Abstract
Patients with gout are more likely to develop most cancers than subjects without gout. Colchicine has been used for the treatment and prevention of gouty arthritis and has been reported to have an anticancer effect in vitro. However, to date no study has evaluated the relationship between colchicine use and incident cancers in patients with gout. This study enrolled male patients with gout identified in Taiwan's National Health Insurance Database for the years 1998 to 2011. Each gout patient was matched with 4 male controls by age and by month and year of first diagnosis, and was followed up until 2011. The study excluded those who were diagnosed with diabetes or any type of cancer within the year following enrollment. We calculated hazard ratio (HR), aged-adjusted standardized incidence ratio, and incidence of 1000 person-years analyses to evaluate cancer risk. A total of 24,050 male patients with gout and 76,129 male nongout controls were included. Patients with gout had a higher rate of incident all-cause cancers than controls (6.68% vs 6.43%, P = 0.006). A total of 13,679 patients with gout were defined as having been ever-users of colchicine and 10,371 patients with gout were defined as being never-users of colchicine. Ever-users of colchicine had a significantly lower HR of incident all-cause cancers than never-users of colchicine after adjustment for age (HR = 0.85, 95% CI = 0.77–0.94; P = 0.001). In conclusion, colchicine use was associated with a decreased risk of incident all-cause cancers in male Taiwanese patients with gout.
INTRODUCTION
Gout is a disorder of purine metabolism and is characterized by hyperuricemia and acute arthritis. Patients with gout and cancer patients have similar risk factors, including obesity and heavy alcohol consumption, as well as inflammation. Gout has been associated with the cytokine and inflammation genes, including the tumor Tumor necrosis factor-α gene,1 cyclic Guanosine monophosphate-dependent protein kinase II gene,2 Interleukin-6,3 and Transforming growth factor-β1 gene.4 In a recent study using Taiwan's National Health Insurance Database (HID), we5 found patients with gout to be more likely to develop most cancers than subjects without gout.
Colchicine is an alkaloid agent that has been used for over a century for the treatment and prevention of gouty arthritis.6–11 Experimentally, colchicine has been demonstrated to dramatically abrogate the inflammatory response to urate crystal stimulation in humans.12 Besides having an antiinflammatory effect, colchicine is also a microtubule destabilizer with a strong capacity to bind to tubulin, perturbing the assembly dynamics of microtubules.13–16 The disruption of microtubule dynamics interferes with the regulation of the mitotic spindle resulting in cell cycle arrest and eventual cell death.17,18 Previous studies have reported that colchicine had an anticancer effect in vitro and in animal models.19–21 However, to date no study has evaluated the relationship between colchicine use and incident cancers in patients with gout. Therefore, we examined the association between colchicine use and incident cancers in a representative national cohort obtained from Taiwan's National HID.
MATERIALS AND METHODS
Study Sample
This study collected the records of 1 million outpatients obtained from National HID in the form of a longitudinal cohort from 1998 to 2011. The dataset represents about 5% of the total population of Taiwan. The selection process is shown in Figure 1. We excluded patients diagnosed with gout during years 1998 to 1999, and included male patients newly with diagnosed with gout (International Classification of Diseases, Ninth Revision [ICD-9] code 274) after January 2000 to ensure that we were identifying and following only new cases. The diagnosis was further confirmed by 3 continuous prescriptions of antigout medications. Our control group was composed of individuals with no diagnosis of gout from years 2000 to 2011. We randomly selected about 4 nongout male controls which we matched with each gouty patient by age and time of their outpatient visit, matching the same month and year of first diagnosis of gout in the study patients. The comorbidities of gout included obesity (ICD-9: 278), hyperlipidemia (ICD-9: 272), and hypertension (ICD-9: 4010, 4011, 4019), all diagnosed within 1 year following enrollment. Patients who were prescribed colchicine after entry were defined as ever-users; those who were not prescribed colchicine were defined as never-users. Benzbromarone, another commonly used gout drug, was used for comparison. Patients who were prescribed benzbromarone after entry were defined as ever-users; those who were not prescribed benzbromarone were defined as never-users. The endpoint was a diagnosis of any cancer (ICD-9: 140–208) after 1 year of enrollment. We excluded any patient who had been diagnosed with type 2 diabetes (ICD-9: 250) and any patient that developed any cancer within 12 months after enrollment both in gout patients and control participants. All participants were followed up from the inclusion date of first diagnosis until 2011. Our matching criteria and exclusion criteria ensured that the follow-up started from the same baseline period and avoided confounding effects of sex and age on the incident cancers. The approval for the analysis of the database was obtained from the Institutional Review Board of Changhua Christian Hospital (CCH IRB 121213).
FIGURE 1.
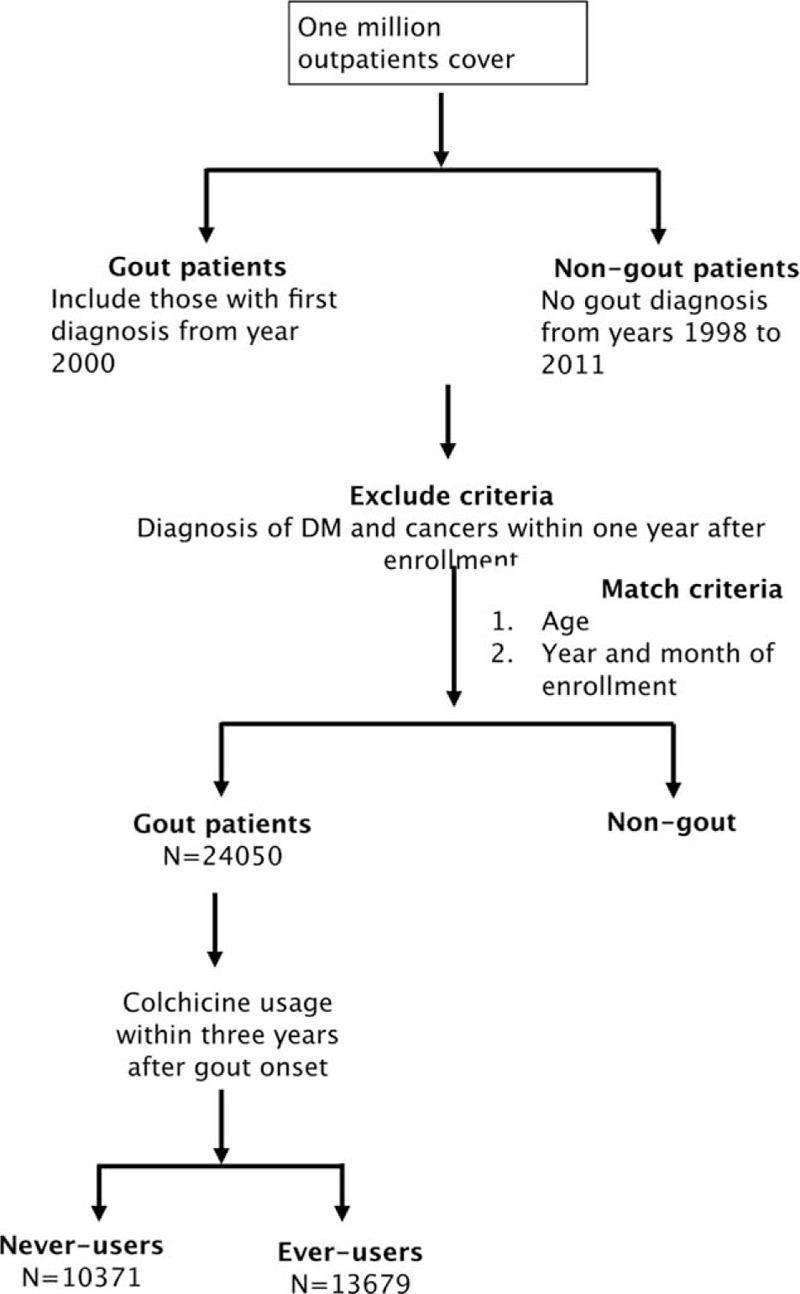
The study population inclusion algorithms.
Statistical Analyses
The mean difference for age and follow-up time were estimated by t-test, and the frequency difference for obesity, hyperlipidemia, hypertension, and all-cause cancers were estimated by Chi-square test between gout patients and controls. The risk of incident all-cause cancers between gout and control groups as well as between ever-users and never-users of colchicine was calculated by hazard ratio (HR), standardized incidence ratio, and 95% confidence intervals (95% CI) after adjustment of age. The significance for cumulative hazard rate was analyzed with Log-Rank test by SAS program (v9.3) after mining the national outpatient records using the PERL (v5.8) program. A P-value less than 0.05 were considered significant.
RESULTS
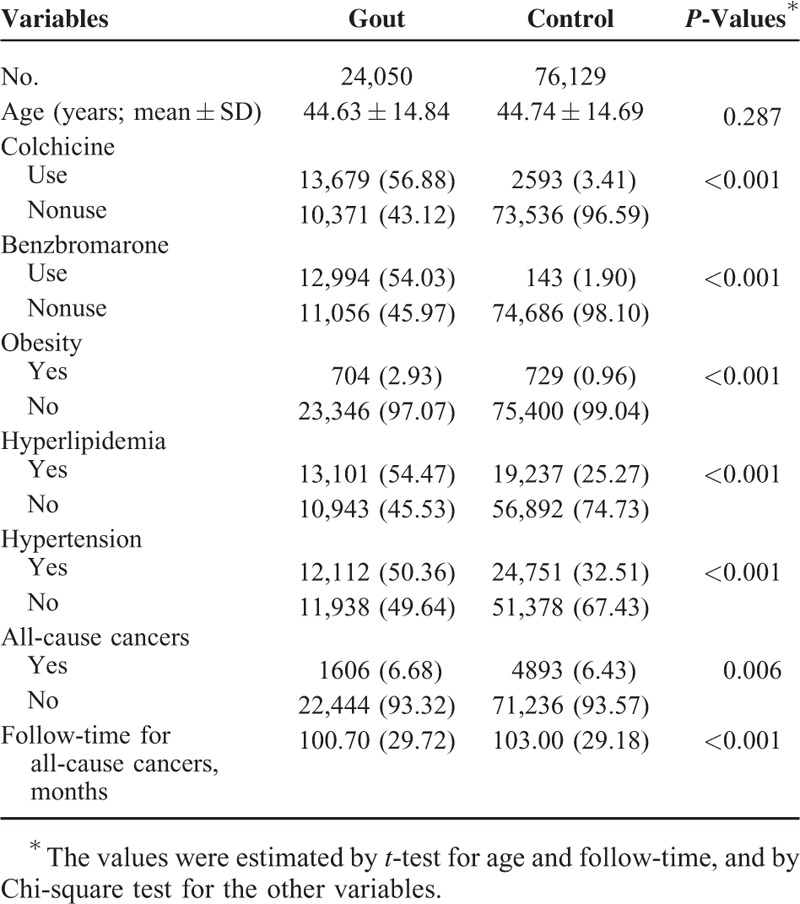
A total of 24,050 male gout patients and 76,129 male nongout controls were included in this study. The mean age of the gout patients was 44.63 ± 14.84 years old and controls 44.74 ± 14.69 years (P = 0.287) (Table 1). Gout patients had a significantly higher prevalence of obesity, hypertension, and hyperlipidemia than controls (all P < 0.001). The prescription rate of colchicine was significantly higher in gout patients than controls (56.88% vs 3.41%, P < 0.001). The prescription rate of benzbromarone was significantly higher in gout patients than controls (54.03% vs 1.90%, P < 0.001). Gout patients had a higher rate of incident all-cause cancers (6.68% vs 6.43%, P < 0.006) compared with controls. The mean follow-up time for gout patients was 100.70 months (±29.72), which was significantly lower than that for controls (103.00 ± 29.18, P < 0.001; Table 1).
TABLE 1.
Baseline Characteristics, Comorbidities, Colchicine Use and Incident All-Cause Cancers in Gout Patients and Controls
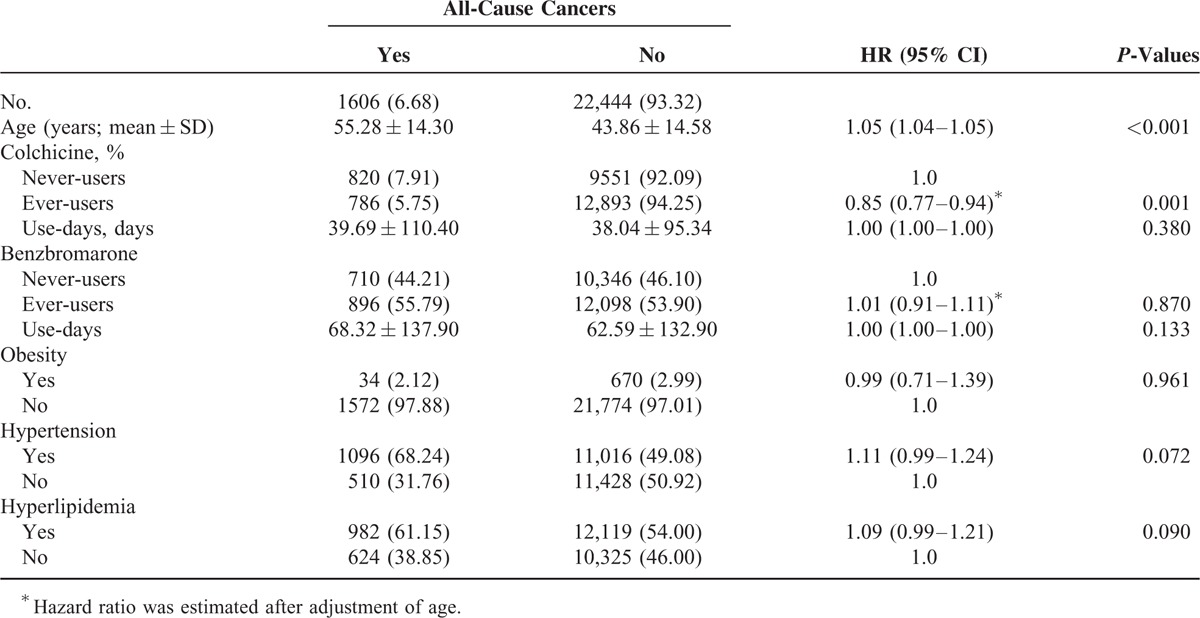
All gout patients were categorized into those with or without incidental cancers (Table 2). In total, 1606 gout patients were diagnosed with incidental cancers during the 12-year follow-up period. Patients with incidental cancers were older and had a significantly lower prescription rate for colchicine (48.9% vs 57.4%) as compared to those without incidental cancers. The prevalence rates of obesity, hypertension, and hyperlipidemia were not significantly different between gout patients with and without incidental cancers. Colchicine ever-users had significantly lower incidence of all cause cancers after adjustment for age, compared with colchicine never-users (HR: 0.85, 95% CI = 0.77–0.94; P = 0.001). The incidence of all cause cancers was not significantly different between benzbromarone ever-users and never-users (HR: 1.01, 95% CI = 0.91–1.11; P = 0.870).
TABLE 2.
The Hazard Ratios of Incidental All-Cause Cancers Among Gout Patients
A total of 13,679 gout patients were ever-users of colchicine and 10,371 were never-users of colchicine (Table 3). Ever-users were younger and had a lower prevalence of hyperlipidemia than never-users. Never-users had a significantly higher HR of incident all-cause cancers than ever-users after adjusting for age and hyperlipidemia (HR = 1.15, 95% CI = 1.04–1.28; P = 0.007; Table 3).
TABLE 3.
The Hazard Ratios of All-Cause Cancers in Colchicine Never-Users and Ever-Users Among Gout Patients
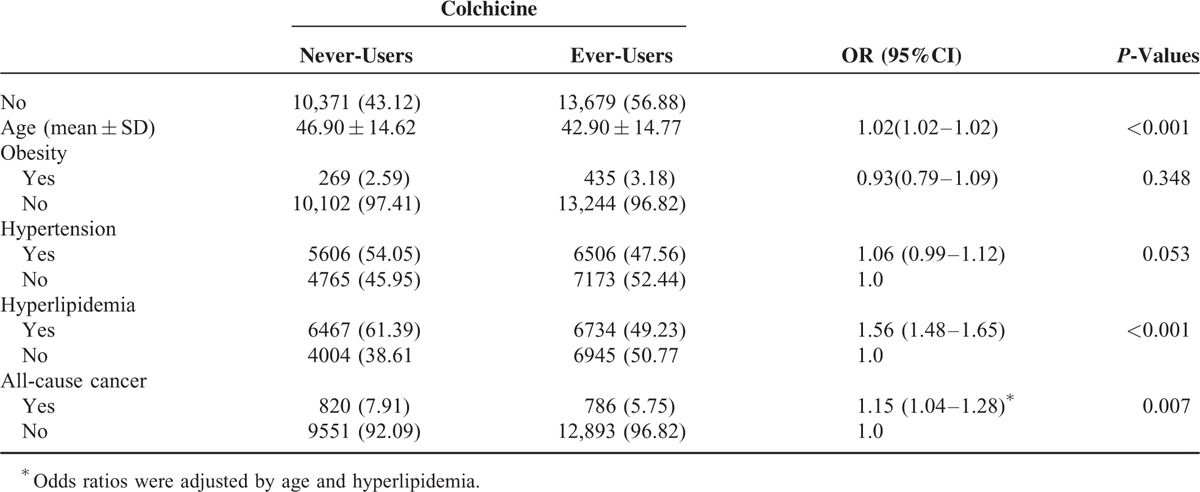
Figure 2 shows the cumulative hazard risks of incident all-cause cancers in gout patients who ever used colchicine and those who never used it. The incidence of all-cause cancers per 1000-person-years was 6.86 cases in those who used colchicine, showing a significant protective effect compared to the 9.41 cases in the nonusers (aged-adjusted standardized incidence ratio = 0.73, 95% CI = 0.66–0.80).
FIGURE 2.
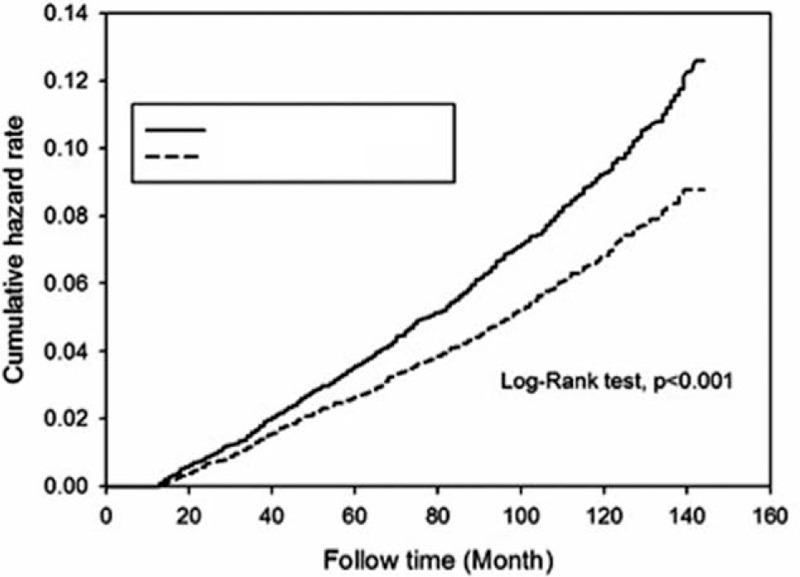
Cumulative hazard risk of incident all-cause cancers in colchicine ever-users and never-users among gout patients (P < 0.001).
Table 4 shows the colchicine exposure days and HR for incident all-cause cancers in gout patients. All analyses showed that colchicine ever-users had a significantly lower risk of incident all cause cancers. Table 5 shows the age-adjusted HR of specific cancers between colchicine ever-users and never-users for gout patients. Compared to never-users of colchicine, ever-users of the drug had a significantly lower risk of prostate cancer (HR = 0.68, 95% CI = 0.52–0.89, P = 0.004) and colorectal cancer (HR = 0.75, 95% CI = 0.60–0.94; P = 0.012). There were no significant differences in incidence of other specific cancers between the 2 groups (all P > 0.05).
TABLE 4.
The Colchicine Exposure Days and Hazard Ratio for Incident All-Cause Cancers
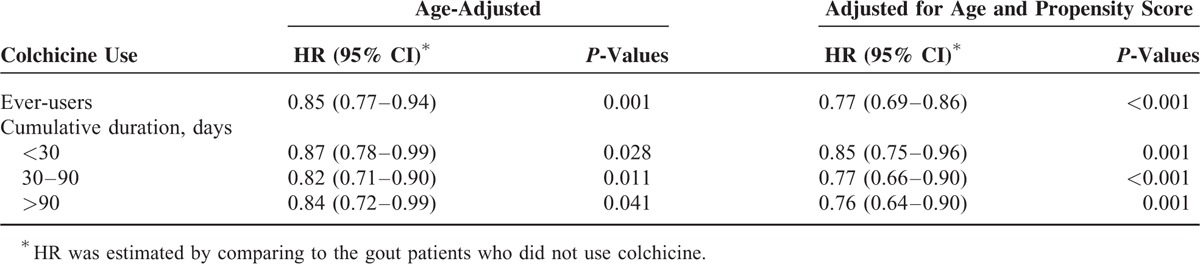
TABLE 5.
The Age-Adjusted Hazard Ratios of Specific Cancers Between Colchicine Ever-Users and Never-Users Among Gout Patients
DISCUSSION
This study found a significant association between reduced risk of incident cancers, particularly in prostate and colorectal cancers, and use of colchicine in male patients with gout.
Gout became the most common inflammatory joint disease in males because the prevalence of gout has increased in recent decades. There are several comorbidities, including but not limited to hypertension, obesity, and type 2 diabetes, associated with gout.22 Choi et al23 found that men with gout had 34% to 66% increased risk of type 2 diabetes compared with those without gout. Many studies have reported patients with type 2 diabetes to be at higher risk of developing cancers.24–29 In addition to one of our previous national database studies finding gout patients to have increased risk of most cancers,5 Kuo et al30 also reported an association between gout and increased risk of cancer, particularly prostate cancer in men. However, those 2 previous studies did not exclude patients with type 2 diabetes who are typically at higher risk of developing cancers. In the current study, we excluded patients with type 2 diabetes, confirming that gout was independently associated with higher risk of incident cancer.
To the best of our knowledge, this study represents the first to report a significant association between colchicine use and lower risk of incidental cancers in male gout patients, who are known to be at higher risk of developing cancers. Colchicine has been found to exhibit an anticancer effect on hepatocellular carcinoma (HCC) by a study using a cell model.19 That study attributed the inhibition of HCC development on the drug's antiproliferative effects. Furthermore, the administration of colchicine has been found to inhibit the development of tumors and impair tumor-free survival in pressure activation of malignant cells in an animal model.20 In a retrospective study of 186 patients, Arrieta et al31 found that long-term colchicine administration in patients with viral hepatitis-related cirrhosis prevented and delayed the development of HCC. Our study, which included more than a hundred thousand male gout patients, found a significant association between colchicine use and a decreased risk of cancer after adjustment (HR = 0.85, 95% CI = 0.77–0.94; P = 0.001, Table 2).
Colchicine use was most significantly associated with a decreased risk of prostate and colorectal cancers in our male gout patients in this study. One well-known anticancer mechanism for colchicine is the direct colchicine–tubulin interaction, which perturbs the assembly dynamics of microtubules.32–34 Fakih et al21 showed that colchicine inhibited the growth prostate adenocarcinoma cells implanted into Dunning rats. Other studies have reported that the antitumor effect of paclitaxel, an anticancer agent, in castrate resistant prostate cancer may be due to its inhibition of androgen receptor activity via its inhibition of microtubule dynamics.35–37 These favorable outcomes which were achieved using microtubule targeted agents have rekindled interest in such therapies for the clinical management of prostate cancer.38 Li et al39 found 2,3′,4,4′,5′-pentamethoxy-trans-stilbene, which targeted microtules, to be a potent inducer of apoptosis in colon cancer cells. Chopra et al40 reported that novel piperazine-based compounds inhibited microtubule dynamics and sensitized colon cancer cells to tumor necrosis factor-induced apoptosis. Although these studies are enlightening, the effects of colchicine on prostate and colorectal cancers need to be further clarified in future studies.
This study has several limitations. First, the potentially important clinical covariates, such as smoking status, alcohol consumption, and the levels of uric acid, were lacked in our study. Second, the numbers of some specific incident cancers were relatively small, so the power of our analysis was limited.
In conclusion, the present study suggests that colchicine use in male patients with gout may decrease the risk of incident cancers, particularly in prostate and colorectal cancers, in this population.
Acknowledgments
The authors thank the grants from the National Science Council in Taiwan (NSC 101-2314-B-390-001).
Footnotes
Abbreviations: HCC = hepatocellular carcinoma, HID = Health Insurance Database, HR = hazard ratio, ICD-9 = International Classification of Diseases, Ninth Revision.
M-CK wrote the manuscript, analyzed the data, and designed the study. S-JC contributed to the discussion and data analysis. M-CH contributed to the collection of research data, manuscript writing, and study design.
This work was supported by the grants from the National Science Council in Taiwan (NSC 101-2314-B-390-001).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Chang SJ, Tsai PC, Chen CJ, et al. The polymorphism −863C/A in tumour necrosis factor-alpha gene contributes an independent association to gout. Rheumatology 2007; 46:1662–1666. [DOI] [PubMed] [Google Scholar]
- 2.Chang SJ, Tsai MH, Ko YC, et al. The cyclic GMP-dependent protein kinase II gene associates with gout disease: identified by genome-wide analysis and case-control study. Ann Rheum Dis 2009; 68:1213–1219. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang SJ, Chen CJ, Tsai FC, et al. Associations between gout tophus and polymorphisms 869T/C and −509C/T in transforming growth factor beta1 gene. Rheumatology 2008; 47:617–621. [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Yen JH, Chang SJ. Gout patients have an increased risk of developing most cancers, especially urological cancers. Scand J Rheumatol 2014; 43:385–390. [DOI] [PubMed] [Google Scholar]
- 6.Cocco G, Chu DC, Pandolfi S. Colchicine in clinical medicine. A guide for internists. Eur J Intern Med 2010; 21:503–508. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein Y, Aks SE, Hutson JR, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol (Phila) 2010; 48:407–414. [DOI] [PubMed] [Google Scholar]
- 8.Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005; 112:2012–2016. [DOI] [PubMed] [Google Scholar]
- 9.Imazio M, Brucato A, Trinchero R, et al. Colchicine for pericarditis: hype or hope? Eur Heart J 2009; 30:532–539. [DOI] [PubMed] [Google Scholar]
- 10.Kallinich T, Haffner D, Niehues T, et al. Colchicine use in children and adolescents with familial Mediterranean fever: literature review and consensus statement. Pediatrics 2007; 119:e474–e483. [DOI] [PubMed] [Google Scholar]
- 11.Levy M, Spino M, Read SE. Colchicine: a state-of-the-art review. Pharmacotherapy 1991; 11:196–211. [PubMed] [Google Scholar]
- 12.Malawista SE, Seegmiller JE. The effect of pretreatment with colchicine on the inflammatory response to microcrystalline urate: a model for gouty inflammation. Ann Intern Med 1965; 62:648–657. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya B, Panda D, Gupta S, et al. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev 2008; 28:155–183. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Chen J, Xiao M, et al. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res 2012; 29:2943–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanton RA, Gernert KM, Nettles JH, et al. Drugs that target dynamic microtubules: a new molecular perspective. Med Res Rev 2011; 31:443–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivakumar G. Colchicine semisynthetics: chemotherapeutics for cancer? Curr Med Chem 2013; 20:892–898. [PubMed] [Google Scholar]
- 17.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 1997; 13:83–117. [DOI] [PubMed] [Google Scholar]
- 18.Mistry SJ, Atweh GF. Role of stathmin in the regulation of the mitotic spindle: potential applications in cancer therapy. Mt Sinai J Med 2002; 69:299–304. [PubMed] [Google Scholar]
- 19.Lin ZY, Wu CC, Chuang YH, et al. Anti-cancer mechanisms of clinically acceptable colchicine concentrations on hepatocellular carcinoma. Life Sci 2013; 93:323–328. [DOI] [PubMed] [Google Scholar]
- 20.Craig DH, Owen CR, Conway WC, et al. Colchicine inhibits pressure-induced tumor cell implantation within surgical wounds and enhances tumor-free survival in mice. J Clin Invest 2008; 118:3170–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fakih M, Yagoda A, Replogle T, et al. Inhibition of prostate cancer growth by estramustine and colchicine. Prostate 1995; 26:310–315. [DOI] [PubMed] [Google Scholar]
- 22.Michael HP, David SG, Obert TK. Gout and its comorbidities. Bull NYU Hosp Jt Dis 2010; 68:199–203. [PubMed] [Google Scholar]
- 23.Choi HK, De Vera MA, Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology 2008; 47:1567–1570. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh MC, Lee TC, Cheng SM, et al. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res 2012; 2012:413782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006; 4:369–380. [DOI] [PubMed] [Google Scholar]
- 26.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007; 121:856–862. [DOI] [PubMed] [Google Scholar]
- 27.Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92:2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson SC, Orsini N, Brismar K, et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006; 49:2819–2823. [DOI] [PubMed] [Google Scholar]
- 29.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:1679–1687. [DOI] [PubMed] [Google Scholar]
- 30.Kuo CF, Luo SF, See LC, et al. Increased risk of cancer among gout patients: a nationwide population study. Joint Bone Spine 2012; 79:375–378. [DOI] [PubMed] [Google Scholar]
- 31.Arrieta O, Rodriguez-Diaz JL, Rosas-Camargo V, et al. Colchicine delays the development of hepatocellular carcinoma in patients with hepatitis virus-related liver cirrhosis. Cancer 2006; 107:1852–1858. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya B, Panda D, Gupta S, et al. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev 2008; 28:155–183. [DOI] [PubMed] [Google Scholar]
- 33.Stanton RA, Gernert KM, Nettles JH, et al. Drugs that target dynamic microtubules: a new molecular perspective. Med Res Rev 2011; 31:443–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sivakumar G. Colchicine semisynthetics: chemotherapeutics for cancer? Curr Med Chem 2013; 20:892–898. [PubMed] [Google Scholar]
- 35.Darshan MS, Loftus MS, Thadani-Mulero M, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res 2011; 71:6019–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan L, Chen S, Wang Y, et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res 2009; 69:8386–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4:253–265. [DOI] [PubMed] [Google Scholar]
- 38.Christiansen JJ, Weimbs T, Bander N, et al. Differing effects of microtubule depolymerizing and stabilizing chemotherapeutic agents on t-SNARE-mediated apical targeting of prostate-specific membrane antigen. Mol Cancer Ther 2006; 5:2468–2473. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Wu WK, Zheng Z, et al. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, is a potent inducer of apoptosis in colon cancer cells via targeting microtubules. Biochem Pharmacol 2009; 78:1224–1232. [DOI] [PubMed] [Google Scholar]
- 40.Chopra A, Anderson A, Giardina C. Novel piperazine-based compounds inhibit microtubule dynamics and sensitize colon cancer cells to tumor necrosis factor-induced apoptosis. J Biol Chem 2014; 289:2978–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]


