study was 2090 individuals with a primary diagnosis of type 2 diabetes who had been newly enrolled in the P4P program of Taiwan between January 1, 2004 and December 31, 2004. Matched by 1:1 ratio, patients in the non-P4P group were selected by propensity score matching (PSM) for sex, age, the first year of diagnosis as diabetes, and 32 other potential confounding factors. Mean (SD) age was 60.91 (12.04) years when diabetes was first diagnosed and mean (SD) duration of diabetes was 4.3 (1.9) years at baseline. The time-dependent Cox regression model was used to explore the impact of P4P on all-cause mortality.
Results:
During a mean of 5.13 years (SD = 1.07 years) of follow-up, 206 and 263 subjects died in the P4P group and the non-P4P group, respectively. After adjusting for the potential confounding factors at baseline, survival was significantly longer in the P4P group than in the non-P4P group (hazard ratio, 0.76 [95% confidence interval, 0.64–0.92], P = 0.004, by log-rank test). This decrease in mortality is equivalent to one less death for every 37 patients who were treated in the P4P program for 5.13 years. In this study, the P4P program significantly increased the medical utilization of physician visits and diabetes-related examinations, improved the adherence of oral hypoglycemic drugs during the first 3 years and that of insulin during the second 3 years, and was negatively associated with risk of cancer and chronic kidney disease. In annual health expense, there was no significant difference between P4P and non-P4P groups, P = 0.430.
Conclusions:
As compared with control, pay-for-performance program significantly improved survival in patients with diabetes without increasing the medical cost. The P4P group had significantly lower risk of cancer and chronic kidney disease.
1. Introduction
Following the Saint Vincent Declaration,[1] pay-for-performance (P4P) programs for diabetes have been increasingly conducted worldwide.[2] In 1999, Anthem Blue Cross and Blue Shield in New Hampshire launched an incentive program, encouraging physicians to provide retinal examinations, lipid and glycated hemoglobin testing for patients with diabetes.[3] In 2004, the United Kingdom introduced a P4P contract for family practitioners, covering the clinical care for 10 chronic diseases, including diabetes.[4] In 2001, a P4P program for 5 diseases, one of which was diabetes, was implemented in Taiwan.[5] Some reports on the effects of P4P were not promising, one with short-term improvement in quality of care [6] and another without significant improvement in processes or outcomes.[7] In Taiwan, increasing annual diabetes-related tests, better medication adherence, and sustained improvement in continuity of care have been achieved with P4P program.[8] In the UK framework, there are 3 main sections: clinical care, practice organization, and patient experience,[9] whereas in the Taiwan framework, the structured care to be implemented in each visit every quarter covers 5 domains: medical history, physical examination, laboratory evaluation, management plan, and diabetes self-management plan (Table 1-1 in Supplementary Appendix).
The impact of P4P programs on long-term mortality for chronic illnesses, especially diabetes mellitus, has been rarely reported. Several studies described the favorable impact of P4P on medical utilizations[8,10,11] or intermediate outcomes,[12] whereas evidence of the long-term impact of P4P programs on mortality was lacking. Therefore, this study aimed to perform a population-based cohort study to investigate the impact of P4P on all-cause mortality in diabetes patients of Taiwan.
2. Material and methods
2.1. Ethics statement
The institutional review board (IRB) of the Kaohsiung Medical University Hospital approved this Taiwan study. Written consent from the study patients was not required because the National Health Insurance (NHI) dataset consists of de-identified secondary data used for research purposes, and the IRB gave a formal written waiver of the need for consent.
2.2. Data source
This retrospective cohort study analyzed data from the Taiwan National Health Insurance Research Database (NHIRD) provided by National Health Research Institute (NHRI), including data for physician visits, emergency department visits, and hospital admissions. By December 2010, more than 23 million people had been covered nationwide, with a coverage rate of 99.6% of residents, which has improved general accessibility to health care.[13] For research purposes, the NHRI adopted a systematic random sampling method to construct a representative database containing data for 1,000,000 randomly selected enrollees in the NHI registry. The resulting database was designated the Longitudinal Health Insurance Database (LHID). The selected sample and the total population did not statistically differ in age, sex, or health care costs.[14] The NHIRD was derived from the reimbursement claims of the NHI program. The database provided details of laboratory and prescription records, including the laboratory tests done, the medications used, dosages, days of supply dispensed, and diagnostic codes based on the International Classifications of Diseases, Ninth Revision, Clinical Modifications (ICD-9-CM). This study used the LHID.
2.3. The P4P program
Since 2001, the Bureau of the National Health Insurance (NHI) has implemented a P4P program for diabetes care. It is patient-centered multidisciplinary team care that engages physicians, registered nurses, nutritionists, pharmacists, and others, who are certified diabetes educators (CDE) by Taiwanese Association of Diabetes Educators (TADE).[15] Four levels of health care facility exist in Taiwan, comprising medical center, regional hospital, district hospital, and community clinic. There is no primary care gatekeeping and referral system[16] in Taiwan, and patients are free to seek health care based on her or his discretion.[17] Health care facility with CDE physicians can voluntarily apply to participate in the NHI P4P program. These certified physicians then can enroll patients individually into the program (Fig. 1).[18] An enrollee of P4P program is advised to visit the physician once every 3 months. In each visit, implemented structured care is clearly defined in initial enrollment visit, continuing care visits, and annual evaluation visit, respectively (Tables 1-1, 1-2 and 1-3 in the Supplementary Appendix). In addition to usual reimbursement for health care services such as physician visits, laboratory evaluations, and medications, the P4P program offers engaged physicians additional “incentive physician fee” and engaged diabetes educators “fee for nursing and nutrition education” in the 3 sequential types of visit. Both fees are included in the New Taiwan Dollar (NTD) 1845, (NTD 32.1 = USD 1.0 in 2009) for initial enrollment visit (Supplementary Appendix: Table 1-1: package P1401C), NTD 875 for continuing care visit (Table 1-2: package P1402C), and NTD 2245 for annual evaluation visit (Table 1-3: package P1403C). To claim the fee of each package, data of the “must-do” laboratory tests and examinations must be electronically uploaded to Bureau of Health Promotion. These “must-dos” include blood sugar, glycated hemoglobin (HbA1C), low-density lipoprotein (LDL), triglyceride, serum creatinine, urine albumin/creatinine ratio, systolic and diastolic blood pressure, eye fundus examination, and foot examination for initial enrollment visit and annual evaluation visit, and include blood sugar, HbA1C, systolic and diastolic blood pressure for continuing care visit. Required and recommended services included in initial enrollment, continuing care, and annual evaluation (e.g., medical history, physical examination, laboratory evaluation, management plan, and diabetes self-management plan) are clearly defined in each package for visit in the P4P program. Between visits, individual phone or cellphone calls or online group communication to care for enrollees to reinforce self-monitoring of blood sugar, intensify physical activity, and boost medication adherence are made for patients whose HbA1C levels are greater than 8.5%. Group education activities at health care facilities once every 2 to 4 weeks are done to promote insulin therapy for patients whose HbA1C levels are greater than 8% despite treatment with 2 or more oral hypoglycemic drugs. In non-P4P group, patients are treated at the discretion of a physician, who may or may not be a CDE. P4P program in Taiwan is to implement the structured cares set up in the initial enrollment visit, continuing care visit, and annual evaluation visit to treat patients to the targets. Because this P4P program presents a chronic care model with patient-centered multidisciplinary team care and structured care implementation (PMTCSCI), the effect of P4P can be isolated separately from the attributes of the treating physicians. There was no coding for CDE physicians in NHIRD, and the proportion of non-P4P group who were cared by CDE physicians could not be ascertained. Additionally, only the physicians who have fulfilled the threshold of enrollment can be listed as a candidate for the “Annual Incentive by Performance.” Annual incentive by performance is calculated according to the following 4 indicators: the proportion of the enrollees who have completed each visit per quarter, 4 visits a year; the proportion of enrollees with HbA1C <7.0%; the proportion of enrollees with HbA1C >9.5%; the proportion of enrollees with low density lipoprotein cholesterol (LDL-C) >130 mg/dL at annual evaluation visit. The CDE physician who ranks in the top 25 percentile of all the candidates will be rewarded by annual incentive (please see Supplementary Appendix).
Figure 1.
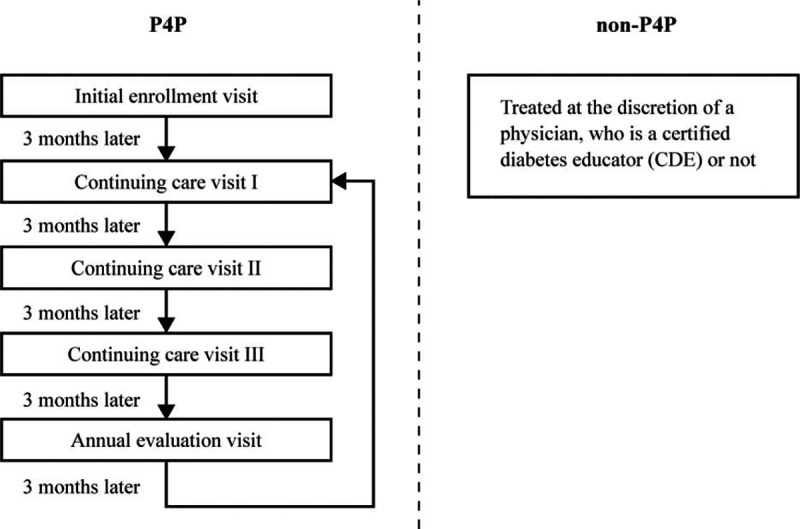
Flowchart of the pay-for-performance (P4P) program for diabetes: Once enrolled into P4P program at the initial enrollment visit, the P4P subject visits physician once each quarter, cycling through 3 continuing care visits and 1 annual evaluation visit. Each visit covers 5 domains: medical history, physical examination, laboratory evaluation, management plan, and diabetes self-management plan.
2.4. Patient involvement
P4P program, a landmark national health policy, involved diabetes patients, health care providers, and administrators in the policymaking. Medical utilization, medication adherence, and prevention of comorbidities were based on structured care implementation to match care to patient need, and the results of the preceding 3 dimensions were revealed to patients in the current or next visits. TADE-certified physicians play a key role in the recruitment to, and conduct of, the study. Their contributions were rewarded by incentives for each patient visit and their annual performance. This quasiexperimental, retrospective cohort study aimed to investigate the impact of P4P to decrease comorbidities and prolong life.
2.5. Study population
Figure 2 shows the collection of our study patients. Using LHID, we identified patients diagnosed with type 2 diabetes [ICD-9-CM, codes 250.xx (excluding 250.x1 or 250.x3)], who had any inpatient diabetes diagnoses or at least 3 outpatient diabetes diagnoses within 1 year. The validation of this definition of diabetes showed a 96.9% sensitivity and 93.9% positive predictive value in a study using a questionnaire assessment of patients with diabetes from NHIRD.[19] All our study patients were diagnosed with diabetes mellitus before December 31, 2003. The intervention group was 2090 individuals aged 18 years or older who had been newly enrolled in the P4P program in Taiwan between January 1, 2004 and December 31, 2004. The starting point of follow-up for intervention group was the date of enrollment into P4P. Each counterpart in comparison group (non-P4P group) was randomly selected by PSM, utilizing 35 covariates including Charlson Comorbidity Index (CCI), Diabetes Severity Comorbidity Index (DSCI), and others. P4P in Taiwan began in 2001, but successful PSM for our study population could not be achieved until 2004 claim data of NHIRD was analyzed.
Figure 2.
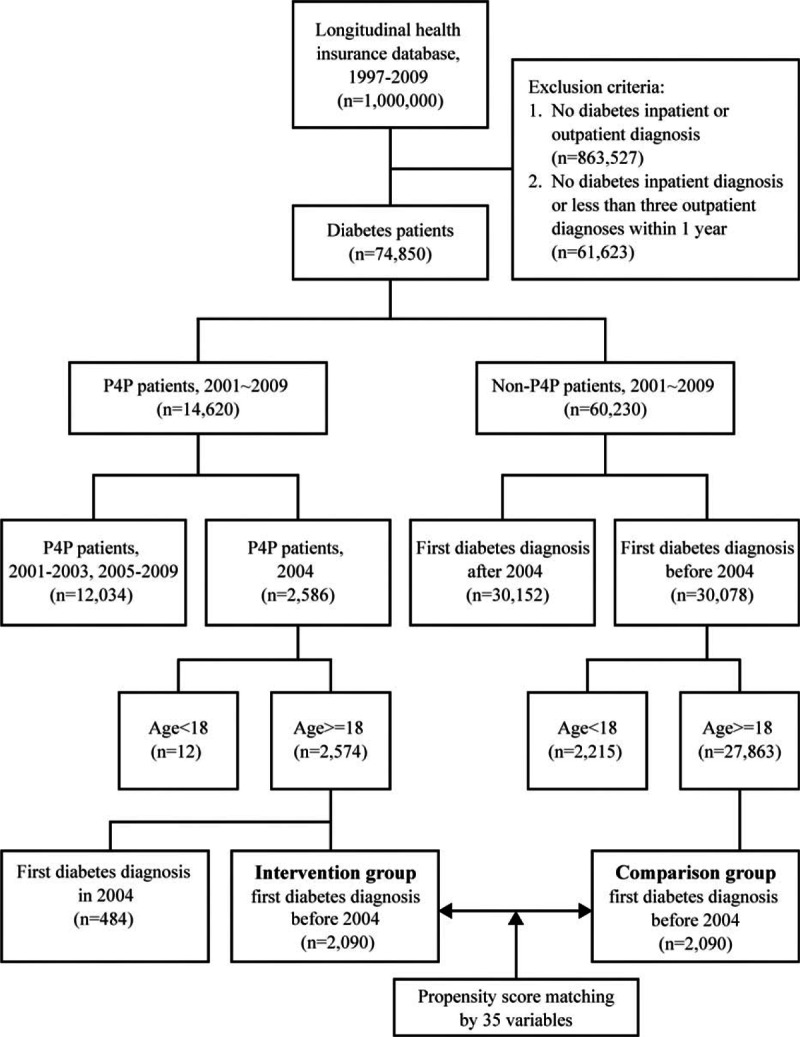
Subject collection flowchart. Using Longitudinal Health Insurance Database (LHID), this study identified diabetes patients diagnosed before December 31, 2003. The intervention group was 2090 individuals who had been newly enrolled into the P4P program in 2004. Utilizing 35 covariates, propensity score matching method was used to perform one-to-one matching between the P4P group and the non-P4P group.
2.6. Study variables for propensity score matching
Propensity score matching method was used to perform one-to-one matching between the P4P group and the non-P4P group based on sex, age, the first year of diagnosis as diabetes, CCI score, DSCI score, catastrophic disabling disease, residence, insurance premium, health care facility level, physician visits, diabetes-related examinations, oral hypoglycemic drugs, insulin, statin, non-statin lipid-lowering drugs, angiotensin-converting-enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), non-ACEI and non-ARB anti-hypertensive drugs, emergency department visits, diabetes-related hospitalization, readmission within 30 days, annual NHI health care expenses, severe hypoglycemia, cancer (ICD-9:140–208, 230–234), hypertension (ICD-9:401), dyslipidemia (ICD-9:272), stroke (ICD-9:430–438), coronary artery disease (ICD-9:410–414, 398.91, 402, 404, 036.1, 036.01, 036.02, 036.05, 036.06, 036.07, 000.66), heart failure (ICD-9:428.0), chronic kidney disease (ICD-9:585), renal disease (ICD-9:580–584, 586–590, 403, 250.40), dementia or Alzheimer (ICD-9:290, 331.0), depressive disorder (ICD-9:296.2x, 296.3x, 300.4, 311), and bipolar disorder(ICD-9:296.0, 296.4, 296.5, 296.7, 296.80, 296.89) at the baseline year. Severe hypoglycemia is an event requiring assistance of another person to actively administer carbohydrate or glucagon.[20] The general medical status before the index date was assessed using a modified version of the Charlson comorbidity index, which was the sum for 19 comorbid conditions.[21] For patient severity/complication, this study used the Diabetes Complications Severity Index (DCSI) developed by Young et al,[22] which includes 7 categories of complications according to the ICD-9-CM codes: cardiovascular complications, nephropathy, retinopathy, peripheral vascular disease, stroke, neuropathy, and metabolic disorders. Certificate of catastrophic disabling disease is issued when a patient is diagnosed with one of thirty categories of catastrophic diseases.[23] Four categories are listed as follows: malignant neoplasms requiring long-term therapy; chronic kidney disease, stage V or dialysis; rheumatologic disorders requiring life-long therapy; mental disorders including dementia, schizophrenia, affective disorders, and others.
Residence was categorized as rural or urban. The insurance premium was a proxy indicator of economic status and was classified into one of 3 categories: fixed premium and dependent, less than NTD 40,000 monthly, and NTD 40,000 or more monthly (NTD 32.1 = USD 1.0 in 2009). The fixed premium group included those receiving social welfare supports such as low-income individuals and veterans. The dependent insurance premium group comprised spouse and dependents who did not have a job or income. Health care facility level was classified according to the Taiwan Joint Commission on Hospital Accreditation criteria as medical center (>500 beds), regional hospital (301–500 beds), district hospital (101–300 beds), and community clinic.
To investigate medication adherence, we used the defined daily dose (DDD). DDD recommended by the World Health Organization is a unit for assessing the standard dose of drug; that is, the dose for a 70-kg adult in a day was called 1 DDD. For example, 1 DDD was 2 g for metformin (biguanide) and 10 mg for enalapril (ACE inhibitor). Cumulative DDD (cDDD), which indicates the duration of drug use, is estimated as the sum of the dispensed DDD of the drug and compared with the mortality risk. Medication use was classified into 1 of 3 categories: 0 cDDD, 1 cDDD to Q3, and ≥Q3. The cutoff point for Q3 was the third quartile of cDDD in our study group. The cDDD were defined similarly to that in another study.[24]
2.7. Statistical analysis
The unit of analysis in this study was the individual patient diagnosed with type 2 diabetes. The main end point in this analysis was the date of death as indicated on the death certificate. Because the diabetes patients were not randomly assigned to the intervention group or comparison group, PSM was used to minimize selection bias and to assign patients with diabetes to the comparison group. The caliper matching method (also known as the greedy algorithm) was used for 1-to-1 matching between the intervention and comparison groups. The baseline characteristics were compared between the P4P and non-P4P groups by χ2 test and t test to make sure the matched controls had similar 35 characteristics as their P4P counterparts at baseline. The Kaplan–Meier method was used to calculate overall survival curves, and the group comparisons of survival rate were delineated by log-rank test. There were 2 main procedures in our analysis. First, the time-dependent Cox regression model was used for multivariate assessment of P4P on mortality by controlling the 35 potential confounding covariates. This model can take dynamic changing of covariates into account and is widely used in pharmacoepidemiology. Volume of medical utilization, annual average dose of medication use, and comorbidity incidence served as time-dependent covariates. Hazard ratios (HRs) and 95% confidence interval (CI) for survival were reported. Secondly, each covariate of medical utilization and medication use was analyzed, in terms of the difference between the averages of P4P and non-P4P groups, through generalized estimating equation by annual longitudinal design. Each comorbidity was also analyzed, in terms of the difference between the risks of P4P and non-P4P groups, through competing risk adjusted Cox regression by controlling mortality. Statistical analyses and data management were performed using SAS 9.4 software (SAS Institute Inc, Cary, NC). All tests were 2-sided, and P values <0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
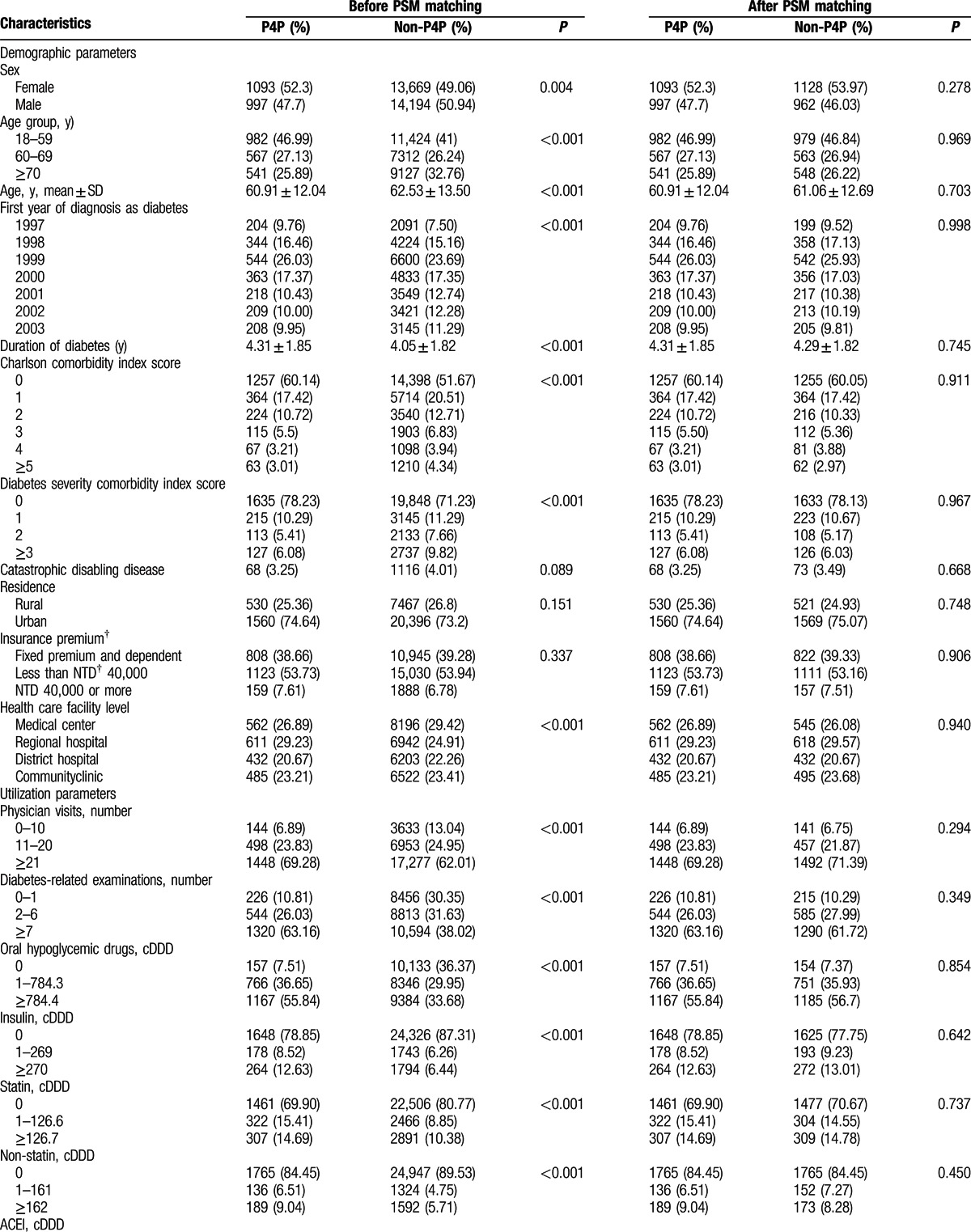
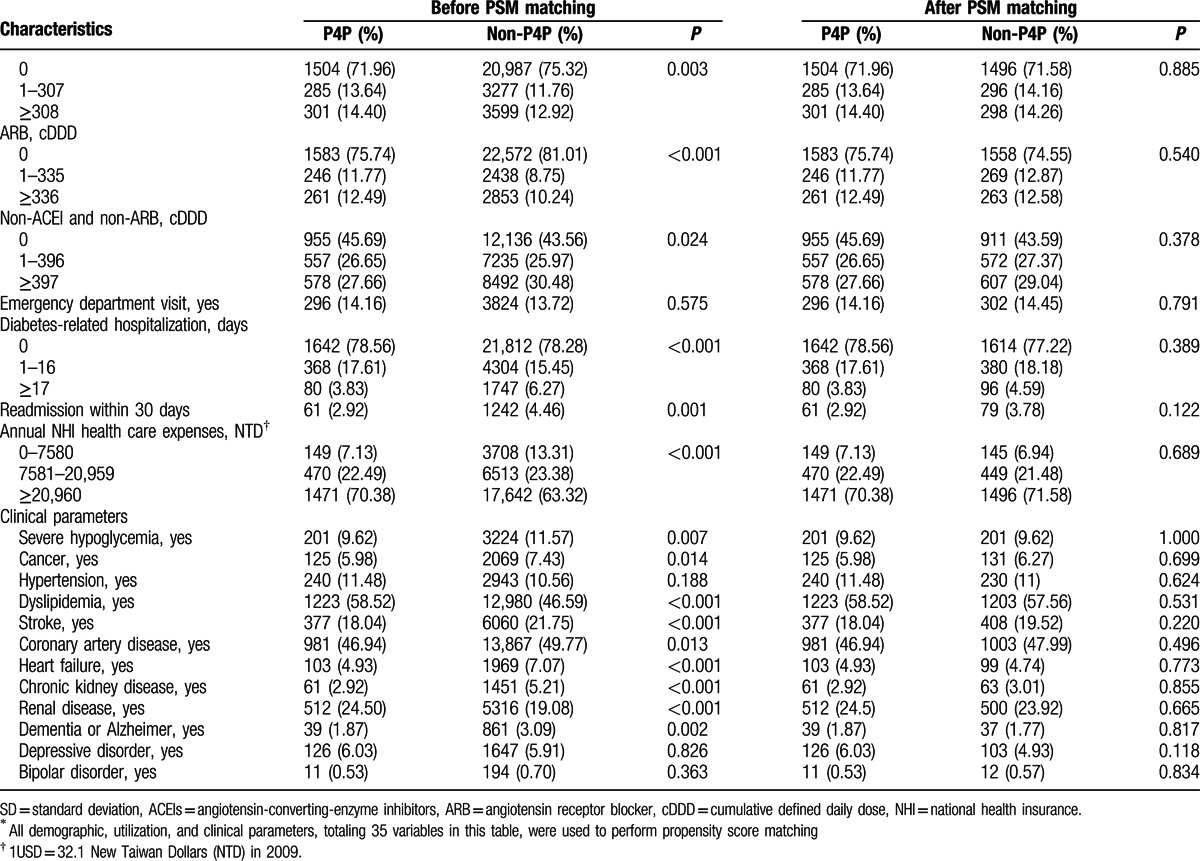
This study analyzed data obtained from 2090 P4P patients and 2090 controls matched by sex, age, the first year of diagnosis as diabetes, and 32 other potential confounding factors. Mean (SD) age was 60.91 (12.04) and 61.06 (12.697) years when diabetes was first diagnosed and mean (SD) duration of diabetes at baseline was 4.3 (1.9) and 4.3 (1.8) years for P4P and non-P4P group, respectively. Approximately 52% of the subjects were female. Table 1 shows that, in the pre-matched sample, the P4P and non-P4P groups significantly (P < 0.05) differed in most of the assessed characteristics. After PSM, all 35 study covariates were similar at baseline between the 2 groups. Figure 2 shows the data collection flow chart.
Table 1.
Demographic, utilization and clinical parameters of study patients by pay-for-performance (P4P) and propensity score matching (PSM)∗.
3.2. Mortality rate after P4P
During an average of 5.13 years of follow-up, 469 of the subjects died (206 P4P and 263 non-P4P subjects). The crude all-cause mortality throughout the follow-up period was 1.90 (per 100 person-years, 95% CI: 1.65–2.18) for the P4P group. In the non-P4P group, it was 2.47 (per 100 person-years, 95% CI: 2.18–2.79). Kaplan–Meier analysis of survival showed that the cumulative survival rate of the P4P group was significantly higher than that of non-P4P group (HR, 0.76 [95% CI, 0.64–0.92], P = 0.004, by log-rank test, Fig. 3).
Figure 3.
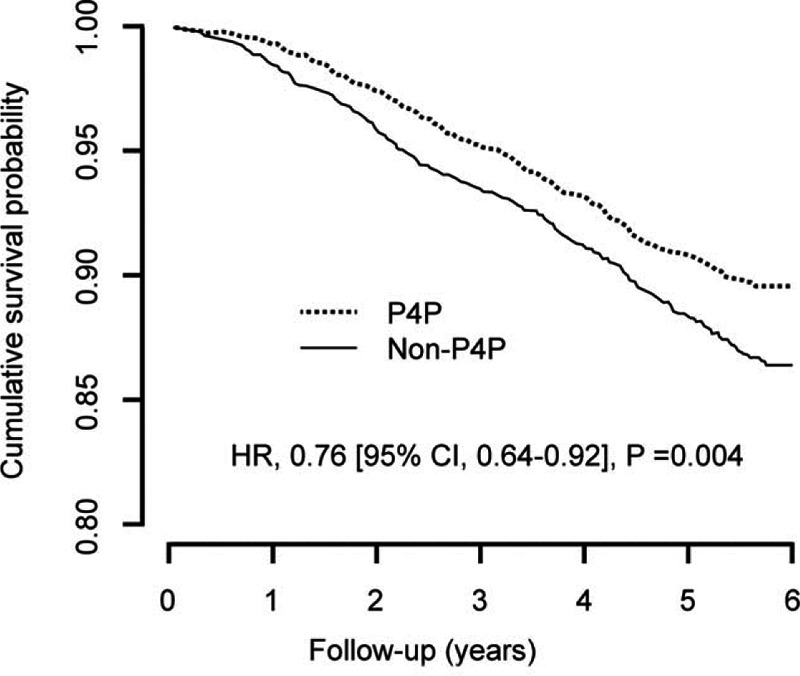
Kaplan–Meier survival curve by pay-for-performance (P4P) in Taiwan. Kaplan–Meier analysis of survival showed that during a mean of 5.13 years of follow-up, the cumulative survival rate of the P4P group was significantly higher than that of non-P4P group (hazard ratio, 0.76 [95% confidence interval, 0.64–0.92], P = 0.004, by log-rank test).
3.3. Impact of P4P on mortality
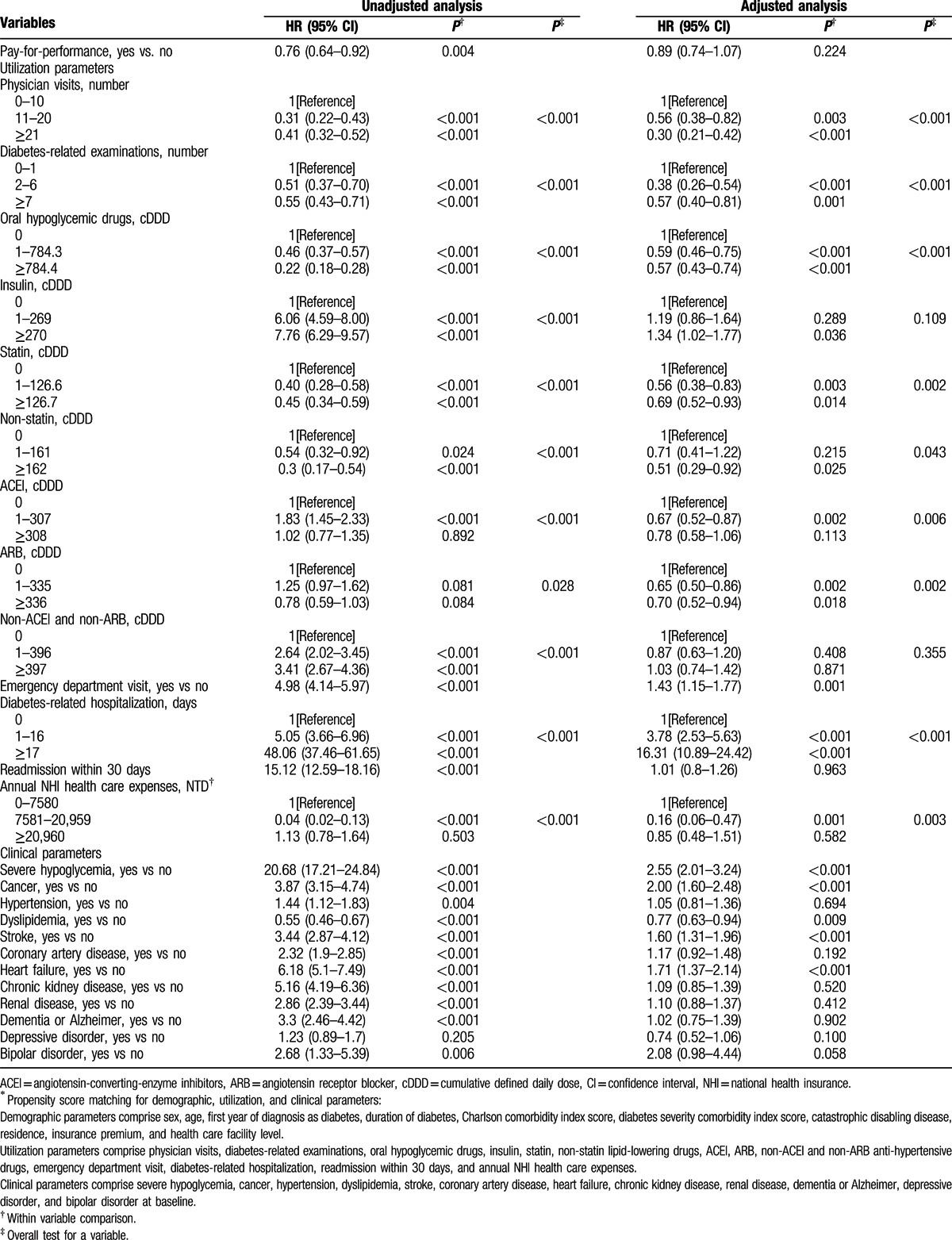
Table 2 shows that during an average 5.13 years follow-up, the mortality rate was significantly lower in the P4P group than in the non-P4P group (HR, 0.76 [95% CI, 0.64–0.92], P = 0.004) in unadjusted univariate analysis. After being adjusted for total 35 covariates in multivariate analysis, no difference in mortality existed between P4P and non-P4P groups (HR, 0.89 [95% CI, 0.74–1.07], P = 0.224), which suggests that some of these 35 covariates account for the impact of P4P on mortality. Multivariate time-dependent Cox regression analysis revealed that mortality was negatively associated with physician visits, diabetes-related examinations, oral hypoglycemic drugs, statin/non-statin lipid-lowering drugs, ACEIs, ARBs, annual NHI health care expenses, and dyslipidemia during the follow-up period. In contrast, emergency department visits, diabetes-related hospitalization, severe hypoglycemia, cancer, stroke, and heart failure were positively associated with mortality in the study patients.
Table 1 (Continued).
Demographic, utilization and clinical parameters of study patients by pay-for-performance (P4P) and propensity score matching (PSM)∗.
3.4. Medical utilization and medication use by P4P patients
Table 3 compares medical utilization and medication use between P4P and non-P4P patients during study period. The mean values for physician visits, diabetes-related examinations, oral hypoglycemic drug use, insulin use, and statin use were significantly higher in the P4P group than in the non-P4P group. P4P group had significantly fewer diabetes-related hospitalizations than non-P4P group. The 2 groups had similar mean values for non-statin lipid-lowering drug use, ACEI use, ARB use, Non-ACEI-non-ARB anti-hypertensive drug use, emergency department visit, readmission within 30 days, annual NHI health care expenses, and severe hypoglycemia.
Table 2.
Time-dependent Cox regression models for mortality based on propensity score matching sample (n = 4180)∗.
3.5. Incidence of comorbidity between P4P and non-P4P groups
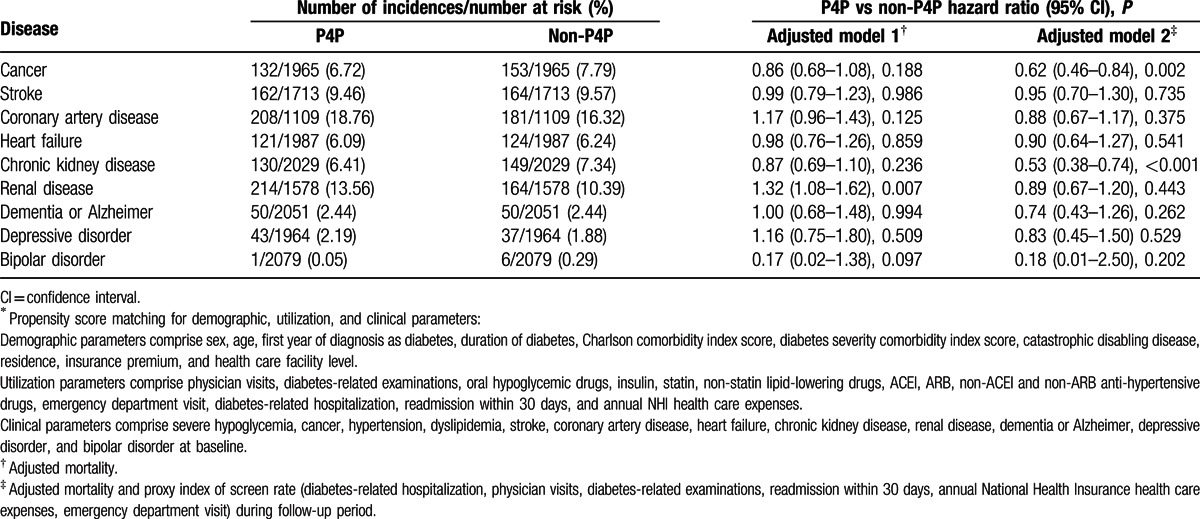
Table 4 compares incidence of comorbidities between P4P and non-P4P patients during study period. The study sample size varied with each comorbidity because in either P4P group or non-P4P group, selected at baseline, were those patients who had no comorbidities to be analyzed, for which they were followed up thereafter. Medical utilization served as a proxy index of screen rate, including physician visits, diabetes-related examinations, emergency department visit, diabetes-related hospitalization, readmission within 30 days, and annual National Health Insurance health care expenses during follow-up period. Without controlling screen rate in the follow-up period, the incidences of all 9 comorbidities were similar between P4P and non-P4P groups (adjusted model 1). Compared with non-P4P group, P4P group had significantly lower incidence of cancer and chronic kidney disease after controlling screen rate (adjusted model 2).
Table 3.
Medical utilization and medication dosage by pay-for-performance (P4P).
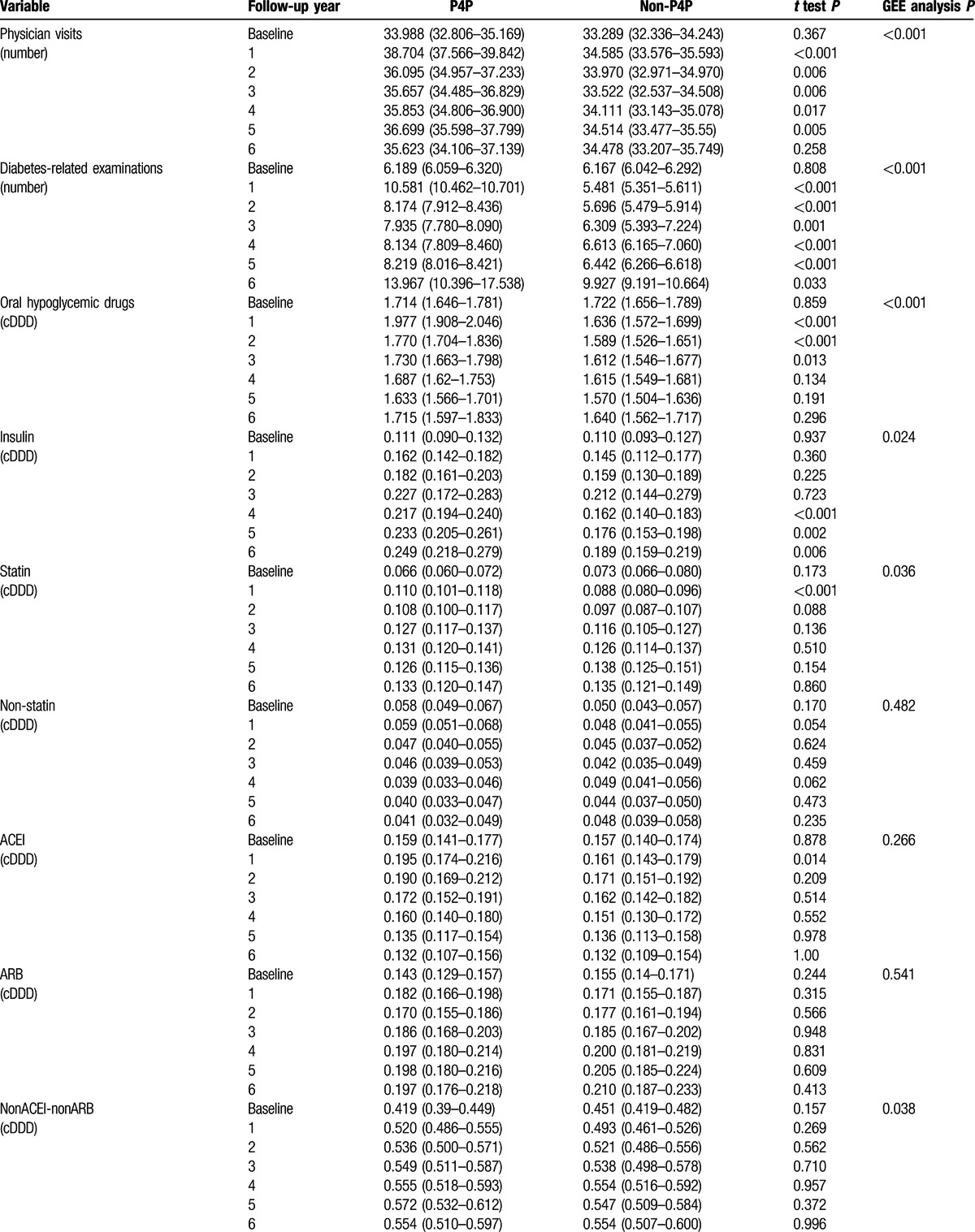
Table 4.
Comorbidities by pay-for-performance (P4P), competing risk adjusted Cox regression∗.
3.6. Dimensions explaining the impact of P4P on mortality
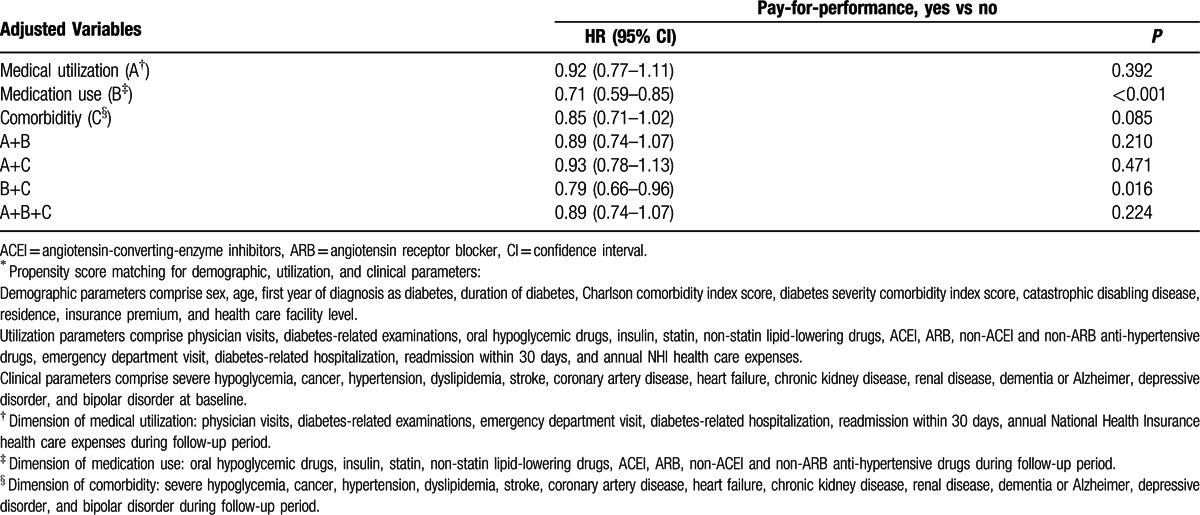
In Table 5, extracted from the 35 covariates, 25 covariates were segregated into 3 dimensions, comprising 6 in medical utilization, 7 in medication use, and 12 in comorbidity. When time-dependent Cox regression models were applied at the level of each dimension or any of their combinations, the results revealed that the impact of P4P on mortality can be explained by analyzing medical utilization, comorbidity, their combination, and combination of medical utilization and medication use.
Table 3 (Continued).
Medical utilization and medication dosage by pay-for-performance (P4P).
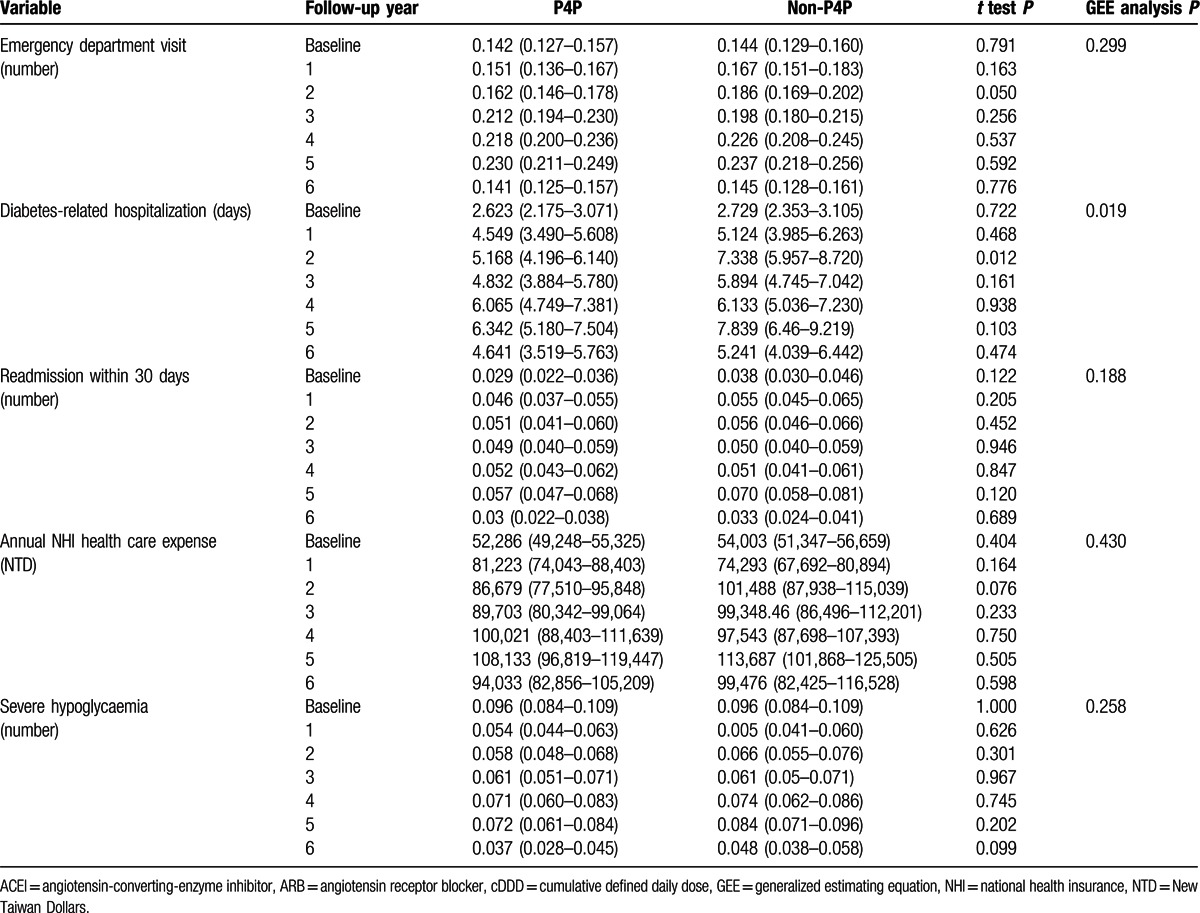
Table 5.
Time-dependent Cox regression models for mortality by controlling 3 dimensions of confounding factors during follow-up period based on propensity score matching sample (n = 4180)∗.
4. Discussion
This study is the first nationwide population-based cohort study to provide evidence that the P4P program improves mortality in patients with diabetes. Using PSM to harness sex, age, the first year of diagnosis as diabetes, and 32 other potential confounding variables, we endeavored to eliminate baseline differences between the P4P and non-P4P groups. The results revealed that P4P program reduced mortality in diabetes patients during an average of 5.13 years of intervention without increasing the medical cost. This decrease in mortality is equivalent to one less death for every 37 patients who were treated in the P4P program for 5.13 years. The impact of P4P on mortality can be explained by the dimension of medical utilization, and the dimension of comorbidity, alone.
4.1. Impact of medical utilization and medication use on mortality
In this study, through PMTCSCI of 3 sequential types of visit, the P4P program significantly increased physician visits and their associated diabetes-related examinations,[8] including measurement of HbA1C (A), blood pressure (B), and low density lipoprotein cholesterol (C). To reach target levels of HbA1C the adherence of oral hypoglycemic drugs was significantly better in P4P group than in non-P4P group during the first 3 years. In Taiwan, diabetes patients are reluctant to accept insulin therapy; and insulin is not utilized until multiple oral hypoglycemic drugs have failed.[25] The adherence of insulin was significantly better in P4P group than in non-P4P group during the second 3 years, which suggested that through PMTCSCI, subjects in P4P group were more likely to accept insulin to achieve HbA1C target than those in non-P4P group.
In Heart Protection Study, simvastatin reduced the rate of first major vascular events by a quarter,[26] and atorvastatin reduced death rate in diabetes subjects in CARDS trial.[27] Our study also revealed that statin use was negatively associated with mortality and P4P group utilized higher dose of statin than non-P4P group. In a retrospective analysis of diabetes subjects by Ho et al,[28] medication nonadherence was significantly associated with increased risks for all-cause hospitalization and for all-cause mortality.
In Taiwan, from 2006 to 2011, the percentages of P4P subjects (n = 720) who had HbA1C lower than 7% (A), both systolic and diastolic blood pressure lower than 130/80 mm Hg (B), and total cholesterol lower than 160 mg/dL or LDL cholesterol lower than 100 mg/dL (C) improved by 6.5% (from 32.4% to 34.5%), 22.0% (from 30.9% to 37.7%), and 57.8% (from 35.3% to 55.7%), respectively, with a resulting total ABC attainment rate from 4.1% to 8.6%.[29,30]
This might be the favorable result of long-term PMTCSCI, which was also echoed in a population study on changes in diabetes self-care behaviors in Taiwan between 2001 and 2005.[31] People (n = 1069) with diabetes in 2005 were significantly more likely to take medication (oral tablets or insulin injections) regularly, control their weight, reduce smoking and drinking, exercise, control their diet, and maintain a regular lifestyle (i.e., getting adequate sleep, not staying up too late, and avoiding stress) than people (n = 797) with diabetes in 2001.[31] One weakness of our study is that no data of ABC from non-P4P group was available for comparison. From the Elderly Nutrition and Health Survey from 1999 to 2000,“dietary diversity scores ≤4 and poor appetite” was associated with lower food and nutrient intakes and an independent risk for mortality in older Taiwanese.[32] Balanced food diversity is the essence of nutrition education highlighted in each visit of P4P program, but its impact on mortality could not be addressed and measured from claim data of NHIRD.
Compared with subjects in non-P4P group in this study, those in P4P group had significantly fewer diabetes-related hospitalizations.[10] Therefore, during the follow-up period, no significant difference in annual NHI health care expense existed between 2 study groups. In terms of annual NHI care expense, mean cost was NTD 81,223 for P4P group and NTD 74,293 for non-P4P group in 2004, and increased to NTD 94,033 for P4P group and NTD 99,476 for non P4P group in 2009. There was no significant difference between P4P and non-P4P groups, P = 0.430, as shown in Table 3 .
4.2. Impact of comorbidities on mortality
The UKPDS looked at patients newly diagnosed with type-2 diabetes and found that for each 1% reduction in HbA1C, there was a relative risk reduction of 21% for diabetes-related deaths.[33] In contrast, the ACCORD study revealed that among patients with type 2 diabetes averaging duration of 10 years and at high risk for cardiovascular disease, glycemic control was significantly better in the intensive-treatment group (HbA1C, 6.4% vs 7.5%). However, during a mean of 3.5 years of follow-up, the intensive-treatment group had significantly higher mortality than the standard-treatment group did,[34] which suggested HbA1C alone might not be a good predictor of mortality.
Using data from the National Health and Human Nutrition Examination Surveys (NHANES) III (1988–1994), body mass index (BMI) category alone could not predict mortality, whereas staging by the presence of risk factors, chronic disease, end-organ damage and end-stage disabilities, the Edmonton obesity staging system independently predicted increased mortality.[35] This ability was independent of BMI.
Therefore, our study investigated the new onset comorbidities during the follow-up period and analyzed their impact on mortality. Subjects of P4P group had lower risk of cancer and chronic kidney disease. Type 2 diabetes and cancer share many risk factors, including aging, diet, physical inactivity and obesity.[36] Diabetes may influence the neoplastic process by several mechanisms, including insulin resistance, hyperinsulinemia, hyperglycemia, or chronic inflammation,[37] which might be improved through medical utilization and medication use by PMTCSCI to a greater extent in the P4P group than in the non-P4P group. Life years saved by successful prevention of end-stage renal disease in elderly patients with diabetes are substantial.[38] In Taiwan, nationwide implementation of predialysis care program and improvement of predialysis chronic kidney disease patient care might be one of the factors for the continuous stabilization of dialysis incidence since 2007.[39] Significantly lower incidence of chronic kidney disease in the P4P might decrease the incidence of end-stage renal disease and contribute to the reduced mortality in the P4P group.
4.3. Impact of each dimension on mortality
The results revealed that the impact of P4P on mortality could be explained by the dimension of medical utilization, and the dimension of comorbidity, alone. Our study revealed statin use in 30.1%, and ACEI use in 28.0% of the P4P group in baseline year, whereas among US adults with diabetes in 2005–2006, there was 51.3% statin use and 60.8% ACEI medication use, respectively.[40] Underuse of statin and ACEIs in Taiwan might be one of the reasons that the dimension of medication use alone could not account for the impact of P4P on mortality in this study.
4.4. National Health Insurance of Taiwan
Taiwan implemented a single-payer National Health Insurance (NHI) system in 1995; in 2008, approximately 92% of the hospitals nationwide were contracted by NHI, and more than 99% of all Taiwanese residents, around 23 million, were covered under NHI.[10] Utilization of medical services increased, whereas cost remained at 5% to 6% of the gross domestic product. Total national health expenditures in Taiwan made up 6.63% of gross domestic product in 2012.[41] The per capita average annual number of visits to the physician's office was 14,[13] because of the absence of referral requirement and free choice of health care providers.[17] High accessibility and structured care implementation of sequential visits, one each quarter, might be 2 of the reasons leading favorably to reduced mortality in the P4P group compared with the non-P4P group.
4.5. Limitations
Certain limitations of this study require consideration. Firstly, there was no coding for CDE physicians in NHIRD, and the proportion of non-P4P group who were cared by CDE physicians could not be ascertained. Secondly, some variables (e.g., health literacy and education) could not be adjusted in the PSM approach used for the research participants. These uncontrolled characteristics might contribute to the preexisting differences between the P4P and non-P4P groups, such as compliance with physician visits and utilization of laboratory examination. Thirdly, the data of HbA1C, LDL-C, and blood pressure could not be compared between the P4P group and the non-P4P group because these data were unavailable in our study database. Fourthly, only the date of death was available; the cause of death could not be delineated in our data. Finally, BMI,[42] which is an important and potentially modifiable factor associated with mortality or developing mobility disability, was also unavailable in our data.
5. Conclusions
In conclusion, the P4P program significantly reduced mortality by 24% in the P4P group in comparison with the non-P4P group during an average of 5.13 years of intervention without increasing the medical cost. In addition, the P4P group had significantly lower risk of cancer and chronic kidney disease.
Acknowledgements
None.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CCI = Charlson comorbidity index, CI = confidence interval, DCSI = diabetes complications severity index, DDD = defined daily dose, DM = diabetes mellitus, DSCI = diabetes severity comorbidity index, HR = hazard ratio, IRB = Institutional Review Board, NHANES = national health and human nutrition examination surveys, NHI = National Health Insurance, P4P = pay-for-performance, PMTCSCI = patient-centered multidisciplinary team care and structured care implementation, PSM = propensity score matching, TADE = Taiwanese Association of Diabetes Educators.
HYS and CTCL have equally contributed to the article.
Authorship: conceptualization and design of study: YCC, HYS, CTCL; interpretation of data: YCC, BJL; draft of manuscript: YCC; acquisition of data: HYS, CTCL; conceptualization and design of study: HYS, CTCL; draft and revision of manuscript: HYS; enlightening teaching of diabetes care history: BJL; interpretation of data: BJL; revision and finalization of manuscript: BJL; critical revision of important intellectual content and approval of final version: YYC; analysis and interpretation of data: CTCL; draft of manuscript: CTCL.
Funding/support: This research was supported by Kaohsiung Medical University Research Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Diabetes care and research in Europe: the Saint Vincent declaration. Diabet Med 1990; 7:360. [PubMed] [Google Scholar]
- 2.Huang J, Yin S, Lin Y, et al. Impact of pay-for-performance on management of diabetes: a systematic review. J Evid Based Med 2013; 6:173–184. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AM, Lee TH, Hamel MB. Paying physicians for high-quality care. N Engl J Med 2004; 350:406–410. [DOI] [PubMed] [Google Scholar]
- 4.Doran T, Fullwood C, Gravelle H, et al. Pay-for-performance programs in family practices in the United Kingdom. N Engl J Med 2006; 355:375–384. [DOI] [PubMed] [Google Scholar]
- 5.Lee TT, Chen CC. A pay-for-performance program for diabetes care in Taiwan: a preliminary assessment. Am J Manag Care 2010; 16:65–69. [PubMed] [Google Scholar]
- 6.Campbell SM, Reeves D, Kontopantelis E, et al. Effects of pay for performance on the quality of primary care in England. N Engl J Med 2009; 361:368–378. [DOI] [PubMed] [Google Scholar]
- 7.Chien AT, Eastman D, Li Z, et al. Impact of a pay for performance program to improve diabetes care in the safety net. Prev Med 2012; 55 (suppl):S80–S85. [DOI] [PubMed] [Google Scholar]
- 8.Cheng JS, Tsai WC, Lin CL, et al. Trend and factors associated with healthcare use and costs in type 2 diabetes mellitus: a decade experience of a universal health insurance program. Med Care 2015; 53:116–124. [DOI] [PubMed] [Google Scholar]
- 9.Roland M. Linking physicians’ pay to the quality of care--a major experiment in the United Kingdom. N Engl J Med 2004; 351:1448–1454. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SH, Lee TT, Chen CC. A longitudinal examination of a pay-for-performance program for diabetes care: evidence from a natural experiment. Med Care 2012; 50:109–116. [DOI] [PubMed] [Google Scholar]
- 11.McWilliams JM, Landon BE, Chernew ME. Changes in health care spending and quality for Medicare beneficiaries associated with a commercial ACO contract. JAMA 2013; 310:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan AM, Doran T. The effect of improving processes of care on patient outcomes: evidence from the United Kingdom's quality and outcomes framework. Medi Care 2012; 50:191–199. [DOI] [PubMed] [Google Scholar]
- 13.Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med 2008; 148:258–267. [DOI] [PubMed] [Google Scholar]
- 14.Institutes NHR. Introduction to the National Health Insurance Research Database (NHIRD), Taiwan Secondary Introduction to the National Health Insurance Research Database (NHIRD), Taiwan; 2013. http://nhird.nhri.org.tw/en/index.html. [Google Scholar]
- 15.Educators TAoD. Diabetes health educator certification scheme. Secondary Diabetes health educator certification Scheme; 2011. https://www.tade.org.tw/en/. [Google Scholar]
- 16.Forrest CB. Primary care in the United States: primary care gatekeeping and referrals: effective filter or failed experiment? BMJ 2003; 326:692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Aff 2003; 22:61–76. [DOI] [PubMed] [Google Scholar]
- 18.Bureau of National Health Insurance (NHI) the Pay-for-Performance (P4P) Program for Diabetes in Taiwan National Health Insurance System. 2001. [in Chinese] http://www.nhi.gov.tw/Resource/webdata/19248_2_%E7%B3%96%E5%B0%BF%E7%97%85%E9%86%AB%E7%99%82%E7%B5%A6%E4%BB%98%E6%94%B9%E5%96%84%E6%96%B9%E6%A1%88.pdf (accessed January 1, 2001). [Google Scholar]
- 19.Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005; 104:157–163. [PubMed] [Google Scholar]
- 20.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36:1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 22.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008; 14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Catastrophic disabling disease. March 24th 2016 [in Chinese] http://www.nhi.gov.tw/webdata/webdata.aspx?menu=18&menu_id=683&webdata_id=3471. [Google Scholar]
- 24.Walley T, Hughes D, Kendall H. Trends and influences on use of antidiabetic drugs in England, 1992–2003. Pharmacoepidemiol Drug Saf 2005; 14:769–773. [DOI] [PubMed] [Google Scholar]
- 25.Tsai ST, Pathan F, Ji L, et al. First insulinization with basal insulin in patients with Type 2 diabetes in a real-world setting in Asia. J Diabetes 2011; 3:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016. [DOI] [PubMed] [Google Scholar]
- 27.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696. [DOI] [PubMed] [Google Scholar]
- 28.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006; 166:1836–1841. [DOI] [PubMed] [Google Scholar]
- 29.Yu NC, Su HY, Tsai ST, et al. ABC control of diabetes: survey data from National Diabetes Health Promotion Centers in Taiwan. Diabetes Res Clin Pract 2009; 84:194–200. [DOI] [PubMed] [Google Scholar]
- 30.Yu NC, Su HY, Chiou ST, et al. Trends of ABC control 2006–2011: a National Survey of Diabetes Health Promotion Institutes in Taiwan. Diabetes Res Clin Pract 2013; 99:112–119. [DOI] [PubMed] [Google Scholar]
- 31.Li CL, Lin NY, Wang HH, et al. A population study on changes in diabetes self-care behaviors in Taiwan between 2001 and 2005. Prev Med 2010; 50:308–309. [DOI] [PubMed] [Google Scholar]
- 32.Huang YC, Wahlqvist ML, Lee MS. Appetite predicts mortality in free-living older adults in association with dietary diversity. A NAHSIT cohort study. Appetite 2014; 83:89–96. [DOI] [PubMed] [Google Scholar]
- 33.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. New Engl J Med 2008; 358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padwal RS, Pajewski NM, Allison DB, et al. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ 2011; 183:E1059–E1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMichael AJ. Food, nutrition, physical activity and cancer prevention. Authoritative report from World Cancer Research Fund provides global update. Public Health Nutr 2008; 11:762–763. [DOI] [PubMed] [Google Scholar]
- 37.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010; 33:1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang DC, Lee LJ, Hsu CC, et al. Estimation of expected life-years saved from successful prevention of end-stage renal disease in elderly patients with diabetes: a nationwide study from Taiwan. Diabetes Care 2012; 35:2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu MS, Wu IW, Shih CP, et al. Establishing a Platform for Battling End-stage Renal Disease and Continuing Quality Improvement in Dialysis Therapy in Taiwan–Taiwan Renal Registry Data System (TWRDS). Acta Nephrologica 2011; 25:148–153. [Google Scholar]
- 40.Mann DM, Woodward M, Ye F, et al. Trends in medication use among US adults with diabetes mellitus: glycemic control at the expense of controlling cardiovascular risk factors. Arch Intern Med 2009; 169:1718–1720. [DOI] [PubMed] [Google Scholar]
- 41.Cheng TM. Reflections on the 20th anniversary of Taiwan's single-payer National Health Insurance System. Health Aff 2015; 34:502–510. [DOI] [PubMed] [Google Scholar]
- 42.Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003; 138:24–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


