Abstract
Osteoarticular mycoses due to non-Aspergillus moulds are uncommon and challenging infections.
A systematic literature review of non-Aspergillus osteoarticular mycoses was performed using PUBMED and EMBASE databases from 1970 to 2013.
Among 145 patients were 111 adults (median age 48.5 [16–92 y]) and 34 pediatric patients (median age 7.5 [3–15 y]); 114 (79.7%) were male and 88 (61.9%) were immunocompromised. Osteomyelitis was due to direct inoculation in 54.5%. Trauma and puncture wounds were more frequent in children (73.5% vs 43.5%; P = 0.001). Prior surgery was more frequent in adults (27.7% vs 5.9%; P = 0.025). Vertebral (23.2%) and craniofacial osteomyelitis (13.1%) with neurological deficits predominated in adults. Lower limb osteomyelitis (47.7%) and knee arthritis (67.8%) were predominantly seen in children. Hyalohyphomycosis represented 64.8% of documented infections with Scedosporium apiospermum (33.1%) and Lomentospora prolificans (15.8%) as the most common causes. Combined antifungal therapy and surgery was used in 69% of cases with overall response in 85.8%. Median duration of therapy was 115 days (range 5–730). When voriconazole was used as single agent for treatment of hyalohyphomycosis and phaeohyphomycosis, an overall response rate was achieved in 94.1% of cases.
Non-Aspergillus osteoarticular mycoses occur most frequently in children after injury and in adults after surgery. Accurate early diagnosis and long-course therapy (median 6 mo) with a combined medical-surgical approach may result in favorable outcome.
INTRODUCTION
Fungal osteomyelitis and arthritis are uncommon diseases that generally present in an indolent manner. Being one of the most challenging complications in orthopedic and trauma surgery, fungal osteoarticular infections often require complex treatments in specialized centers. The majority of these infections are caused by Aspergillus1–3 and Candida species.4,5 Other osteoarticular infections are reported with dimorphic fungi and Cryptococcus neoformans, which demonstrate distinctive clinical presentations, occur predominantly in immunocompetent patients, and develop from hematogenous dissemination.6
Opportunistic infections due to other groups of fungi are increasingly reported as potential emerging pathogens, but with limited description and relatively few reports of osteoarticular mycoses. Although a management algorithm was proposed recently for such fungal bone and joint infections,7 no comprehensive literature analysis addresses the demographic, clinical aspects, microbiology, therapy, and outcome of osteoarticular infections caused by non-Aspergillus moulds. The possible mechanisms of infection that cause osteomyelitis or arthritis are also not well documented. The portal of entry and the ability to disseminate may differ for each group of fungi. Furthermore, many clinical, diagnostic, and therapeutic questions remain uncertain.
We therefore conducted an extensive literature review to study bone and joint infections by hyaline hyphomycetes, Mucorales, and dematiaceous moulds. Using highly detailed case criteria of host factors, symptoms, physical findings, disease features, diagnostic imaging, management, and outcome, we compiled the characteristic clinical manifestations and treatment modalities of these serious invasive fungal diseases.
METHODS
Search Criteria
To identify fungal osteomyelitis and arthritis caused by hyaline hyphomycetes, Mucorales, and dematiaceous fungi, we used the OvidSP search platform in the MEDLINE and EMBASE databases using the following keywords: fungi, Ascomycota, Pseudallescheria, Chaetomium, Schizophyllum, Mucorales, mitosporic fungi, Acremonium, Alternaria, Beauveria, Chrysosporium, Cladosporium, Exophiala, Fusarium, Helminthosporium, Madurella, Phialophora, Scedosporium, Scopulariopsis, Trichoderma, Ascomycetes, Basidiomycetes, blastocladiomycota, Deuteromycetes, zygomycetes, zygomycosis, systemic mycosis, entomophthoromycosis, mucormycosis, bone diseases, bone infection, osteitis, osteomyelitis, periostitis, spondylitis, discitis, osteochondritis, osteomyelitis, periostitis, infectious arthritis, bone and joint infections, and reactive arthritis. Qatar Foundation proposal number NPRP 5-298-3-086 approved the study; the ethical approval was not necessary for the retrospective literature review nature of the research.
We retrieved a total of 2421 references published from January 1970 to September 2013. Figure 1 shows the selection process applied to identify the osteoarticular infections.
FIGURE 1.
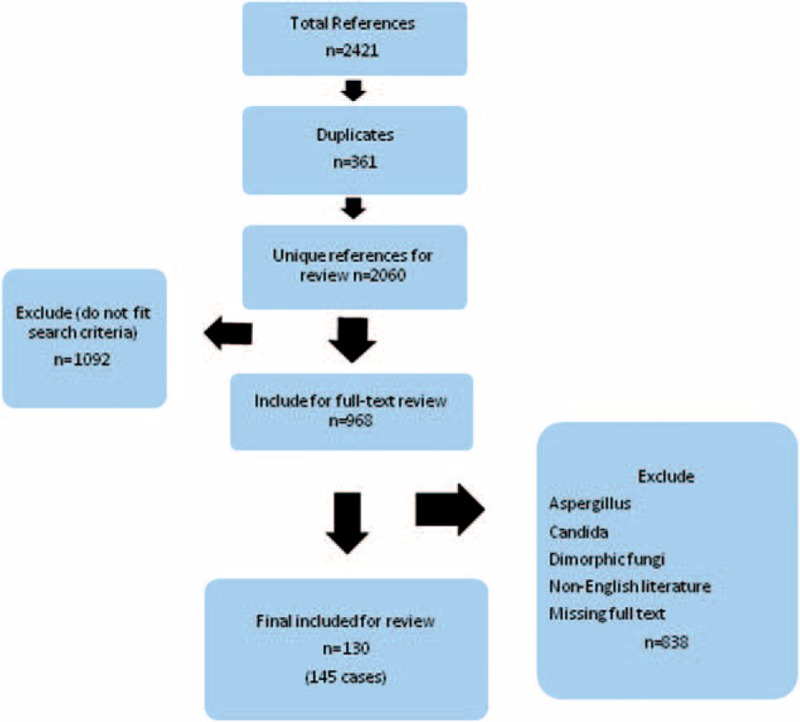
Flow diagram of search and included studies.
We included cases in the final analysis with data on osteomyelitis and or arthritis, site of infection, underlying disease, and medical and surgical therapy. Other parameters also considered in case analysis were radiological images, inflammatory markers, and disease manifestations. We excluded cases with bone extension from rhinosinusitis, outbreak of Exserohilum rostratum infections after injection of contaminated glucocorticosteroids in USA,8 as well as cases with missing full texts, and cases of non-English literature.
Data Extraction
The following parameters were extracted from each study when present: age, sex, risk factors, prior surgery, treatment, antifungal agent, duration of treatment, time to diagnosis, fever, inflammatory markers, neutropenia, radiological features, type of bone infection, surgical intervention, histopathology, microscopy, culture, fungal species, and outcome.
Synonyms of Fungi
Due to taxonomic changes since the year 1970,9–12 fungi in this study are referred with their current name: Scedosporium boydii (formerly Pseudallescheria boydii, or Petriellidium boydii, or Allescheria boydii), Scedosporium apiospermum (formerly Monosporium apiospermum, or Pseudoallescheria apiosperma), Lomentospora prolificans (formerly Scedosporium prolificans, or Scedosporium inflatum), Lichtheimia corymbifera (formerly Absidia corymbifera and Mycocladus corymbifera), and Fusarium falciforme (formerly Acremonium falciforme).
Definitions
All definitions used throughout this study were referred to the previously published definitions.1–5 The following definitions were related to the mechanism of bone infection, criteria for diagnostic probability, onset of infection, and therapeutic response.
Direct inoculation: Local bone or joint infection after a skin breakdown.
Hematogenous: Seeding to bone or joint by dissemination from a distant site of inoculation/infection.
Contiguous: Seeding to bone or joint from an adjacent infection site.
Proven fungal osteomyelitis: Evidence of a positive culture, and/or histology from bone tissue, joint fluid, or metal hardware.
Probable fungal osteomyelitis: Compatible clinical and radiological features of osteomyelitis with evidence of positive histology and/or fungal culture from an extraosteoarticular site.
De novo fungal osteomyelitis: Clinically apparent onset of fungal osteomyelitis in a patient not concomitantly receiving antifungal agents for an invasive fungal disease.
Overall response: Complete or partial resolution of clinical and radiological findings of osteomyelitis.
Children definition: Patients ≤15 years.
C-reactive protein (CRP) was elevated when >1 mg/dL; white blood cell (WBC) count was elevated when >10,000/mm3
Data Analysis and Statistical Methods
Descriptive statistics were used to summarize all demographic and clinical characteristics of the patients. Quality of data (review of completeness, data verification, and validation and accuracy of data) was assessed by the lead investigators. Quantitative variables are presented as mean ± standard deviation (SD) or as medians (with range). Differences between continuous variables of at least 2 independent groups were analyzed using unpaired t test and 1-way analysis of variance (ANOVA). When an overall group difference was found to be statistically significant, pairwise comparisons were made using the appropriate post-hoc test. Differences between categorical variables were analysed using chi-square test and Fisher exact test, as appropriate. Relationships between 2 continuous variables were further examined using Pearson correlation coefficients. Pictorial presentations of the key results were made using appropriate statistical graphs. All P values presented were 2-tailed, and P values <0.05 were considered as statistically significant. All statistical analyses were done using statistical packages SPSS 19.0 (SPSS Inc. Chicago, IL).
RESULTS
Population, Demographic Characteristics, and Comorbidities
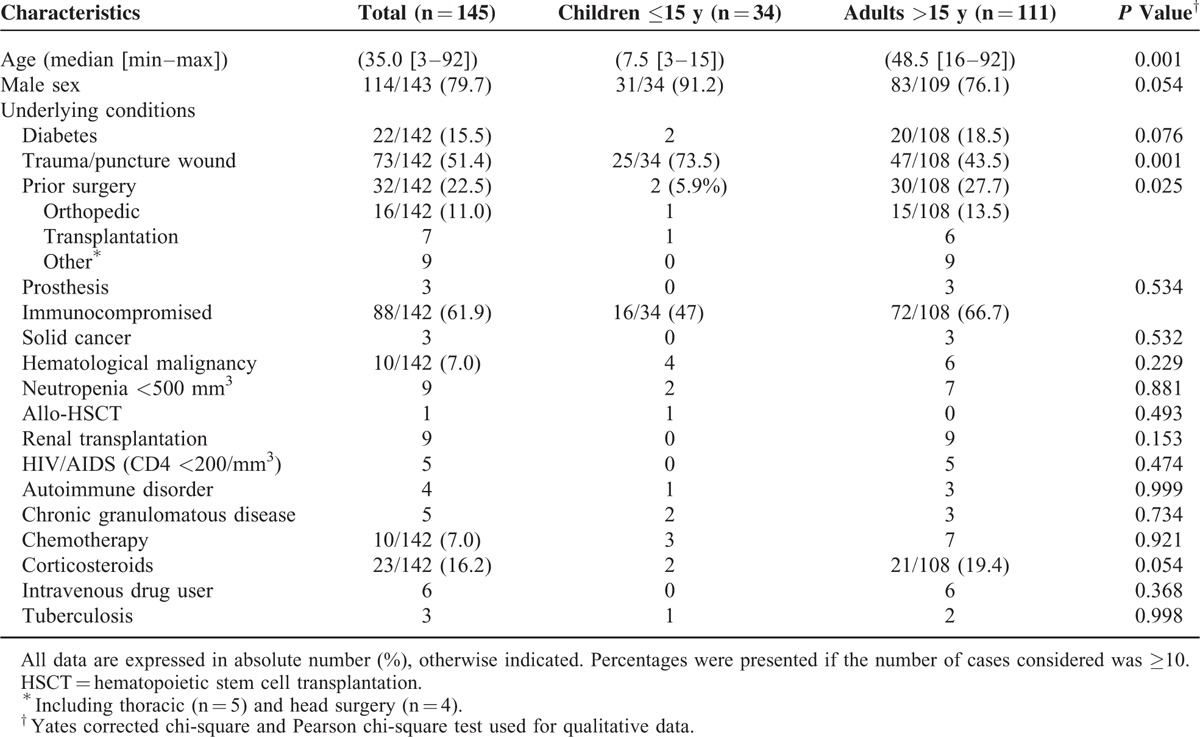
A total of 145 individual cases from 130 publications13–142 of osteoarticular infections fulfilled the definition criteria. Cases were classified as proven in 51% (n = 74) and probable in 49% (n = 71). The number of reports increased over time particularly in the adult population (Fig. 2). The demographic characteristics of the 145 cases are described in Table 1. Among the 111 adults and 34 children, male patients predominated (79.7%). The underlying conditions identified for the majority of patients included trauma or puncture wound (51.4%), prior surgery (22.5%), and diabetes (15.5%). Corticosteroid use was reported in 16.2% of the cases. Severe immunocompromised patients including solid cancer, hematological malignancy, transplantation, chronic granulomatous disease (CGD), human immunodeficiency virus (HIV/AIDS), and autoimmune disorder accounted for 61.9% (n = 88).
FIGURE 2.
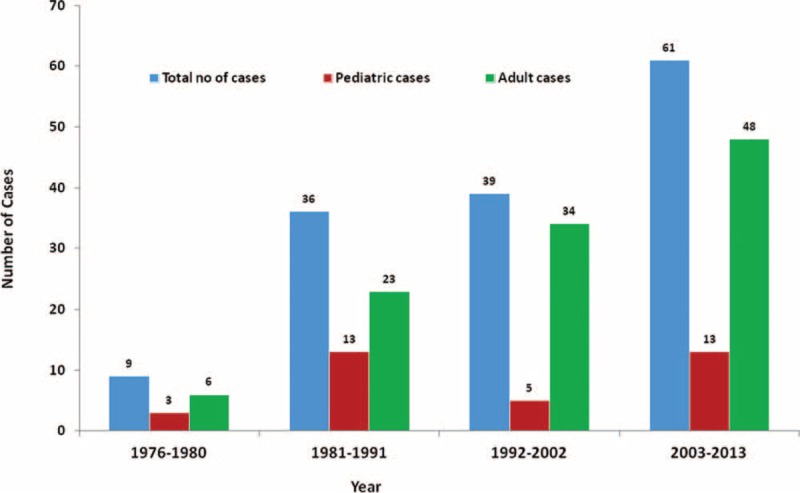
Number of osteoarticular infections caused by non-Aspergillus fungal species reported in the literature from 1970 to 2013.
TABLE 1.
Demographic Characteristic of Non-Aspergillus Filamentous Fungi Osteoarticular Infections Reported in Adults and Children Between 1970 and 2003
Etiologies and Mechanism of Infection
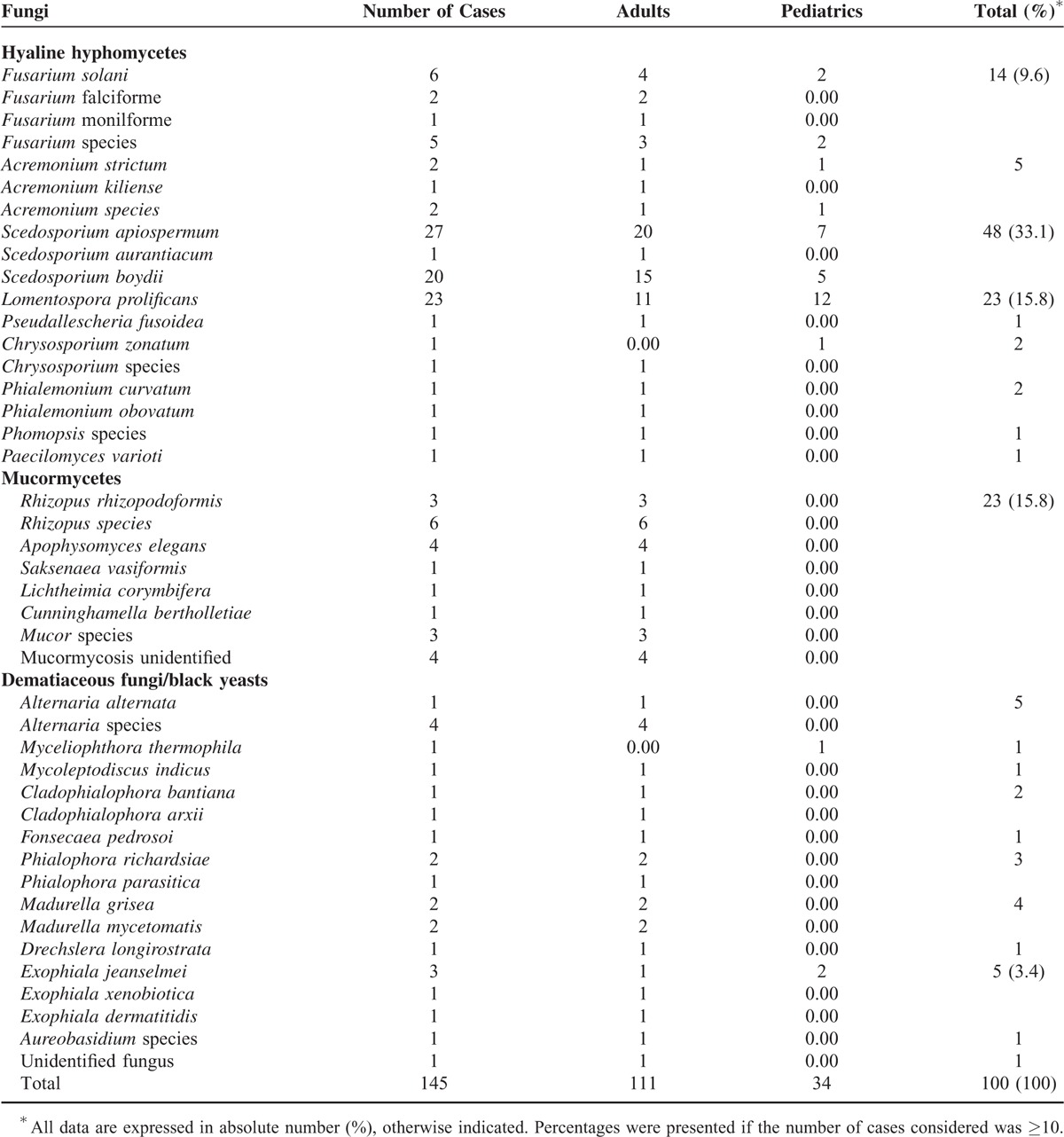
Overall, the most common groups of fungi involved in non-Aspergillus filamentous fungal osteoarticular infections were hyalohyphomycetes (n = 97), dematiaceous moulds (n = 25), and Mucorales (n = 23). The most common species involved in osteoarticular infections were S. apiospermum, L. prolificans, and S. boydii, followed by Fusarium solani. Most patients were infected with 1 fungal species. The fungal species was not specified in 30 cases (Table 2). The distribution of non-hyalohyphomycete species depended on the patient's age. Mucormycosis and the majority of phaeohyphomycosis cases were responsible for osteomyelitis in adults. Chrysosporium zonatum (hyalohyphomycosis) and Myceliophthora thermophila (phaeohyphomycosis) were isolated only in children. Hyalohyphomycoses were more frequently localized in lower limbs and axial skeleton compared with other groups of fungi.
TABLE 2.
Distribution of Filamentous Fungi Causing Osteoarticular Infections in Adult and Pediatric Patients
The main mechanism of infection was direct inoculation 79 (54.5%), especially in children (n = 27/34, 79.4%), followed by hematogenous dissemination (24.8%), and contiguous spread (20.7%). Infection by direct inoculation in children was significantly higher than that observed in adults (79.4% vs 46.8%; P = 0.0007). Contiguous types of infections were significantly higher in adult patients (P = 0.0007). No significant difference was found in hematogenous dissemination between adults and children (P > 0.5).
Among the underlying conditions in immunocompetent patients were road accidents with fracture and puncture of the knee or penetrating wounds. Figure 3 shows a significant association between injury and types of apparent infections (P < 0.001). The highest risk surgical procedure was orthopedic followed by transplantation (Table 1). Prior surgery was significantly more frequent in adults (27.7% vs 5.9%; P = 0.025).
FIGURE 3.
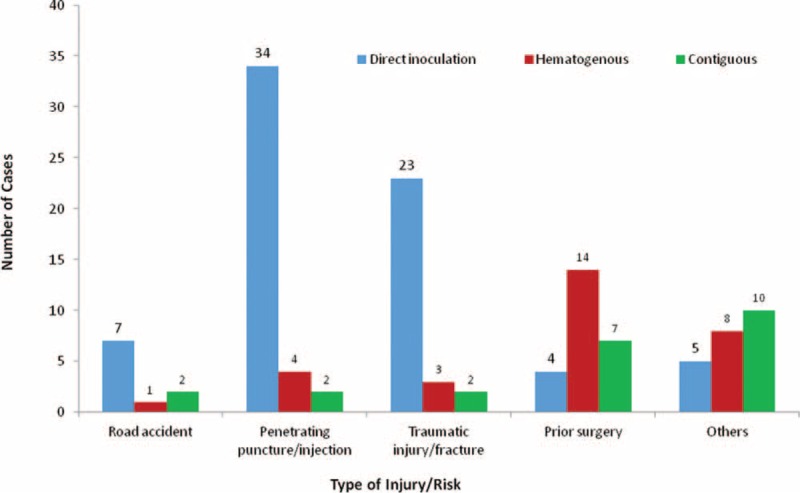
Significant association between injury and types of osteoarticular infections due to non-Aspergillus filamentous fungi (Overall P < 0.001).
Diagnostic Procedures and Cocultured Bacteria
Median time to diagnosis from onset of symptoms was 90 (7–1825) days. Among 139 diagnostic procedures, diagnosis of fungal osteomyelitis was performed by open biopsy in 96 (69%), percutaneous biopsy in 31 (22.3%), arthroscopy in 7 (5%), and other surgical procedures in 5 (3.6%). From these specimens, fungi were detected by culture, direct microscopy, and histology. Bacteria were cocultured from the same specimen in 22 cases; documented bacteria included Staphylococcus aureus followed by Gram-negative organisms, including Escherichia coli and Pseudomonas aeruginosa (data not shown).
Clinical and Laboratory Features
The most frequently reported clinical manifestations were local pain and tenderness (69.0%), local inflammatory signs (44.1%), and restricted movements (53.5%). Children presented with significantly more local inflammatory signs than adults, 76.4% vs 34.2% (P = 0.0001). Fever was reported in only 31.5% of patients, significantly more frequently in the pediatric patients than in adults (61.2% vs 22.2%; P = 0.001). Dissemination of the infection was documented in 21/145(14.4%) patients, including 12 (57.1%) immunocompromised patients. De novo fungal osteoarticular infections occurred in 93.1% (n = 135) of the patients (Table 3).
TABLE 3.
Clinical Characteristics and Anatomical Distribution of Osteoarticular Infections due to Non-Aspergillus Filamentous Fungi Reported in the Literature From 1970 to 2013

Among 99 cases of osteomyelitis caused by non-Aspergillus moulds, the most commonly involved sites were the lower limbs, particularly the foot (25.3%), vertebrae (23.2%), and the skull (13.1%). The lower limbs were more frequently involved in children than in adults (78.9% vs. 40.0%; P = 0.001). Vertebral osteomyelitis arose from hematogenous spread from pulmonary infections or occasionally by direct inoculation. Vertebral destruction/compression or cranial osteomyelitis led to neurological deficits in 8 and 9 cases, respectively. All such cases occurred in adult patients (n = 15, 10.3%). Among 56 cases of septic arthritis caused by non-Aspergillus filamentous fungi, the most common joint infected was the knee (67.8%) in both adults and children.
Elevated biomarkers of inflammation were detected in most tested patients. The median CRP value was 45 mg/dL (1.1–362), which was elevated to >5 mg/dL in 87.8% (n = 33) and to >1.0 mg/dL in 100% of tested patients. Significantly higher mean values of CRP were reported for pediatric patients than adult patients (110.2 ± 130.1 vs 46.7 ± 39.0; P = 0.034). An elevated WBC count was observed in 47.5% of the patients (n = 40), although the median value was 9850 cells/mm3 (1900–33,500).
Diagnostic Imaging
The radiological abnormalities seen by different radiological techniques included osteolytic lesions, lucencies, vertebral compressions, abscesses, and increase of radionuclide uptake. Epidural, paraspinal, and psoas abscesses were detected only in adult patients. Magnetic resonance imaging (MRI) showed decreased signal intensity on T1-weighted images, as well as increased signal intensity on T2-wieghted images. Vertebral compression or decreased intervertebral space detected by plain radiographs was most commonly encountered in adult patients (Table 3).
Treatment and Outcome
Surgical intervention and/or medical therapy was reported in 137 (94.5%) patients (Table 4). The majority of patients (69.3%) were treated with antifungal agents and surgery, 25.5% with antifungal agents only, and 5% (n = 7) with surgical treatment only. Amphotericin B (AmB) and voriconazole (VRC) were the 2 most commonly used antifungal agents in both children and adults. Combinations of antifungal therapy were reported in 25.4% of patients.
TABLE 4.
Treatment Strategy and Outcome of Non-Aspergillus Fungal Osteoarticular Infections in Pediatric and Adult Patients Reported in the Literature From 1970 to 2013
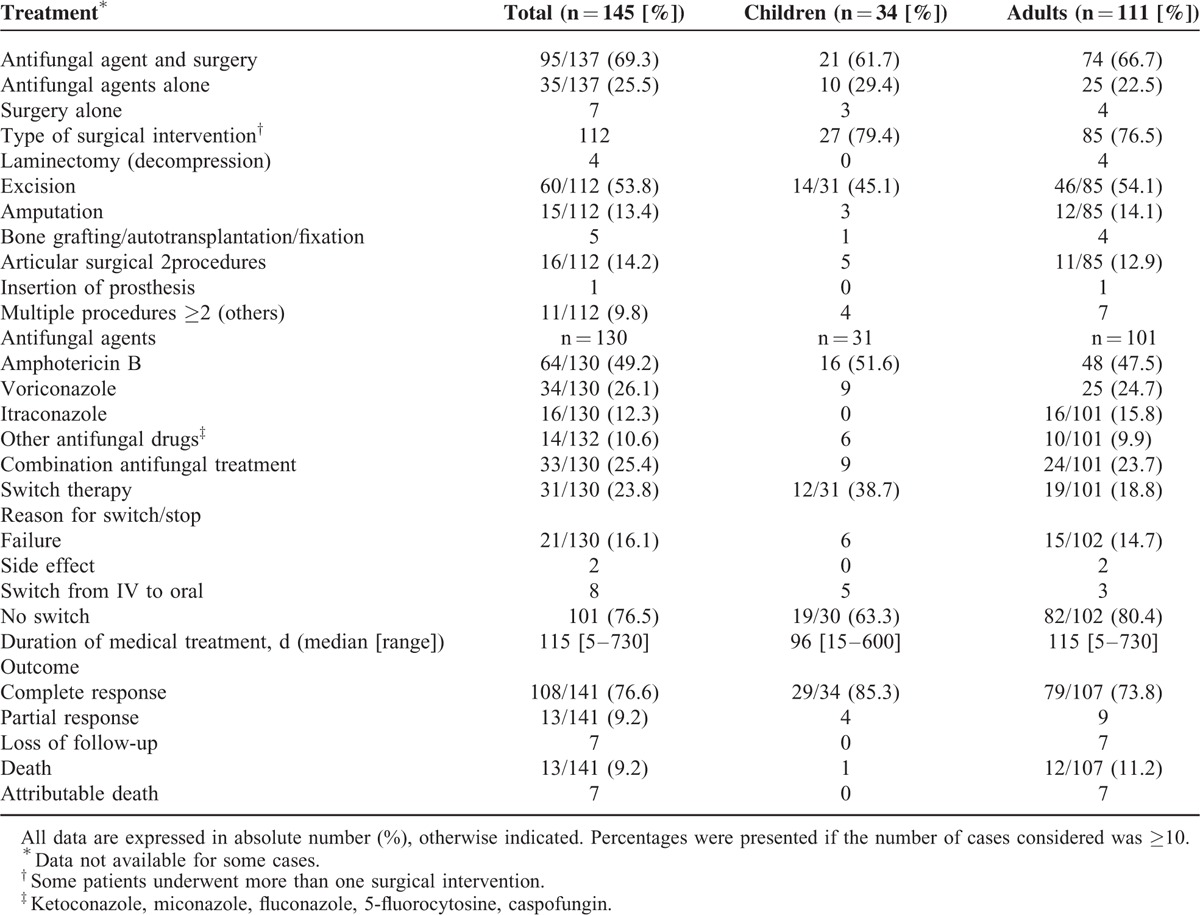
Hyalohyphomycosis was treated with AmB and VRC in 36 and 30 cases, respectively; overall responses included 3 deaths. All cases of mucormycosis were treated with AmB; treatment failure leading to death was noticed in 3 cases. Patients with phaeohyphomycosis were treated with AmB (n = 8), itraconazole (IT) (n = 9), and VRC (n = 3); 1 attributable death due to Exophiala jeanselmei was reported with AmB. A switch therapy was observed in 31 (23.5%) patients mainly due to treatment failure (15.9%). Switch therapy includes VRC, IT, posaconazole (POS) + terbinafine, or VRC + terbinafine.
Excision was the most common surgical intervention (52.6%), followed by articular surgical procedures (14%), amputation (13.1), and bone grafting. Regional infusion of antifungal agents was performed in 9 patients.
Median duration of medical treatment was 115 (5–730) days (Table 4). An overall response rate of 85.8% was achieved in the treatment of 141 non-Aspergillus mould osteomyelitis. complete response was reported in 108 (76%) patients, partial response in 13 (9.2%), and loss of follow-up in 7. Survival was 90.8% and overall mortality rate was 9.2% (n = 13/141). Irrespective of antifungal treatments, death was attributed to fungal osteoarticular infections in 7 adult patients.
DISCUSSION
Reviewing 145 cases of fungal osteomyelitis and arthritis caused by non-Aspergillus filamentous fungi over 43 years, we found 43 fungal species belonging to 25 fungal genera of opportunistic and uncommon pathogens in immunocompetent and immunocompromised patients. This study displayed that 38.1% of osteoarticular infections due to these fungi occurred in apparently immunocompetent patients. Among the non-Aspergillus moulds, hyalohyphomycetes are the major group reported to cause osteoarticular infections. Two main pathogenic mechanisms of infection were observed: the first one was a community-acquired infection by direct inoculation during trauma, and the second one was a healthcare-associated infection after surgical procedures. Osteoarticular infections due to non-Aspergillus filamentous fungi were most of the times de novo infections and the rate of secondary dissemination was low. Fever was not a good clinical sign of osteoarticular infection, but local pain and inflammatory signs should alert the clinicians. Despite a low mortality rate, these osteoarticular infections were difficult to treat, and led to sequelae, especially when axial skeleton was involved.
As with Aspergillus and Candida osteoarticular infections,1–5 there is a high male predominance with >3.9:1 male-to-female ratio, and there were significant differences between the pediatric (≤15 y) and adult (>15 y) populations. In particular, trauma and puncture wounds, as well as lower limbs infections, were significantly more frequent in pediatric patients. Although there was no significant difference in the number of immunocompromised patients, adults had less clinical signs such as fever, movement limitation, or local inflammatory signs than children. Corticosteroids, which were mostly used in adults, could have masked the clinical signs in part of the adult population due to their anti-inflammatory effects. Another explanation could be driven by the differences in fungal species between children and adults. Hyalohyphomycosis predominated in children, whereas phaeohyphomycosis and mucormycosis were uncommon or absent in pediatric patients. Vertebral osteomyelitis occurred mainly in adults and was associated with neurological deficitsas in Aspergillus osteomyelitis.1
Biological markers may be useful in diagnosis and follow-up of some osteoarticular mycoses. Our findings revealed that an elevated level of CRP may support the clinical findings for early diagnosis of non-Aspergillus mould osteoarticular infections, whereas WBC count was minimally elevated. Unlike Candida osteomyelitis,5 CRP also may be useful for therapeutic monitoring of osteoarticular infections caused by non-Aspergillus spp.1
Surgical debridement, irrigation, and drainage of joints, combined with antifungal therapy, is widely considered the standard treatment option of fungal osteomyelitis and joint infections.143It is worthwhile mentioning that the majority of patients with Aspergillus (67%) and Candida (48%) osteoarticular infections received combined antifungal therapy and surgery.1,5
The treatment strategy for bone and joint infections due to non-Aspergillus filamentous fungi in the present study combined surgical and antifungal therapy in most cases. Therapeutic success, however, also depended upon the etiologic agent, the severity of the disease, type and location of infected bone, and the choice of antifungal agent. For example, S. apiospermum is more susceptible to antifungal agents than is L. prolificans. S. apiospermum, the related species L. prolificans, and other hyalohyphomycetes constituted 48.9% of osteoarticular infections. Treatment of osteomyelitis due to S. apiospermum often relies on a combination of antifungal therapy, surgical procedures, and occasionally amputation, when a poor prognosis is expected.41,59 The most potent in vitro activity has been observed with VRC.144,145 VRC, which was initially introduced in year 2001, has been used with good clinical results against S. apiospermum infections in immunocompromised patients, mostly for pulmonary and soft-tissue infections,146,147 and was recommended in the therapeutic standard in the European guidelines.148 Moreover, this agent has proved to be effective in treating Acremonium osteomyelitis,22and it has been shown to be effective in the treatment of other cases of S. apiospermum osteomyelitis where surgical amputation was avoided.32
As the antifungal susceptibility profile for L. prolificans showed higher resistance than S. apiospermum,144,145 identification of the fungus (from bone specimen) to the species level is important for the management of osteoarticular infections. The emergence of Scedosporium aurantiacum84and another related fungus, Pseudallescheria fusoidea,37 as new etiological agents of osteomyelitis, indicates that this group is an emerging cause of serious invasive diseases of bone in both immunocompetent and immunocompromised patients, and should be considered in the differential diagnosis of fungal osteomyelitis. In such cases, negative culture of synovial biopsy should be treated with a great deal of care, and a proper specimen based on bone biopsy is highly recommended. In addition, Scedosporium and Fusarium spp. are morphologically similar in histopathology sections.149 However, identification to species level is important to plan treatment strategy, as susceptibility to antifungal drugs is not similar.144,145,150
Osteoarticular mucormycosis constitutes a serious diagnostic and therapeutic challenge. Among 23 cases of osteomyelitis due to mucormycosis, all were treated with AmB and 3 had an unfavorable outcome. Osteoarticular mucormycosis is a highly destructive infection with poor prognosis, if not diagnosed early.151
Fusarium is highly refractory to treatment by antifungal agents with partial response and treated with either AmB17 or VRC.14 Susceptibility to antifungal agents is species specific.152 Koehler et al,7 based on combined treatment with VRC and AmB, suggested a management algorithm for treatment of Fusarium osteomyelitis.
The treatment option of these groups of fungi is based on combination of surgery and antifungal therapy in adults and pediatrics. It is interesting to note a considerably higher proportion of patients receiving the combination of surgery and antifungal therapy with a good survival rate (90.8%). Our findings also are consistent with case series by Koehler et al,7 who reported 61 cases of non-Aspergillus bone and joint infections, with a survival rate of 88%.
Unlike Candida and Aspergillus osteoarticular infections, which were reported as a result of hematogenous spread,1,5 direct inoculation is the cause of infection for the majority of non-Aspergillus filamentous fungal species reported herein. Patients with trauma, wounds, and punctures in this study comprised 51.4% of all cases of fungal osteoarticular infections. It constitutes the key risk factor of underlying conditions for development of knee arthritis and lower-limb osteomyelitis. Fungi rarely cause disease in healthy immunocompetent hosts. Disease results when fungi accidentally penetrate host barriers or when immunologic defects or other debilitating conditions exist, which favor fungal entry and infection. The etiologic agents gain entrance through transcutaneous puncture wounds, usually by a thorn or a splinter, or other kinds of trauma, such as road accident fractures, can be identified as the portal of entry of the fungus.153 Fungal spores may gain entrance to the host's soft tissue via plant fragments, or by injection with foreign body; the topography of the lesions on lower limbs, that is, feet and legs is typical presentation of direct inoculation particularly in pediatric patients of this study. A primary lesion can evolve to polymorphic skin lesions, including tenderness, swollen, erythema, or drainage sinus/abscess, which slowly enlarges and progress to osteomyelitis or arthritis.
This study identified previously unreported distinctions in the clinical manifestations, etiology, pathogenesis, and laboratory features between pediatric and adult patients with non-Aspergillus mould osteoarticular infections. Children presented with significantly more local signs than adults. Fever was present in twice the number of children than in adults. Epidural, paraspinal, and psoas abscesses were detected only in adult cases. As a preceding event, trauma and puncture wounds were nearly twice more frequent in children, whereas prior surgery was found more than 4 times more often in adults. Whereas lower-limb osteomyelitis and knee arthritis predominantly occurred in children, vertebral and craniofacial osteomyelitis with neurological deficits were common in adults. Infection by direct inoculation was a more frequent mechanism of infection in children than in adults. Finally, osteoarticular mucormycosis and phaeohyphomycosis predominated in adults, possibly as a reflection of a greater frequency of diabetes mellitus and immune impairment.
This study has several limitations. It is retrospective and thus will not capture all cases. There is the potential for publication bias, suggesting that cases that may have more favorable outcome are more likely to be published than those with poor outcome. Cognizant of these limitations, we believe that this study is the largest compilation and most detailed analysis of osteoarticular infections caused by medically important non-Aspergillus filamentous fungi. Moreover, due to the scarcity of data on these uncommon filamentous fungi causing osteoarticular infections, we believe that the case reports included in this study are likely representative enough of other cases. The data analysis of this study was based on detailed parameters of each single case to permit a high degree of analysis of several variables. Whereas an early study of literature reviewing 61 case series was highly informative in respect to detailed etiology and treatment, it lacked the numerical data for analysis of detailed clinical parameters, laboratory markers, as well as reporting only ten pediatric (≤15 y) patients.7
Non-Aspergillus osteoarticular mycoses occur most frequently in children after injury and in adults after surgery. These fungi can cause a serious illness and more virulent in individuals with impaired immune systems. Accurate early diagnosis and long-course therapy (median 6 mo) with a combined medical-surgical approach may result in favorable outcome.
Footnotes
Abbreviations: AmB = amphotericin B, ANOVA = analysis of variance, CGD = chronic granulomatous disease, CRP = C-reactive protein, HIV = human immunodeficiency virus, IT = itraconazole, MRI = magnetic resonance imaging, POS = posaconazole, VRC = voriconazole, WBC = white blood cell.
Funding: Supported by Grant NPRP 5-298-3-086 from the Qatar National Research Fund (a member of Qatar Foundation) to Saad J. Taj-Aldeen.
Disclosures: Walsh receives support from the Henry Schueler Foundation (Scholar in Mucormycosis), Sharpe Family Foundation (Scholar in Pediatric Infectious Diseases), and from the Save Our Sick Kids Foundation.
DPK is a consultant and board member and received payment for lectures from Schering-Plough, Pfizer, and Astellas Pharma US, and has received grant support from Astellas Pharma US and Merck; and has received honorarium from Enzon Pharmaceuticals. Walsh has received research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Cubist, Novartis, Pfizer, and Theravance. He has served as consultant to Astellas, ContraFect, Cubist, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. OL is a Consultant for Gilead Sciences; boards or speaker's bureau Pfizer, Astellas, Merck. Other authors have no declarations.
REFERENCES
- 1.Gamaletsou MN, Rammaert B, Bueno MA, et al. Aspergillus osteomyelitis: epidemiology, clinical manifestations, management, and outcome. J Infect 2014; 68:478–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrielli E, Fothergill AW, Brescini L, et al. Osteomyelitis caused by Aspergillus species: a review of 310 reported cases. Clin Microbiol Infect 2014; 20:559–565. [DOI] [PubMed] [Google Scholar]
- 3.Koehler P, Tacke D, Cornely OA. Aspergillosis of bones and joints: a review from 2002 until today. Mycoses 2014; 57:323–335. [DOI] [PubMed] [Google Scholar]
- 4.Slenker AK, Keith SW, Horn DL. Two hundred and eleven cases of Candida osteomyelitis: 17 case reports and a review of the literature. Diag Microbiol Infect Dis 2012; 73:89–93. [DOI] [PubMed] [Google Scholar]
- 5.Gamaletsou MN, Kontoyiannis DP, Sipsas NV, et al. Candida osteomyelitis: analysis of 207 pediatric and adult cases (1970–2011). Clin Infect Dis 2012; 55:1338–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rammaert B, Gamaletsou MN, Zeller V, et al. Dimorphic fungal osteoarticular infections. Eur J Clin Microbiol Infect Dis 2014; 33:2131–2140. [DOI] [PubMed] [Google Scholar]
- 7.Koehler P, Tacke D, Cornely OA. Bone and joint infections by Mucorales, Scedosporium, Fusarium and even rarer fungi. Crit Rev Microbiol 2014; 9:1–14. [DOI] [PubMed] [Google Scholar]
- 8.Malani AN, Vandenberg DM, Singal B, et al. Magnetic resonance imaging screening to identify spinal and paraspinal infections associated with injections of contaminated methylprednisolone acetate. JAMA 2013; 309:2465–2472. [DOI] [PubMed] [Google Scholar]
- 9.Lackner M, Hagen F, Meis JF, et al. Susceptibility and diversity in the therapy-refractory genus Scedosporium. Antimicrob Agents Chemother 2014; 58:5877–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Hermoso D, Hoinard D, Gantier JC, et al. Molecular and phenotypic evaluation of Lichtheimia corymbifera (formerly Absidia corymbifera) complex isolates associated with human mucormycosis: rehabilitation of L. ramosa. J Clin Microbiol 2009; 47:3862–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summerbell RC, Schroers HJ. Analysis of phylogenetic relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani species complex and a review of similarities in the spectrum of opportunistic infections caused by these fungi. J Clin Microbiol 2002; 40:2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Hoog GS, Haase G, Chaturvedi V, et al. Taxonomy of medically important fungi in the molecular era. Lancet Infect Dis 2013; 13:385–386. [DOI] [PubMed] [Google Scholar]
- 13.Edupuganti S, Rouphael N, Mehta A, et al. Fusarium falciforme vertebral abscess and osteomyelitis: case report and molecular classification. J Clin Microbiol 2011; 49:2350–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sierra-Hoffman M, Paltiyevich-Gibson S, Carpenter JL, et al. Fusarium osteomyelitis: case report and review of the literature. Scand J Infect Dis 2005; 37:237–240. [DOI] [PubMed] [Google Scholar]
- 15.Bourguignon RL, Walsh AF, Flynn JC, et al. Fusarium species osteomyelitis. Case report. J Bone Joint Surg Am 1976; 58:722–723. [PubMed] [Google Scholar]
- 16.Page JC, Friedlander G, Dockery GL. Postoperative Fusarium osteomyelitis. J Foot Surg 1982; 21:174–176. [PubMed] [Google Scholar]
- 17.Nuovo MA, Simmonds JE, Chacho MS, et al. Fusarium solani osteomyelitis with probable nosocomial spread. Am J Clin Pathol 1988; 90:738–741. [DOI] [PubMed] [Google Scholar]
- 18.Brint JM, Flynn PM, Pearson TA, et al. Disseminated fusariosis involving bone in an adolescent with leukemia. Pediatr Infect Dis J 1992; 11:965–968. [DOI] [PubMed] [Google Scholar]
- 19.Bader M, Jafri AK, Krueger T, et al. Fusarium osteomyelitis of the foot in a patient with diabetes mellitus. Scand J Infect Dis 2003; 35:895–896. [DOI] [PubMed] [Google Scholar]
- 20.Wu CY, Chen GS, Lan CC. Onychomycosis caused by Fusarium solani in a woman with diabetes. Clin Exp Dermatol 2009; 34:e772–e774. [DOI] [PubMed] [Google Scholar]
- 21.Gradon JD, Lerman A, Lutwick LI. Septic arthritis due to Fusarium moniliforme. Rev Infect Dis 1990; 12:716–717. [DOI] [PubMed] [Google Scholar]
- 22.Moschovi M, Trimis G, Anastasopoulos J, et al. Subacute vertebral osteomyelitis in a child with diabetes mellitus associated with Fusarium. Pediatr Int 2004; 46:740–742. [DOI] [PubMed] [Google Scholar]
- 23.Keynan Y, Sprecher H, Weber G. Acremonium vertebral osteomyelitis: molecular diagnosis and response to voriconazole. Clin Infect Dis 2007; 45:e5–e6. [DOI] [PubMed] [Google Scholar]
- 24.Beaudreuil S, Buchler M, Al Najjar A, et al. Acute septic arthritis after kidney transplantation due to Acremonium. Nephrol Dial Transplant 2003; 18:850–851. [DOI] [PubMed] [Google Scholar]
- 25.Noble RC, Salgado J, Newell SW, et al. Endophthalmitis and lumbar diskitis due to Acremonium falciforme in a splenectomized patient. Clin Infect Dis 1997; 24:277–278. [DOI] [PubMed] [Google Scholar]
- 26.Bassiri-Jahromi S, Doostkam A. Fungal infection and increased mortality in patients with chronic granulomatous disease. J Mycol Med 2012; 22:52–57. [DOI] [PubMed] [Google Scholar]
- 27.Charles JF, Eberle C, Daikh DI, et al. Resolution of recurrent Fusarium arthritis after prolonged antifungal therapy. J Clin Rheumatol 2011; 17:44–45. [DOI] [PubMed] [Google Scholar]
- 28.Miyakis S, Velegraki A, Delikou S, et al. Invasive Acremonium strictum infection in a bone marrow transplant recipient. Pediatr Infect Dis J 2006; 25:273–275. [DOI] [PubMed] [Google Scholar]
- 29.Szombathy SP, Chez MG, Laxer RM. Acute septic arthritis due to Acremonium. J Rheumatol 1988; 15:714–715. [PubMed] [Google Scholar]
- 30.Brabender W, Ketcherside J, Hodges GR, et al. Acremonium kiliense osteomyelitis of the calvarium. Neurosurgery 1985; 16:554–556. [PubMed] [Google Scholar]
- 31.Hell M, Neureiter J, Wojna A, et al. Post-traumatic Pseudallescheria apiosperma osteomyelitis: positive outcome of a young immunocompetent male patient due to surgical intervention and voriconazole therapy. Mycoses 2011; 54 Suppl 3:43–47. [DOI] [PubMed] [Google Scholar]
- 32.Gompels MM, Bethune CA, Jackson G, et al. Scedosporium apiospermum in chronic granulomatous disease treated with an HLA matched bone marrow transplant. J Clin Pathol 2002; 55:784–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porte L, Khatibi S, Hajj LE, et al. Scedosporium apiospermum mycetoma with bone involvement successfully treated with voriconazole. Trans R Soc Trop Med Hyg 2006; 100:891–894. [DOI] [PubMed] [Google Scholar]
- 34.Stripeli F, Pasparakis D, Velegraki A, et al. Scedosporium apiospermum skeletal infection in an immunocompetent child. Med Mycol 2009; 47:441–444. [DOI] [PubMed] [Google Scholar]
- 35.Gottesman-Yekutieli T, Shwartz O, Edelman A, et al. Pseudallescheria boydii infection of a prosthetic hip joint--an uncommon infection in a rare location. Am J Med Sci 2011; 342:250–253. [DOI] [PubMed] [Google Scholar]
- 36.Cetrulo CL, Jr, Leto Barone AA, Jordan K, et al. A multi-disciplinary approach to the management of fungal osteomyelitis: current concepts in post-traumatic lower extremity reconstruction: a case report. Microsurgery 2012; 32:144–147. [DOI] [PubMed] [Google Scholar]
- 37.Lindsley MD, Guarro J, Khairy RN, et al. Pseudallescheria fusoidea, a new cause of osteomyelitis. J Clin Microbiol 2008; 46:2141–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanafani ZA, Comair Y, Kanj SS. Pseudallescheria boydii cranial osteomyelitis and subdural empyema successfully treated with voriconazole: a case report and literature review. Eur J Clin Microbiol Infect Dis 2004; 23:836–840. [DOI] [PubMed] [Google Scholar]
- 39.Sydnor MK, Kaushik S, Knight TE, Jr, et al. Mycotic osteomyelitis due to Scedosporium apiospermum: MR imaging-pathologic correlation. Skeletal Radiol 2003; 32:656–660. [DOI] [PubMed] [Google Scholar]
- 40.Studahl M, Backteman T, Stalhammar F, et al. Bone and joint infection after traumatic implantation of Scedosporium prolificans treated with voriconazole and surgery. Acta Paediatr 2003; 92:980–982. [DOI] [PubMed] [Google Scholar]
- 41.Steinbach WJ, Schell WA, Miller JL, et al. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J Clin Microbiol 2003; 41:3981–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piper JP, Golden J, Brown D, et al. Successful treatment of Scedosporium apiospermum suppurative arthritis with itraconazole. Pediatr Infect Dis J 1990; 9:674–675. [PubMed] [Google Scholar]
- 43.Levine NB, Kurokawa R, Fichtenbaum CJ, et al. An immunocompetent patient with primary Scedosporium apiospermum vertebral osteomyelitis. J Spinal Disord Tech 2002; 15:425–430. [DOI] [PubMed] [Google Scholar]
- 44.Dellestable F, Kures L, Mainard D, et al. Fungal arthritis due to Pseudallescheria boydii (Scedosporium apiospermum). J Rheumatol 1994; 21:766–768. [PubMed] [Google Scholar]
- 45.Malekzadeh M, Overturf GD, Auerbach SB, et al. Chronic, recurrent osteomyelitis caused by Scedosporium inflatum. Pediatr Infect Dis J 1990; 9:357–359. [DOI] [PubMed] [Google Scholar]
- 46.Mesfin FB, Tobin E, Adamo MA, et al. Fungal vertebral osteomyelitis due to Scedosporium apiospermum after near-drowning. J Neurosurg Spine 2008; 9:58–61. [DOI] [PubMed] [Google Scholar]
- 47.German JW, Kellie SM, Pai MP, et al. Treatment of a chronic Scedosporium apiospermum vertebral osteomyelitis. Case report. Neurosurg 2004; 17:E9. [DOI] [PubMed] [Google Scholar]
- 48.Taj-Aldeen SJ, Taj-Aldeen WS, Guarro J, et al. Osteomyelitis caused by Scedosporium apiospermum in immunocompetent patient. J Invasive Fungal Infect 2008; 2:96–99. [Google Scholar]
- 49.Kesson AM, Bellemore MC, O’Mara TJ, et al. Scedosporiumprolificans osteomyelitis in an immunocompetent child treated with a novel agent, hexadecylphospocholine (miltefosine), in combination with terbinafine and voriconazole: a case report. Clin Infect Dis 2009; 48:1257–1261. [DOI] [PubMed] [Google Scholar]
- 50.Howden BP, Slavin MA, Schwarer AP, et al. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur J Clin Microbiol Infect Dis 2003; 22:111–113. [DOI] [PubMed] [Google Scholar]
- 51.Ginter G, de Hoog GS, Pschaid A, et al. Arthritis without grains caused by Pseudallescheria boydii. Mycoses 1995; 38:369–371. [DOI] [PubMed] [Google Scholar]
- 52.Ong A, Blyth CC, Bency R, et al. Fatal mycotic aneurysms due to Scedosporium and Pseudallescheria infection. J Clin Microbiol 2011; 49:2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guignard S, Hubert D, Dupont B, et al. Multifocal Scedosporium apiospermum spondylitis in a cystic fibrosis patient. J Cyst Fibros 2008; 7:89–91. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Vidal C, Cabellos C, Ayats J, et al. Fungal postoperative spondylodiscitis due to Scedosporium prolificans. Spine J 2009; 9:e1–e7. [DOI] [PubMed] [Google Scholar]
- 55.Lonser RR, Brodke DS, Dailey AT. Vertebral osteomyelitis secondary to Pseudallescheria boydii. J Spinal Disord 2001; 14:361–364. [DOI] [PubMed] [Google Scholar]
- 56.Ochiai N, Shimazaki C, Uchida R, et al. Disseminated infection due to Scedosporium apiospermum in a patient with acute myelogenous leukemia. Leuk Lymphoma 2003; 44:369–372. [DOI] [PubMed] [Google Scholar]
- 57.Li JY, Yong TY, Grove DI, et al. Successful control of Scedosporium prolificans septic arthritis and probable osteomyelitis without radical surgery in a long-term renal transplant recipient. Transpl Infect Dis 2008; 10:63–65. [DOI] [PubMed] [Google Scholar]
- 58.Matlani M, Kaur R, Shweta A case of Scedosporium prolificans osteomyelitis in an immunocompetent child, misdiagnosed as tubercular osteomyelitis. Indian J Dermatol 2013; 58:80–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung LH, Norwood LA. Osteomyelitis due to Pseudallescheria boydii. South Med J 1993; 86:231–234. [DOI] [PubMed] [Google Scholar]
- 60.Tirado-Miranda R, Solera-Santos J, Brasero JC, et al. Septic arthritis due to Scedosporium apiospermum: case report and review. J Infect 2001; 43:210–212. [DOI] [PubMed] [Google Scholar]
- 61.Dalton PA, Munckhof WJ, Walters DW. Scedosporium prolificans: an uncommon cause of septic arthritis. ANZ J Surg 2006; 76:661–663. [DOI] [PubMed] [Google Scholar]
- 62.Pickles RW, Pacey DE, Muir DB, et al. Experience with infection by Scedosporium prolificans including apparent cure with Fluconazole therapy. J Infect 1996; 33:193–197. [DOI] [PubMed] [Google Scholar]
- 63.Vasoo S, Yeo SB, Lim PL, et al. Efficacy of voriconazole for Scedosporium apiospermum skull base osteomyelitis: case report and literature review. Int J Antimicrob Agents 2008; 31:184–185. [DOI] [PubMed] [Google Scholar]
- 64.Busaba NY, Poulin M. Invasive Pseudallescheria boydii fungal infection of the temporal bone. Otolaryngol Head Neck Surg 1997; 117:S91–94. [DOI] [PubMed] [Google Scholar]
- 65.Gatto J, Paterson D, Davis L, et al. Vertebral osteomyelitis due to Pseudallescheria boydii. Pathology 1997; 29:238–240. [DOI] [PubMed] [Google Scholar]
- 66.Holmes NE, Trevillyan JM, Kidd SE, et al. Locally extensive angio-invasive Scedosporium prolificans infection following resection for squamous cell lung carcinoma. Med Mycol Case Rep 2013; 2:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanhooteghem O, Gillard P, Dezfoulian B, et al. Scedosporium apiospermum septicemia following a wedge excision of an ingrown toenail. Int J Dermatol 2009; 48:1137–1139. [DOI] [PubMed] [Google Scholar]
- 68.Gosbell IB, Toumasatos V, Yong J, et al. Cure of orthopaedic infection with Scedosporium prolificans, using voriconazole plus terbinafine, without the need for radical surgery. Mycoses 2003; 46:233–236. [DOI] [PubMed] [Google Scholar]
- 69.Angelini A, Drago G, Ruggieri P. Post-tsunami primary Scedosporium apiospermum osteomyelitis of the knee in an immunocompetent patient. Int J Infect Dis 2013; 17:e646–e649. [DOI] [PubMed] [Google Scholar]
- 70.Frazier DD, Campbell DR, Garvey TA, et al. Fungal infections of the spine. Report of eleven patients with long-term follow-up. J Bone Joint Surg Am 2001; 560–565.83-A. [PubMed] [Google Scholar]
- 71.Wilson CM, O’Rourke EJ, McGinnis MR, et al. Scedosporium inflatum: clinical spectrum of a newly recognized pathogen. J Infect Dis 1990; 161:102–107. [DOI] [PubMed] [Google Scholar]
- 72.Wood GM, McCormack JG, Muir DB, et al. Clinical features of human infection with Scedosporium inflatum. Clin Infect Dis 1992; 14:1027–1033. [DOI] [PubMed] [Google Scholar]
- 73.Menon S, Edwards JC. Mycotic arthritis of the knee due to Madurella grisea. Br J Rheumatol 1994; 33:292–295. [DOI] [PubMed] [Google Scholar]
- 74.Lutwick LI, Galgiani JN, Johnson RH, et al. Visceral fungal infections due to Petriellidium boydii (Allescheria boydii). In vitro drug sensitivity studies. Am J Med 1976; 61:632–640. [DOI] [PubMed] [Google Scholar]
- 75.Hayden G, Lapp C, Loda F. Arthritis caused by Monosporium apiospermum treated with intraarticular amphotericin B. Am J Dis Child 1977; 131:927. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Guerrero ML, Ruiz Barnes P, Ales JM. Postcraniotomy mycetoma of the scalp and osteomyelitis due to Pseudallescheria boydii. J Infect Dis 1987; 156:855. [DOI] [PubMed] [Google Scholar]
- 77.Hung CC, Chang SC, Yang PC, et al. Ivasive pulmonary Pseudoallescheriasis with direct invasion of the thoracic spine in an immunocompetent patient. Eur J Clin Microbiol Infect Dis 1994; 13:749–751. [DOI] [PubMed] [Google Scholar]
- 78.Haapasaari J, Essen RV, Kahanpaa A, et al. Fungal arthritis simulating juvenile rheumatoid arthritis. Br Med J 1982; 285:923–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kemp HB, Bedford AF, Fincham WJ. Petriellidium boydii infection of the knee: a case report. Skeletal Radiol 1982; 9:114–117. [DOI] [PubMed] [Google Scholar]
- 80.1987; Ansari RA, Hindson DA, Stevens DL, et al. Pseudallescheria boydii arthritis and osteomyelitis in a patient with Cushing's disease. 80:90–92. [DOI] [PubMed] [Google Scholar]
- 81.Dirschl DR, Henderson RC. Patellar overgrowth after infection of the knee. A case report. J Bone Joint Surg A 1991; 73:940–941. [PubMed] [Google Scholar]
- 82.Halpern AA, Nagel DA, Schurman DJ. Allescheria boydii osteomyelitis following multiple steroid injections and surgery. Clin Orthop Rel Res 1977; 126.:232–234. [PubMed] [Google Scholar]
- 83.Lang AG, Peterson HA. Osteomyelitis following puncture wounds of the foot in children. J Trauma 1976; 16:993–999. [DOI] [PubMed] [Google Scholar]
- 84.Kooijman CM, Kampinga GA, de Hoog GS, et al. Successful treatment of Scedosporium aurantiacum osteomyelitis in an immunocompetent patient. Surg Infect (Larchmt) 2007; 8:605–610. [DOI] [PubMed] [Google Scholar]
- 85.Toy EC, Rinaldi MG, Savitch CB, et al. Endocarditis and hip arthritis associated with Scedosporium inflatum. South Med J 1990; 83:957–960. [DOI] [PubMed] [Google Scholar]
- 86.McCall RE. Maduromycosis Allescheria boydii septic arthritis of the knee: a case report. Orthopedics 1981; 4:1144–1146. [DOI] [PubMed] [Google Scholar]
- 87.Drouhet E, Dupont B. Laboratory and clinical assessment of ketoconazole in deep-seated mycoses. Am J Med 1983; 74:30–47. [DOI] [PubMed] [Google Scholar]
- 88.Tadros TS, Workowski KA, Siegel RJ, et al. Pathology of hyalohyphomycosis caused by Scedosporium apiospermum (Pseudallescheria boydii): an emerging mycosis. Hum Pathol 1998; 29:1266–1272. [DOI] [PubMed] [Google Scholar]
- 89.Talbot TR, Hatcher J, Davis SF, et al. Scedosporium apiospermum pneumonia and sternal wound infection in a heart transplant recipient. Transplantation 2002; 74:1645–1647. [DOI] [PubMed] [Google Scholar]
- 90.Galgiani JN, Stevens DA, Graybill JR. Pseudallescheria boydii infections treated with ketoconazole. Clinical evaluations of seven paients and in vitro susceptibility results. Chest 1984; 86:219–224. [DOI] [PubMed] [Google Scholar]
- 91.Lichtman DM, Johnson DC, Mack GR, et al. Maduromycosis (Allescheria boydii) infection of the hand. A case report. J Bone Joint Surg Am 1978; 60:546–548. [PubMed] [Google Scholar]
- 92.Capoor MR, Khanna G, Nair D, et al. Eumycetoma pedis due to Exophiala jeanselmei. Indian J 2007; 25:155–157. [DOI] [PubMed] [Google Scholar]
- 93.Dan M, Yossepowitch O, Hendel D, et al. Phialemonium curvatum arthritis of the knee following intra-articular injection of a corticosteroid. Med Mycol 2006; 44:571–574. [DOI] [PubMed] [Google Scholar]
- 94.Roncoroni AJ, Smayevsky J. Arthritis and endocarditis from Exophiala jeanselmei infection. Ann Intern Med 1988; 108:773. [DOI] [PubMed] [Google Scholar]
- 95.Lim A, Speers D, Inderjeeth C. Cladophialophora (Xylohypha) bantiana: an unusual cause of septic arthritis. Rheumatology (Oxford) 2013; 52:958–959. [DOI] [PubMed] [Google Scholar]
- 96.Karuppal R, Kumaran CM, Marthya A, et al. Tibial osteomyelitis due to Fonsecaea pedrosoi in an immunocompetent patient: case report. J Foot Ankle Surg 2009; 48:569–572. [DOI] [PubMed] [Google Scholar]
- 97.Sridhar S, Cheong D, Fontaine JP, et al. Alternaria-infected sternoclavicular. Infect Dis Clin Pract 2013; 21:e21–e23. [Google Scholar]
- 98.Destino L, Sutton DA, Helon AL, et al. Severe osteomyelitis caused by Myceliophthora thermophila after a pitchfork injury. Ann Clin Microbiol Antimicrob 2006; 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dewar CL, Sigler L. Fungal arthritis of the knee caused by Mycoleptodiscus indicus. Clin Rheumatol 2010; 29:1061–1065. [DOI] [PubMed] [Google Scholar]
- 100.Shigemura T, Agematsu K, Yamazaki T, et al. Femoral osteomyelitis due to Cladophialophora arxii in a patient with chronic granulomatous disease. Infection 2009; 37:469–473. [DOI] [PubMed] [Google Scholar]
- 101.Woollons A, Darley CR, Pandian S, et al. Phaeohyphomycosis caused by Exophiala dermatitidis following intra-articular steroid injection. Br J Dermatol 1996; 135:475–477. [PubMed] [Google Scholar]
- 102.Sutton DA, Timm WD, Morgan-Jones G, et al. Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phomopsis saccardo 1905: criteria for identification, case history, and therapy. J Clin Microbiol 1999; 37:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khan SA, Hasan AS, Capoor MR, et al. Calcaneal osteomyelitis caused by Exophiala jeanselmei in an immunocompetent child. A case report. J Bone Joint Surg Am 2007; 89:859–862. [DOI] [PubMed] [Google Scholar]
- 104.O’Riordan E, Denton J, Taylor PM, et al. Madura foot in the U.K.: fungal osteomyelitis after renal transplantation. Transplantation 2002; 73:151–153. [DOI] [PubMed] [Google Scholar]
- 105.Morio F, Berre JY, Garcia-Hermoso D, et al. Phaeohyphomycosis due to Exophiala xenobiotica as a cause of fungal arthritis in an HIV-infected patient. Med Mycol 2012; 50:513–517. [DOI] [PubMed] [Google Scholar]
- 106.Magnon KC, Jalbert M, Padhye AA. Osteolytic phaeohyphomycosis caused by Phialemonium obovatum. Arch Pathol Lab Med 1993; 117:841–843. [PubMed] [Google Scholar]
- 107.Katsolis JG, Sudduth EJ, Chen N, et al. Alternaria osteomyelitis in an immunocompetent host treated with voriconazole. Infect Dis Clin Pract 2012; 20:164–166. [Google Scholar]
- 108.Lee DK, Schwartz AK. Primary mycetoma osteomyelitis of the calcaneus with active subcutaneous nodules. J Foot Ankle Surg 2007; 46:302–306. [DOI] [PubMed] [Google Scholar]
- 109.Lespessailles E, Kerdraon R, Michenet P, et al. Alternaria infection of the skin and joints. A report of two cases involving the hand. Rev Rhum Engl (Ed) 1999; 66:509–511. [PubMed] [Google Scholar]
- 110.Murtagh J, Smith JW, Mackowiak PA. Case report: Alternaria osteomyelitis: eight years of recurring disease requiring cyclic courses of amphotericin B for cure. Am J Med Sci 1987; 293:399–402. [Google Scholar]
- 111.Yangco BG, TeStrake D, Okafor J. Phialophora richardsiae isolated from infected human bone: morphological, physiological and antifungal susceptibility studies. Mycopathologia 1984; 86:103–111. [DOI] [PubMed] [Google Scholar]
- 112.Kaell AT, Weitzman I. Acute monoarticular arthritis due to Phialophora parasitica. Am J Med 1983; 74:519–522. [DOI] [PubMed] [Google Scholar]
- 113.Koppang HS, Olsen I, Stuge U, et al. Aureobasidium infection of the jaw. J Oral Pathol Med 1991; 20:191–195. [DOI] [PubMed] [Google Scholar]
- 114.Uberti-Foppa C, Fumagalli L, Gianotti N, et al. First case of osteomyelitis due to Phialophora richardsiae in a patient with HIV infection. AIDS 1995; 9:975–976. [PubMed] [Google Scholar]
- 115.Beeram V, Challa S, Vannemreddy P. Cerebral mycetoma with cranial osteomyelitis. J Neurosurg Pediatrics 2008; 1:493–495. [DOI] [PubMed] [Google Scholar]
- 116.Roilides E, Sigler L, Bibashi E, et al. Disseminated infection due to Chrysosporium zonatum in a patient with chronic granulomatous disease and review of non-Aspergillus fungal infections in patients with this disease. J Clin Microbiol 1999; 37:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stillwell WT, Rubin BD, Axelrod JL. Chrysosporium, a new causative agent in osteomyelitis. A case report. Clin Orthop 1984; 190–192. [PubMed] [Google Scholar]
- 118.Cohen-Abbo A, Edwards KM. Multifocal osteomyelitis caused by Paecilomyces varioti in a patient with chronic granulomatous disease. Infection 1995; 23:55–57. [DOI] [PubMed] [Google Scholar]
- 119.Pierce PF, Wood MB, Roberts GD, et al. Saksenaea vasiformis osteomyelitis. J Clin Microbiol 1987; 25:933–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen F, Lu G, Kang Y, et al. Mucormycosis spondylodiscitis after lumbar disc puncture. Eur Spine J 2006; 15:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meis JF, Kullberg BJ, Pruszczynski M, et al. Severe osteomyelitis due to the zygomycete Apophysomyces elegans. J Clin Microbiol 1994; 32:3078–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Echols RM, Selinger DS, Hallowell C, et al. Rhizopus osteomyelitis. A case report and review. Am J Med 1979; 66:141–145. [DOI] [PubMed] [Google Scholar]
- 123.Eaton ME, Padhye AA, Schwartz DA, et al. Osteomyelitis of the sternum caused by Apophysomyces elegans. J Clin Microbiol 1994; 32:2827–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chaudhuri R, McKeown B, Harrington D, et al. Mucormycosis osteomyelitis causing avascular necrosis of the cuboid bone: MR imaging findings. AJR Am J Roentgenol 1992; 159:1035–1037. [DOI] [PubMed] [Google Scholar]
- 125.Weinberg WG, Wade BH, Cierny G, et al. Invasive infection due to Apophysomyces elegans in immunocompetent hosts. Clin Infect Dis 1993; 17:881–884.3rd. [DOI] [PubMed] [Google Scholar]
- 126.Dinasarapu CR, Auerbach J, Levi MH, et al. Mucormycosis as a pathogen in polymicrobial necrotizing fasciitis. Infect Dis ClinPract 2010; 18:417–418. [Google Scholar]
- 127.Oo MM, Kutteh LA, Koc ON, et al. Mucormycosis of petrous bone in an allogeneic stem cell transplant recipient. Clin Infect Dis 1998; 27:1546–1547. [DOI] [PubMed] [Google Scholar]
- 128.Adler N, Seitz IA, Gottlieb LJ. Acute wound closure and reconstruction following head zygomycosis: presentation of two cases and review of literature. J Reconstr Microsurg 2008; 24:507–513. [DOI] [PubMed] [Google Scholar]
- 129.Parra-Ruiz J, Pena-Monje A, Tomas-Jimenez C, et al. Septic arthritis due to Absidia corymbifera in a patient with HIV-1 infection. Infection 2008; 36:279–281. [DOI] [PubMed] [Google Scholar]
- 130.Buhl MR, Joseph TP, Snelling BE, et al. Temporofacial zygomycosis in a pregnant woman. Infection 1992; 20:230–232. [DOI] [PubMed] [Google Scholar]
- 131.Vashi N, Avedian R, Brown J, et al. Successful surgical and medical treatment of Rhizopus osteomyelitis following hematopoietic cell transplantation. Orthopedics 2012; 35:e1556–e1561. [DOI] [PubMed] [Google Scholar]
- 132.Fortun J, Cobo J, Canal J, et al. Post-traumatic cranial mucormycosis in an immunocompetent patient. J Oral Maxillofac Surg 1995; 53:1099–1102. [DOI] [PubMed] [Google Scholar]
- 133.Huffnagle KE, Southern PM, Jr, Byrd LT, et al. Apophysomyces elegans as an agent of zygomycosis in a patient following trauma. J Med Vet Mycol 1992; 30:83–86. [DOI] [PubMed] [Google Scholar]
- 134.Holtom PD, Obuch AB, Ahlmann ER, et al. Mucormycosis of the tibia: a case report and review of the literature. Clin Orthop 2000; 222–228. [PubMed] [Google Scholar]
- 135.Shaw CJ, Thomason AJ, Spencer JD. Fungal osteomyelitis of the foot. A report of an unusual case. J Bone Joint Surg Br 1994; 76:137–139. [PubMed] [Google Scholar]
- 136.Buruma OJ, Craane H, Kunst MW. Vertebral osteomyelitis and epidural abcess due to mucormycosis, a case report. Clin Neurol Neurosurg 1979; 81:39–44. [DOI] [PubMed] [Google Scholar]
- 137.Maliwan N, Reyes CV, Rippon JW. Osteomyelitis secondary to cutaneous mucormycosis. Report of a case and a review of the literature. Am J Dermatopathol 1984; 6:479–481. [DOI] [PubMed] [Google Scholar]
- 138.Moore PH, Jr, McKinney RG, Mettler FA., Jr Radiographic and radionuclide findings in Rhizopus osteomyelitis. Radiology 1978; 127:665–666. [DOI] [PubMed] [Google Scholar]
- 139.Ratnayake G, Judson IR, Scurr M, et al. Fungal spinal cord compression in metastatic synovial sarcoma. Acta Oncol 2011; 50:158–159. [DOI] [PubMed] [Google Scholar]
- 140.Mostaza JM, Barbado FJ, Fernandez-Martin J, et al. Cutaneoarticular mucormycosis due to Cunninghamella bertholletiae in a patient with AIDS. Rev Infect Dis 1989; 11:316–318. [DOI] [PubMed] [Google Scholar]
- 141.Stevanovic MV, Mirzayan R, Holtom PD, et al. Mucormycosis osteomyelitis in the hand. Orthopedics 1999; 22:449–450. [DOI] [PubMed] [Google Scholar]
- 142.Jakle C, Leek JC, Olson DA, et al. Septic arthritis due to Fusarium solani. J Rheumatol 1983; 10:151–153. [PubMed] [Google Scholar]
- 143.Katragkou A, Walsh TJ, Roilides E. Why is mucormycosis more difficult to cure than more common mycoses? Clin Microbiol Infect 2014; 20. Suppl 6:74–81. [DOI] [PubMed] [Google Scholar]
- 144.Guarro J, Kantarcioglu AS, Horre R, et al. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol 2006; 44:295–327. [DOI] [PubMed] [Google Scholar]
- 145.Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev 2008; 21:157–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schaenman JM, DiGiulio DB, Mirels LF, et al. Scedosporium apiospermum soft tissue infection successfully treated with voriconazole: potential pitfalls in the transition from intravenous to oral therapy. J Clin Microbiol 2005; 43:973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Walsh TJ, Lutsar I, Driscoll T, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr Infect Dis J 2002; 21:240–248. [DOI] [PubMed] [Google Scholar]
- 148.Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect 2014; 20 Suppl 3:27–46. [DOI] [PubMed] [Google Scholar]
- 149.Schell WA. Histopathology of fungal rhinosinusitis. Otolaryngol Clin North Am 2000; 33:251–276. [DOI] [PubMed] [Google Scholar]
- 150.Al-Hatmi AM, van Diepeningen AD, Curfs-Breuker I, et al. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J Antimicrob Chemother 2015; 70:1068–1071. [DOI] [PubMed] [Google Scholar]
- 151.Harrasser N, Banke IJ, Hauschild M, et al. Clinical challenge: fatal mucormycotic osteomyelitis caused by Rhizopus microsporus despite aggressive multimodal treatment. BMC Infect Dis 2014; 14:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nucci M, Marr KA, Vehreschild MJ, et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect 2014; 20:580–585. [DOI] [PubMed] [Google Scholar]
- 153.Esterre P, Queiroz-Telles F. Management of chromoblastomycosis: novel perspectives. Curr Opin Infect Dis 2006; 19:148–152. [DOI] [PubMed] [Google Scholar]


