Abstract
Low vitamin D status has been implicated in several chronic medical conditions and unfavorable health outcomes. Our goal was to investigate whether serum 25-hydroxyvitamin D (25OHD) levels are a potentially modifiable risk factor for anemia in a nationally representative cohort of community-dwelling individuals in the United States.
We performed a cross-sectional study of 5456 individuals (≥17 years) from the National Health and Nutrition Examination Survey from 2001 to 2006. Locally weighted scatterplot smoothing (LOWESS) was used to graphically depict the relationship between serum 25OHD levels and the cumulative frequency of anemia. Multivariable logistic regression models were then used to assess the independent association of 25OHD levels with anemia, while controlling for age, sex, race, body mass index, chronic kidney disease, as well as serum levels of C-reactive protein, ferritin, iron, vitamin B12, and folic acid.
The mean (standard error) 25OHD and hemoglobin levels in the analytic group were 23.5 (0.4) ng/mL and 14.4 (0.1) g/dL, respectively. Prevalence of anemia was 3.9%. Locally weighted scatterplot smoothing analysis demonstrated a near-linear relationship between vitamin D status and cumulative frequency of anemia up to 25OHD levels of approximately 20 ng/mL. With increasing 25OHD levels, the curve flattened out progressively. Multivariable regression analysis demonstrated an inverse association of 25OHD levels with the risk of anemia (adjusted odds ratio 0.97; 95% confidence interval 0.95–0.99 per 1 ng/mL change in 25OHD). Compared to individuals with ≥20 ng/mL, individuals with 25OHD levels <20 ng/mL were more likely to be anemic (adjusted odds ratio 1.64; 95% confidence interval 1.08–2.49).
In a nationally representative sample of community-dwelling individuals in the United States, low 25OHD levels were associated with increased risk of anemia. Randomized controlled trials are needed to determine whether optimizing vitamin D status can reduce the burden of anemia in the general population.
INTRODUCTION
Anemia affects 1 of every 3 individuals worldwide and is estimated to have a global disease burden surpassing that of major depression and chronic respiratory ailments.1 Although the prevalence of anemia has been declining globally during the last 2 decades,1,2 the prevalence of anemia, and its associated comorbidities, in the general population of the United States has been on the rise.3 The health and economic impacts of anemia are even more pronounced in hospitalized individuals, where patients with anemia may have up to a 3-fold higher risk of mortality compared with patients without anemia.3–5 Similarly, the costs of medical care for hospitalized patients double in the presence of anemia, independent of any baseline comorbidities.6
The burden of anemia persists despite its known causes and the availability of effective treatments. Iron deficiency remains the overwhelming cause of anemia, affecting over 2 billion individuals globally.2,7 The United States Centers for Disease Control and Prevention (CDC) has emphasized primary prevention on a population level through a healthful diet with adequate iron sources as an inexpensive, widely accessible, and effective approach to reducing the risk of anemia in the general population.8 Nonetheless, adequate dietary intake of iron remains a challenge in both, developing and developed countries.2 Recent evidence suggests that other dietary factors, such as adequate vitamin D consumption, may affect iron regulation and erythropeisis.9–14 Although previous reports suggest that serum 25-hydroxyvitamin D (25OHD), which is widely regarded as the best marker of total body vitamin D status, is associated with hemoglobin (Hgb) levels, these studies either had limited sample sizes,15–17 or were primarily focused on children,18–20 the elderly,21 only women,22,23 or adults in a healthcare setting.24,25 Therefore, our objective was to investigate the association of 25OHD levels with the risk of anemia in a large, nationally representative, community-dwelling sample of individuals from the United States.
METHODS
Data Source
The National Center for Health Statistics (Atlanta, GA) conducted a nationally representative cross-sectional survey of the noninstitutionalized civilian population in the United States from 2001 to 2006, know as the National Health and Nutrition Examination Survey (NHANES).26 The NHANES survey data was gathered in 3 phases from 1971 to 1994 and then annually from 1999 onwards. Survey data related to the 31,509 participants from 2001 to 2006 represent the most current data with 25OHD assessment. Oversampling was used for the following groups to produce more accurate population estimates: low-income White; Non-Hispanic Black; Mexican American; ages 12 to 19; and ages ≥70 years. After local Institutional Review Board approvals (Partners Human Research Committee and Tulane University), we conducted a cross-sectional analysis of the NHANES 2001 to 2006 dataset.
Survey Methods
Previous reporting on the NHANES methodology specifies the informed consent, sampling, interview, examination, laboratory tests, and ethics approval processes.26 Written informed consent and/or assent was obtained from all participants ≥17 years of age. A stratified complex, multistage probability sample design was used to produce samples that are nationally representative during 2-year cycles, each with approximately 12,000 individuals. Data on demographics, health, and nutrition were collected through in-home interviews. Mobile examination center or home-based laboratory testing and physical examinations were subsequently performed. Centrifuged and aliquoted blood samples collected on site were shipped to central laboratories on dry ice and stored at −70 °C until biomarker analysis.
Data Abstraction
We included all individuals, 17 years and older, in the NHANES 2001 to 2006 databases. Exclusion criteria were missing values of either 25OHD or Hgb. We then abstracted the following demographic information: age, sex, race, body mass index (BMI), and poverty-to-income ratio (PIR: as an indicator of socioeconomic status). Values of Hgb less than the NHANES III-defined age- and sex-specific fifth percentile thresholds27 were used to categorize anemia as the primary outcome for this study. Body mass index was calculated from individual height and weight records from physical examinations. NHANES calculated the family PIR based on family income and poverty thresholds, defined by the United States Census Bureau according to the year, state of residence, and family size. We also abstracted information related to the presence of chronic kidney disease (CKD), which was defined as a urine albumin-to-creatinine ratio of ≥30 mg/g according to clinical guidelines.28 In addition, we abstracted information related to serum levels of C-reactive protein (CRP), ferritin, iron, vitamin B12, and folic acid.
Biomarker Processing
After acentonitrile-based extraction, the National Center for Environmental Health (Atlanta, GA) measured 25OHD levels with a radioimmunoassay kit (DiaSorin, Stillwater, MN). The National Institute of Standards and Technology assigned certified values for 25OHD to produce assay standards using isotope dilution tandem mass spectrometry (liquid chromatography–mass spectrometry/mass spectrometry) candidate reference measurement. The release of these July 2009 standards were followed by the CDC release of a revised analytic note using National Institute of Standards and Technology standards from liquid chromatography–mass spectrometry/mass spectrometry and regression equations to adjust 25OHD levels for the NHANES 2001 to 2006 datasets. In November 2010, the 25OHD data from 2003 to 2006 were further adjusted by NHANES in response to observed assay performance drift with fluctuation in reagent and calibration lot.29 Because empirical trends would be independent of values from sample subjects, a statistical model was used to adjust for the drift period values with quality control pool data.
A Coulter Counter Model S-Plus JR (Beckman Coulter, Brea, CA) was used to measure Hgb levels. Latex-enhanced nephelometry was used to measure CRP levels with a Dade Behring Nephelometer II Analyzer System (Dade Behring Diagnostics Inc., Somerville, NJ). Quantimmune Ferritin IRMA kit (Bio-Rad Laboratories, Hercules, CA) was used to measure serum ferritin in 2001 to 2004, and 2005 to 2006 levels were measured using Hitachi 912 clinical analyzers with the Roche Tina-quant kit (Roche Diagnostics Corporation, Indianapolis, IN). Serum iron level was measured in 2001 to 2002 by the colorimetrical method using RFA Analyzers (Alpkem Corporation, Clackamas, OR), and in 2003 to 2006 by the timed-endpoint method with Beckman Synchron LX20 (Beckman Coulter, Brea, CA). Quantaphase Folate radioassay kit (Bio-Rad Laboratories, Hercules, CA) was used to measure vitamin B12 and folic acid levels.
Statistical Analyses
We used the appropriate survey commands to apply the subsample weights for the interview and examination data, as recommended by NHANES. All results are reported as weighted values to account for unequal selection probability and thus provide accurate estimates for the United States population. Using the Taylor series linearization method, we calculated variance from the masked variance units provided by NHANES. We calculated proportions or means with standard errors (SEs) for demographic information and other variables believed to be associated with Hgb levels. Proportions (of variables describing anemic versus nonanemic participants) were compared using χ2 tests, whereas means were compared using independent sample t-tests.
We first constructed a multivariable linear regression model to assess the independent association between 25OHD (as a continuous variable) and Hgb levels by gradually adding covariates suspected of confounding this relationship. We then constructed a multivariable logistic regression model to assess the independent association between 25OHD levels (as a continuous variable) and anemia (using the NHANES age and sex-specific fifth percentiles). To graphically represent the relationship between 25OHD levels and the cumulative frequency of anemia in our analytic cohort, we used locally weighted scatterplot smoothing (LOWESS). This nonparametric regression technique begins with limited assumptions on the magnitude and form of the association of 2 variables to model their relationship across given sections of their ranges using local fitting.30,31 Based on the results of our LOWESS curve, we then constructed a multivariable logistic regression model to assess the independent association of 25OHD levels as a dichotomous variable with anemia. Beta values are reported for the linear regression analysis, and the odds ratio (OR) with 95% confidence interval (CI) are reported for the logistic regression analyses.
In all analyses, the following covariates were dichotomized: sex (female versus male), race (Non-White versus White), PIR (≤ federal poverty level versus >federal poverty level), and presence of CKD (albumin-to-creatinine ratio < 30 mg/g versus ≥30 mg/g). All other covariates were considered as continuous variables. Missing data points were replaced with plausible values based on the existing measurements; this was done by multiple imputation with multivariate normal regression and Bayesian Markov chain Monte Carlo procedure including 3000 iterations for the burn-in period.32 All statistical analyses were conducted with Stata 14.0 (StataCorp LP, College Station, TX); 2-tailed P < 0.05 or OR with 95% CI not spanning 1 were considered statistically significant.
RESULTS
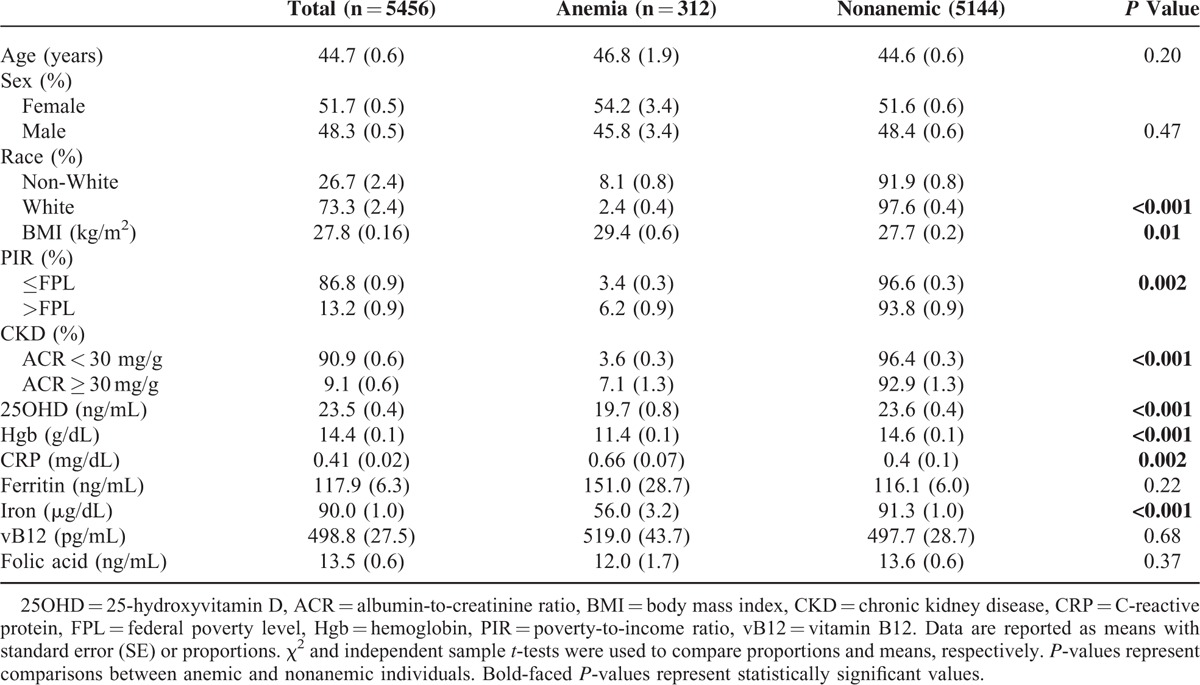
The demographic and serologic characteristics for the 5456 individuals who met study criteria are shown in Table 1. The overall mean age was 44.7 years (SE 0.6), 51.7% (SE 0.5) were women, and 73.3% (SE 2.4) were White. The mean serum 25OHD and Hgb levels of the analytic cohort were 23.5 ng/mL (SE 0.4) and 14.4 g/dL (SE 0.1), respectively. Overall, 3.9% (SE 0.3) of participants were anemic.
TABLE 1.
Demographic Information and Laboratory Data of Individuals ≥17 years of Age Who Had 25-hydroxyvitamin D and Hemoglobin Levels Assessed During the National Health and Nutrition Surveys 2001–2006
Multivariable linear regression, while controlling for age, sex, race, BMI, presence of CKD, as well as CRP, ferritin, vitamin B12, folic acid, and iron levels demonstrated that each 1 ng/mL increase in 25OHD was associated with 0.01 g/dL increase in Hgb levels (β = 0.01: 95% CI 0.00–0.02; P = 0.03).
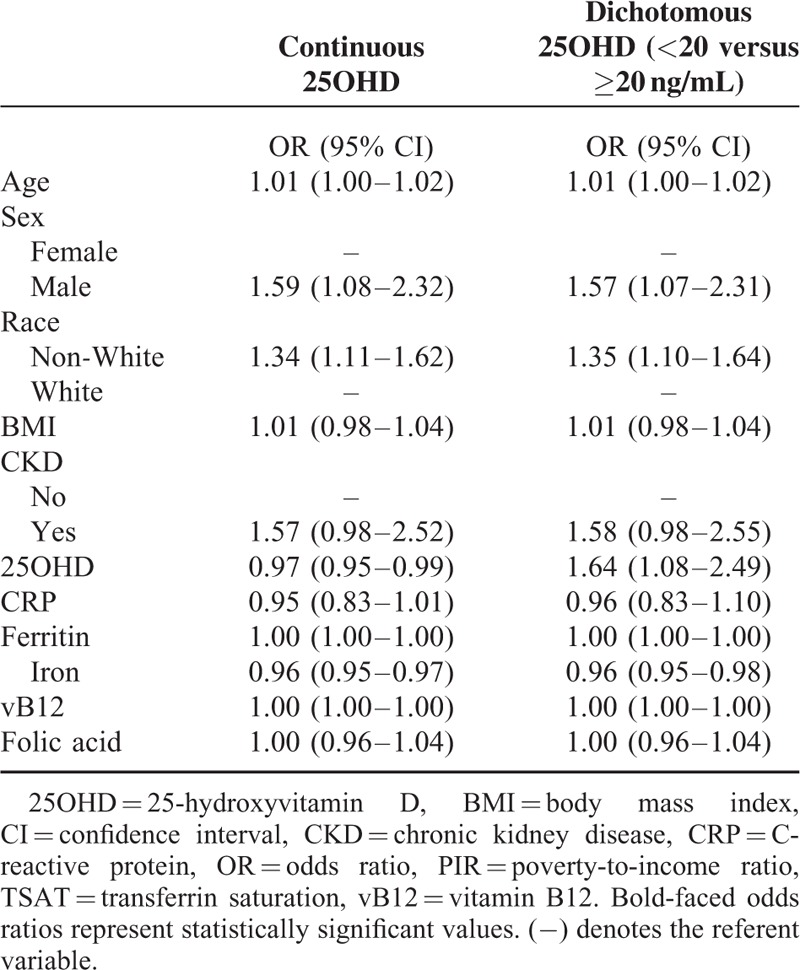
Multivariable logistic regression analysis, while controlling for age, sex, race, BMI, presence of CKD, as well as CRP, ferritin, vitamin B12, folic acid, and iron levels demonstrated that each 1 ng/mL increase in 25OHD was associated with an 3% lower likelihood of anemia (OR 0.97; 95% CI 0.95–0.99; P = 0.049). Locally weighted scatterplot smoothing curve analysis demonstrated a near-linear relationship between vitamin D status and the cumulative frequency of anemia up to 25OHD approximately 20 ng/mL, with the curve increasingly flattening out between 20 and 60 ng/mL, and remaining flat beyond 60 ng/mL (Fig. 1). We therefore dichotomized 25OHD level in our final logistic regression model as <20 ng/mL versus ≥20 ng/mL; and despite controlling for age, sex, race, BMI, presence of CKD, as well as CRP, ferritin, iron, vitamin B12, and folic acid levels, participants with 25OHD levels <20 ng/mL were almost 1.5 times more likely to be anemic compared with participants with levels ≥20 ng/mL (OR 1.64; 95% CI 1.08–2.49; P = 0.03). Other variables found to be independently associated with the risk of anemia in our regression analyses were gender, race, and iron (Table 2).
FIGURE 1.
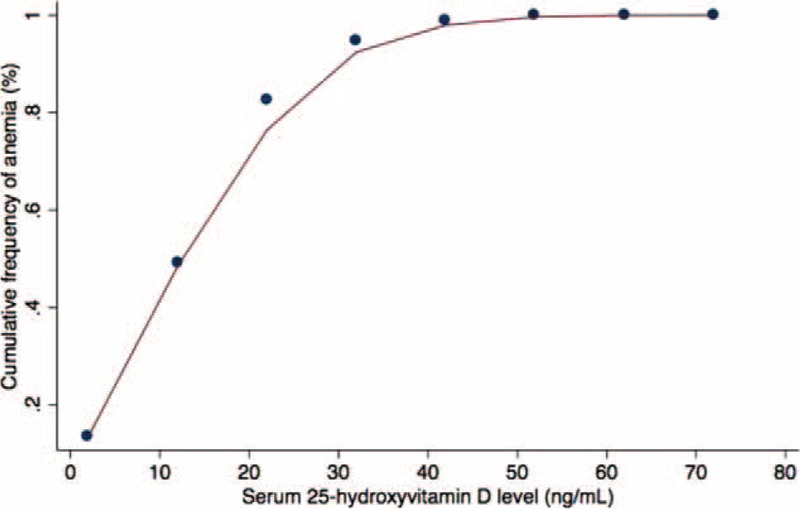
Locally weighted scatterplot smoothing analysis demonstrating a near-linear relationship of vitamin D status and cumulative frequency of anemia up to 25-hydroxyvitamin D levels of 20 ng/mL. Between levels of 20 and 60 ng/mL there is progressive flattening of the curve. Beyond 60 ng/mL the curve remains flat (ie, no additional patients with anemia).
TABLE 2.
Adjusted Risk for Anemia Among Individuals ≥17 years of Age in Multivariable Models Where 25-hydroxyvitamin D Levels are Treated as a Continuous Versus Dichotomous Variable
For our primary analysis, we categorized anemia based on the NHANES III-defined age- and sex-specific fifth percentile thresholds.27 We reported results using these thresholds instead of the more common 1968 World Health Organization (WHO) definition,33 because of recent controversy34 and stronger evidence in support of the NHANES definition (ie, the use of improved laboratory techniques and estimation from a significantly larger sample of participants). To facilitate comparisons to previous studies related to anemia, we repeated the same final multivariable regression models as above, however, they were modified with the WHO thresholds for anemia instead of those from NHANES III. Results from these modified models were similar to those from our original models, as each increasing 25OHD unit was associated with a 4% lower likelihood of anemia (OR 0.96; 95% CI 0.93–0.98; P = 0.003) and a 2-fold increased likelihood of anemia when 25OHD was dichotomized as <20 ng/mL versus ≥20 ng/mL (OR 1.95; 95% CI 1.22–3.10; P = 0.008).
DISCUSSION
We assessed the relationship between 25OHD levels and anemia in a large, nationally representative sample of community-dwelling individuals, ≥17 years of age, in the United States. Our results suggest that low vitamin D status is associated with an increased risk of anemia in the general population and that this relationship is most evident when comparing individuals with 25OHD levels <20 ng/mL to those with levels ≥20 ng/mL. These results are similar to the recent analysis by Atkinson et al that reported in a nationally representative sample of 10,410 children and adolescents that subjects with 25OHD levels <20 ng/mL were significantly more likely to be anemic even after controlling for age, sex, race, obesity, CRP, B12, and folate.20 Our study demonstrates this increased risk of anemia is also present in a nationally representative sample of noninstitutionalized individuals 17 years and older even after controlling for a more comprehensive set of covariates. Although the cross-sectional design of both studies limits any causal inferences about the effect of low 25OHD levels and higher risk of anemia, the biologic plausibility is compelling, as discussed below.
The association between vitamin D status and anemia is of potentially great public health importance because over half of the US population suffers from low 25OHD.35–37 As is increasingly appreciated, the potential benefits of vitamin D extend well beyond skeletomuscular maintenance9–14 with growing evidence suggesting that 25OHD levels are associated with cardiovascular health, glycemic regulation, angiotensin-regulated vascular responses, immune function, and cell differentiation.38–40 Indeed, low 25OHD levels may increase the risk of heart disease, hypertension, stroke, and diabetes.41 Moreover, the immunomodulatory effects of vitamin D have been described in various diseases.13,14
With regard to anemia, there is a well-documented inverse association between 25OHD levels and the need for exogenous erythropoietin (EPO) among patients with anemia related to renal disease.42,43 Insufficient production of EPO by the kidneys, and thus attenuated erythroid maturation in bone marrow, is the main pathophysiologic mechanism believed to be responsible for anemia in CKD.44 Erythroid precursors also require hepcidin as a major mediator of iron absorption and utilization; hepcidin is pathologically upregulated by inflammatory cytokines and results in a reduction in circulating iron levels.45 Activation of the vitamin D receptor in bone marrow (stromal and accessory cells) inhibits production of interleukin (IL)-1, IL-6, interferon-γ, tumor necrosis factor-α, as well as other proinflammatory cytokines,46,47 and upregulates production of the anti-inflammatory cytokine, IL-10.48 These alterations in cytokine expression have been shown to enhance erythroid proliferation and blunt hepcidin overproduction.49
Published epidemiological studies, each with different limitations, suggest a relationship between vitamin D status and anemia. In a moderately sized cross-sectional study of ambulatory care patients (n = 554) in the west coast of the United States, 25OHD levels <30 ng/mL were shown to be associated with an almost 2-fold increased risk of anemia after adjusting for age, sex, and presence of CKD.17 Moreover, in German patients presenting for cardiac surgery (n = 4428), low vitamin D status was associated with an increased risk of preoperative anemia, after adjusting for various cardiovascular and respiratory comorbidities as well as BMI, CRP, and renal disease.24 In another German study of preoperative assessment in cardiac surgery patients (n = 3615), those with dual suboptimal 25OHD and 1,25-dihydroxyvitamin D levels were at highest risk for anemia.25 On the contrary, in ambulatory care individuals from Bahrain (n = 421), low 25HOD levels were associated with increased risk of anemia in women, but not in men.15 Similarly, in a study of Korean patients (n = 500), 25OHD level and female sex were found to be independently associated with anemia.16 In a large study of community dwelling Koreans (n = 5786),22 women—but not men—in the lowest quartile for 25OHD levels were at an increased risk of anemia compared with women in the highest quartile, after controlling mainly for age, renal function, and smoking status. It, however, is important to recognize that the study investigators used a stepwise regression modeling approach based on sex, and as a result, the final models to test the association of 25OHD level and anemia in men versus premenopausal women versus postmenopausal women were slightly different in each patient. In a larger study of noninstitutionalized adults in Korea (n = 11,206),23 again women—but not men—in the lowest quintile for 25OHD levels were at an increased risk of anemia compared with women in the highest quintile, after controlling for age, sex, smoking status, cumulative weekly exercise, hypertension, diabetes mellitus, cardiovascular disease, BMI, season, daily iron intake, estimated glomerular filtration rate, as well as ferritin, iron, TIBC, cholesterol, and triglyceride levels. Interestingly, among elderly men (n = 1666) in Australia,21 serum 1,25-dihydroxyvitamin D (the most biologically active vitamin D metabolite), but not 25OHD levels were found to be independently associated with Hgb levels in both cross-sectional and longitudinal analyses. This raises the question whether different vitamin D metabolites may influence anemia states through sex hormone-dependent biologic pathways.
Although our results build on previous studies15–20,22–25 and provide further evidence to suggest that vitamin D status may be a modifiable risk factor for anemia in the general population, it is important to discuss the potential limitations of the current study. As with all observational studies, and any cross-sectional research design, there is potential for confounding because of the lack of a randomly distributed exposure. Selection bias may be present, because 25OHD and Hgb levels were not recorded for all participants in the NHANES 2001 to 2006 survey. And despite adjusting for multiple potential confounders, there may still be residual confounding, which could account for the observed differences in outcomes. In particular, low 25OHD and/or Hgb levels may be a reflection of poor general health or suboptimal nutritional state, for which we are unable to fully adjust. Given the confines of the NHANES survey, a further limitation is that we were unable to fully adjust for lack of sun exposure, use of sunscreens, physical activity, and less common causes of anemia (eg, anemia of chronic disease, hemolytic disorders, bone marrow suppression, sickle cell disease, and thalassemia). Another potential limitation considering the known link between anemia and age is that our results could have been affected by failing to implement appropriate upper thresholds for age even though our model controlled for the age of subjects. We therefore performed a separate analysis with various age thresholds based on the literature and confirmed these did not materially change the observed results. It should also be noted that the LOWESS curve, which was used to determine the dichotomous threshold for 25OHD in the logistic regression model, graphically displayed 2 points slightly outside the curve. This is acceptable given the majority of the points are captured by the curve using a technique that is a well-supported tool for verifying model calibration, or agreement between an adequate amount of observed values and predicted probabilities.30 And finally, there continues to be controversy over consensus on thresholds to define anemia (WHO versus NHANES III). Although either definition did not materially change the results of our analyses, it may significantly impact future investigations. As such, these issues will need to be addressed in prospective studies to replicate and extend our findings.
SUMMARY
Our analyses suggest that low 25OHD levels are strongly associated with anemia in a large, nationally representative sample of community-dwelling individuals from the United States. We hypothesize that suboptimal vitamin D status may contribute to anemia via inadequate EPO production, and/or by promoting a persistent inflammatory state, which in turn decreases erythroid production and results in hepcidin overproduction. Longitudinal studies are required to confirm our findings and to establish the mechanisms underlying the observed relationship between 25OHD levels and anemia. Moreover, high-quality, randomized controlled trials are warranted to determine whether vitamin D supplementation in individuals with low vitamin D status may affect the prevalence of anemia in the general population.
Footnotes
Abbreviations: 25OHD = 25-hydroxyvitamin D, BMI = body mass index, CDC = Centers for Disease Control and Prevention, CI = confidence interval, CKD = chronic kidney disease, CRP = C-reactive protein, Hgb = hemoglobin, LOWESS = locally weighted scatterplot smoothing, NHANES = National Health and Nutrition Examination Survey, OR = odds ratio, PIR = poverty-to-income, Vit D = vitamin D.
Institution where work was performed: ViDIS Laboratory, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA.
Funding Disclosure: SAQ received support from the US National Institutes of Health T32 GM007592 and L30 TR001257.
Statement of author contributions to manuscript DJM and SAQ jointly conceived the study as well as designed and implemented the analysis with assistance from CAC and JTM. DJM assembled input data, wrote code, ran the model, and analyzed output data. DJM and SAQ wrote the manuscript. CAC and JTM edited the manuscript and provided conceptual advice.
The authors report no conflicts of interest.
REFERENCES
- 1.Pasricha S-R. Anemia: a comprehensive global estimate. Blood 2014; 123:611–612. [DOI] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014; 123:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rachoin J-S, Cerceo E, Milcarek B, et al. Prevalence and impact of anemia in hospitalized patients. South Med J 2013; 106:202–206. [DOI] [PubMed] [Google Scholar]
- 4.Potter LJ, Doleman B, Moppett IK. A systematic review of pre-operative anaemia and blood transfusion in patients with fractured hips. Anaesthesia 2015; 70:483–500. [DOI] [PubMed] [Google Scholar]
- 5.Reade MC, Weissfeld L, Angus DC, et al. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm Med 2010; 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade SW, Knight K, Wilson A, et al. The economic burden of anemia in an insured population. Value Health 2004; 7:315–316. [Google Scholar]
- 7.McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr 2009; 12:444–454. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 1998; 47:1–29. [PubMed] [Google Scholar]
- 9.Toxqui L, Pérez-Granados AM, Blanco-Rojo R, et al. Effects of an iron or iron and vitamin D–fortified flavored skim milk on iron metabolism: a randomized controlled double-blind trial in iron-deficient women. J Am Coll Nutr 2013; 32:312–320. [DOI] [PubMed] [Google Scholar]
- 10.Santoro D, Caccamo D, Lucisano S, et al. Interplay of vitamin D, erythropoiesis, and the renin-angiotensin system. Biomed Res Int 2015; 2015:145828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riccio E, Sabbatini M, Bruzzese D, et al. Effect of paricalcitol vs calcitriol on hemoglobin levels in chronic kidney disease patients: a randomized trial. PLoS One 2015; 10:e0118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sooragonda B, Bhadada SK, Shah VN, et al. Effect of vitamin D replacement on hemoglobin concentration in subjects with concurrent iron-deficiency anemia and vitamin D deficiency: a randomized, single-blinded, placebo-controlled trial. Acta Haematol 2015; 133:31–35. [DOI] [PubMed] [Google Scholar]
- 13.Zughaier SM, Alvarez JA, Sloan JH, et al. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J Clin Transl Endocrinol 2014; 1:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med 1989; 320:980–991. [DOI] [PubMed] [Google Scholar]
- 15.Golbahar J, Altayab D, Carreon E, et al. Association of vitamin D deficiency and hyperparathyroidism with anemia: a cross-sectional study. J Blood Med 2013; 4:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo E-H, Cho H-J. Prevalence of 25-hydroxyvitamin D deficiency in Korean patients with anemia. J Clin Lab Anal 2015; 29:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim JJ, Lac PT, Liu ILA, et al. Vitamin D deficiency and anemia: a cross-sectional study. Ann Hematol 2010; 89:447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin HJ, Lee JH, Kim MK. The prevalence of vitamin D deficiency in iron-deficient and normal children under the age of 24 months. Blood Res 2013; 48:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JA, Hwang JS, Hwang IT, et al. D levels are associated with both iron deficiency and anemia in children and adolescents. Pediatr Hematol Oncol 2015; 32:99–108. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson MA, Melamed ML, Kumar J, et al. Vitamin D, race, and risk for anemia in children. J Pediatr 2014; 164:153–158e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirani V, Cumming RG, Blyth F, et al. Cross-sectional and longitudinal associations between the active vitamin D metabolite (1,25 dihydroxyvitamin D) and haemoglobin levels in older Australian men: the Concord Health and Ageing in Men Project. Age 2015; 37:9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin JY, Shim JY. Low vitamin D levels increase anemia risk in Korean women. Clin Chim Acta 2013; 421:177–180. [DOI] [PubMed] [Google Scholar]
- 23.Han SS, Kim M, Kim H, et al. Non-linear relationship between serum 25-hydroxyvitamin D and hemoglobin in Korean females: the Korean National Health and Nutrition Examination Survey 2010-2011. PLoS One 2013; 8:e72605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zittermann A, Kuhn J, Dreier J, et al. Association of 25-hydroxyvitamin D with anemia risk in patients scheduled for cardiac surgery. Int J Lab Hematol 2014; 36:29–36. [DOI] [PubMed] [Google Scholar]
- 25.Ernst JB, Becker T, Kuhn J, et al. Independent association of circulating vitamin D metabolites with anemia risk in patients scheduled for cardiac surgery. PLoS One 2015; 10:e0124751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zipf G, Chiappa M, Porter KS, et al. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat 1 2013; 56:1–37. [PubMed] [Google Scholar]
- 27.Hollowell JG, van Assendelft OW, Gunter EW, et al. Hematological and iron-related analytes: reference data for persons aged 1 year and over: United States, 1988-94. Vital Health Stat 11 2005; 247:1–156. [PubMed] [Google Scholar]
- 28.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: 2:S1–266. [PubMed] [Google Scholar]
- 29.Centers for Disease Control & Prevention. Revised analytical note for NHANES 2000–2006 and NHANES III (1988–1994) 25-hydroxyvitamin D analysis. National Health and Nutrition Examination Survey. 2010. http://www.cdc.gov/nchs/nhanes/nhanes2005–2006/VID_D.htm Accessed August 13, 2015. [Google Scholar]
- 30.Austin PC, Steyerberg EW. Graphical assessment of internal and external calibration of logistic regression models by using loess smoothers. Stat Med 2014; 33:517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 1988; 83:596–610. [Google Scholar]
- 32.Chapman and Hall/CRC, Schafer JL. Analysis of Incomplete Multivariate Data. 1997. [Google Scholar]
- 33.Nutritional Anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser 1968; 405:5–37. [PubMed] [Google Scholar]
- 34.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 2006; 107:1747–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85:649–650. [DOI] [PubMed] [Google Scholar]
- 36.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281. [DOI] [PubMed] [Google Scholar]
- 37.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 38.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014; 21:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilz S, Kienreich K, Rutters F, et al. Role of vitamin D in the development of insulin resistance and type 2 diabetes. Curr Diab Rep 2013; 13:261–270. [DOI] [PubMed] [Google Scholar]
- 40.Andersen LB, Przybyl L, Haase N, et al. Vitamin D depletion aggravates hypertension and target-organ damage. J Am Heart Assoc 2015; 4:e001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danik JS, Manson JE. Vitamin d and cardiovascular disease. Curr Treat Options Cardiovasc Med 2012; 14:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Icardi A, Paoletti E, De Nicola L, et al. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrol Dial Transplant 2013; 28:1672–1679. [DOI] [PubMed] [Google Scholar]
- 43.Kiss Z, Ambrus C, Almasi C, et al. Serum 25(OH)-cholecalciferol concentration is associated with hemoglobin level and erythropoietin resistance in patients on maintenance hemodialysis. Nephron Clin Pract 2011; 117:c373–c378. [DOI] [PubMed] [Google Scholar]
- 44.Nangaku M, Eckardt K-U. Pathogenesis of renal anemia. Semin Nephrol 2006; 26:261–268. [DOI] [PubMed] [Google Scholar]
- 45.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis 2010; 55:726–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int 2006; 69:33–43. [DOI] [PubMed] [Google Scholar]
- 47.Borges MC, Martini LA, Rogero MM. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition 2011; 27:399–404. [DOI] [PubMed] [Google Scholar]
- 48.Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010; 10:482–496. [DOI] [PubMed] [Google Scholar]
- 49.Aucella F, Scalzulli RP, Gatta G, et al. Calcitriol increases burst-forming unit-erythroid proliferation in chronic renal failure. Nephron Clin Pract 2004; 95:c121–c127. [DOI] [PubMed] [Google Scholar]


