Supplemental Digital Content is available in the text
Abstract
Liver-related death in human immunodeficiency virus (HIV)-infected individuals is about 10 times higher compared with the general population, and the prevalence of significant liver fibrosis in those with HIV approaches 15%. The present study aimed to assess risk factors for development of hepatic fibrosis in HIV patients receiving a modern combination anti-retroviral therapy (cART).
This cross-sectional prospective study included 432 HIV patients, of which 68 (16%) patients were anti-hepatitis C virus (HCV) positive and 23 (5%) were HBsAg positive.
Health trajectory including clinical characteristics and liver fibrosis stage assessed by transient elastography were collected at inclusion. Liver stiffness values >7.1 kPa were considered as significant fibrosis, while values >12.5 kPa were defined as severe fibrosis. Logistic regression and Cox regression uni- and multivariate analyses were performed to identify independent factors associated with liver fibrosis.
Significant liver fibrosis was detected in 10% of HIV mono-infected, in 37% of HCV co-infected patients, and in 18% of hepatitis B virus co-infected patients. The presence of diabetes mellitus (odds ratio [OR] = 4.6) and FIB4 score (OR = 2.4) were independently associated with presence of significant fibrosis in the whole cohort. Similarly, diabetes mellitus (OR = 5.4), adiposity (OR = 4.6), and the FIB4 score (OR = 3.3) were independently associated with significant fibrosis in HIV mono-infected patients. Importantly, cumulative cART duration protected, whereas persistent HIV viral replication promoted the development of significant liver fibrosis along the duration of HIV infection.
Our findings strongly indicate that besides known risk factors like metabolic disorders, HIV may also have a direct effect on fibrogenesis. Successful cART leading to complete suppression of HIV replication might protect from development of liver fibrosis.
BACKGROUND
In the era of modern combination anti-retroviral therapy (cART), life expectancy of human immunodeficiency virus (HIV)-infected patients approaches that of the general population if cART is initiated at high CD4 cell counts.1 Therefore, non-AIDS defining events are increasingly responsible for morbidity and mortality among aging HIV-infected individuals.2 In particular, liver-related deaths occur 10 times higher than in the general population,3 ranging between 7% and 14% in different cohorts4,5 and rendering liver diseases an important non-AIDS condition in HIV patients. Chronic viral hepatitis B (HBV) or C (HCV), as well as alcohol abuse or nonalcoholic steatohepatitis (NASH) induce liver injury with consecutive fibrosis and might progress to liver cirrhosis with an increased risk for the development of hepatocellular carcinoma.6 However, one-third of liver-related deaths is not associated with viral hepatitis,7 indicating that other than the classic risk factors play an important role. Several experimental studies indicate that HIV may also have a direct effect on hepatic fibrogenesis. HIV enters via CCR5 and CXCR4 and activates the hepatic stellate cells (HSC), the principle fibrogenic cell type in the liver.8–11 Thus, uncontrolled replication of HIV might enhance hepatic fibrogenesis directly.
To date, sparse data are available on the prevalence and potential risk factors for hepatic injury among HIV mono-infected patients. Interestingly, uncontrolled HIV viral replication, low CD4+ T cell count, as well as long-term exposure to antiretroviral regimes were identified as risk factors for the development of significant liver fibrosis.12 The later seems somehow contradictory, since cART on one side might protect from liver fibrosis by suppressing HIV viral replication, and on the other side might promote fibrosis due to hepatotoxic effects. Therefore, this study aimed to assess the risk factors associated with the development of clinical relevant liver fibrosis in a large HIV-cohort from the Infectious Diseases outpatient clinic at Bonn University, screened by transient elastography (TE) (FibroScan® ECHOSENS, Creteil, France).
METHODS
Patients
This cross-sectional prospective study included 432 HIV-infected patients followed at the Infectious Diseases outpatient clinic at Bonn University between August 2013 and August 2014. All patients with confirmed HIV infection were eligible for inclusion. Blood collection and liver stiffness (LS) measurement were performed on a regular scheduled visit in our outpatient clinic. Informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the approval by the institution's human research committee (279/14).
Data Acquisition and Follow-Up
Laboratory work-up of metabolic (HbA1c, cholesterol, LDH, HDL, triglycerides, transaminases, bilirubin, creatinine, platelets, albumin, and TSH), inflammatory/immunologic (C reactive protein (CRP), CD4, and CD8), and HIV-relevant disease markers (viral load) was performed at the time of TE. Demographic and clinical characteristics, as well as health trajectory of HIV-infection and antiretroviral treatments were collected from the patient's health record and our department's database. Current co-medication, alcohol consumption, and smoking status were recorded and the actual body mass index (BMI) was assessed.
Liver Stiffness Measurement
LS measurement was assessed by TE using FibroScan 502 touch® (ECHOSENS, Creteil, France). TE was performed by experienced and trained operators using BMI-corresponding probes. According to manufacturer's recommendations, only examinations with at least 10 valid measurements, a low variability defined as an interquartile range (IQR/M) smaller than 30% of the median value from an individual scan, and a successful acquisition rate of >60% were considered reliable and were approved for analysis. LS values >7.1 kPa (equivalent to Metavir F ≥ 2) were considered abnormal. LS ranging from 7.1 to 12.4 kPa was considered significant fibrosis and values ≥12.5 kPa (equivalent to Metavir F ≥ 3–4) as severe fibrosis.13,14
Statistical Analysis
Demographic, clinical, and laboratory characteristics stratified by the severity of liver fibrosis were compared. Differences in continuous variables, expressed as medians and first and third quartiles were assessed using nonparametric Mann–Whitney test. Categorical variables, expressed as absolute frequencies and percentages, were compared using Pearson's Chi-squared test or Fisher's exact tests. A correlation analysis estimated possible impacts on liver fibrosis determined by TE and serum scores. Uni- and multivariate analyses were performed using logistic and Cox regression models. SPSS version 22 (IBM Corporation, Armonk, NY) was used for statistical analysis.
RESULTS
General Characteristics of the Cohort
Among 432 adults, 78% were male and 79% Caucasian with a median age of 46 (IQR, 38–53) years. Half of the patients were men who have sex with men. The baseline characteristics of the subjects included are shown in Table 1.
TABLE 1.
Baseline Subject Characteristics, Overall and by Significant Fibrosis as Determined by LS > 7.1 kPa
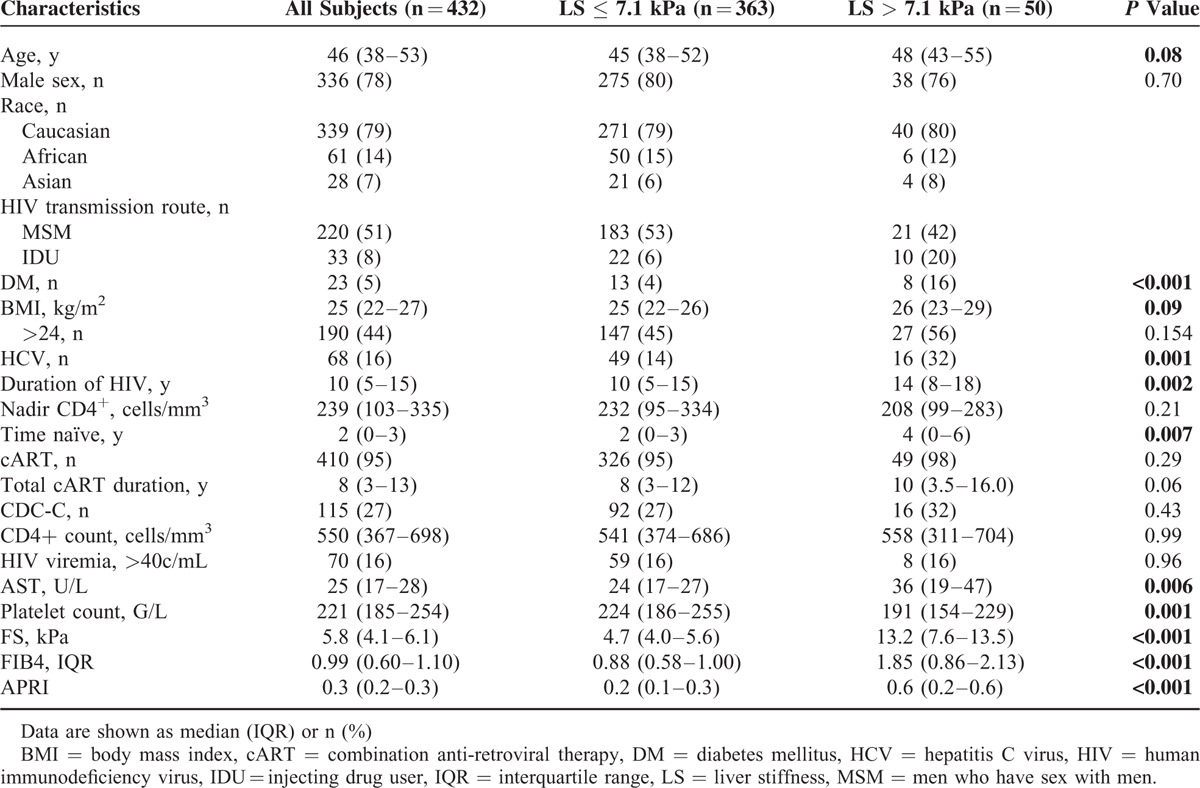
Twenty-three (5%) patients were HBsAg positive, 68 (16%) were anti-HCV positive, of which 37 (54%) had achieved sustained virologic response (SVR) and 31 (46%) were HCV-RNA positive. Three (0.7%) were HBsAg positive and anti-HCV positive, and 343 (80%) were HIV mono-infected. The vast majority (95%) received cART, and the median duration of HIV infection was 10 (IQR, 5–15) years. One hundred fifteen (27%) patients were classified as CDC category C. The median CD4+ T cell count was 550 cells/mm3 (IQR 367–698). Seventy patients of the total population (17%) had detectable HIV viremia (defined as >40 copies/mL), of which 19 (27%) were ART naive and 51 (73%) were under cART. Of all patients receiving cART 87% had undetectable HIV viremia and 5% had a viral load <200 copies/mL. With regard to the 68 HIV/HCV co-infected patients, 37 (54%) achieved an SVR, 21 (31%) were HCV therapy naïve, and 10 (15%) either relapsed or were nonresponder to previously administrated HCV treatments.
LSM at Inclusion
Among all patients with valid LS measurements (n = 413), 9% (37/413) had significant fibrosis (FibroScan value 7.1–12.4 kPa) and 3% (13/413) had severe fibrosis (FibroScan value ≥12.5 kPa). As expected, a markedly higher prevalence of fibrosis was found in the patients with chronic hepatitis C (patients with positive HCV-RNA, n = 31) or hepatitis B co-infection (patients with positive HBsAg, n = 23), namely 37% and 18%, respectively. However, patients with HCV antibodies and a history of SVR after successful HCV therapy (n = 37) did not show a significantly higher rate of abnormal LS (13%). Most interestingly, 8% (26/327) of the HIV patients without hepatitis co-infection had significant and 2% (5/327) severe fibrosis (Fig. 1A).
FIGURE 1.
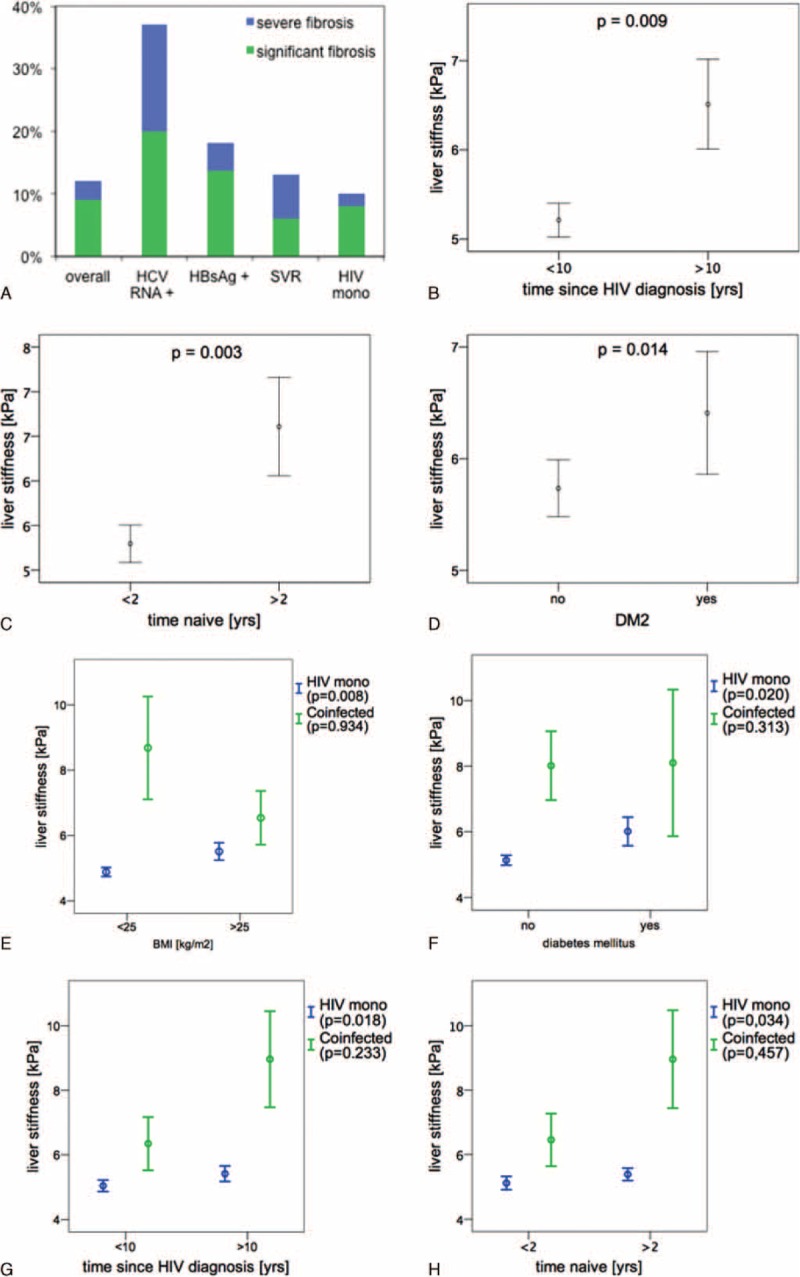
(A) Distribution of fibrosis among subgroups. (B–D) Association between LS and HIV duration, time naive, and DM. (E–H) Association between LS and BMI, DM, HIV duration, and time naive in HIV mono-infected patients compared with coinfected patients. Data are presented as mean ± SEM. BMI = body mass index, DM = diabetes mellitus, HIV = human immunodeficiency virus, LS = liver stiffness, SEM = standard error of the mean.
Variables Associated With Significant Liver Fibrosis
There were significant differences between the patients with significant and severe fibrosis compared to patients with LS values ≤7.1 kPa with regard to metabolic conditions like DM (P < 0.001), duration of HIV infection (P = 0.002), exposure to antiretroviral drug regimens containing azidothymidine (AZT), stavudine (d4T), or didanosine (DDI) and interestingly also to the duration the patient was treatment naive (P = 0.007; Table 1). Also if LS values were not stratified, duration of HIV, treatment naive time, and diabetes maintained a level of statistical significance (Fig. 1B–D). In contrast, age, sex, BMI, CD4+ T cell count (nadir and actual values), and detectable HIV viremia were not associated with abnormal LS.
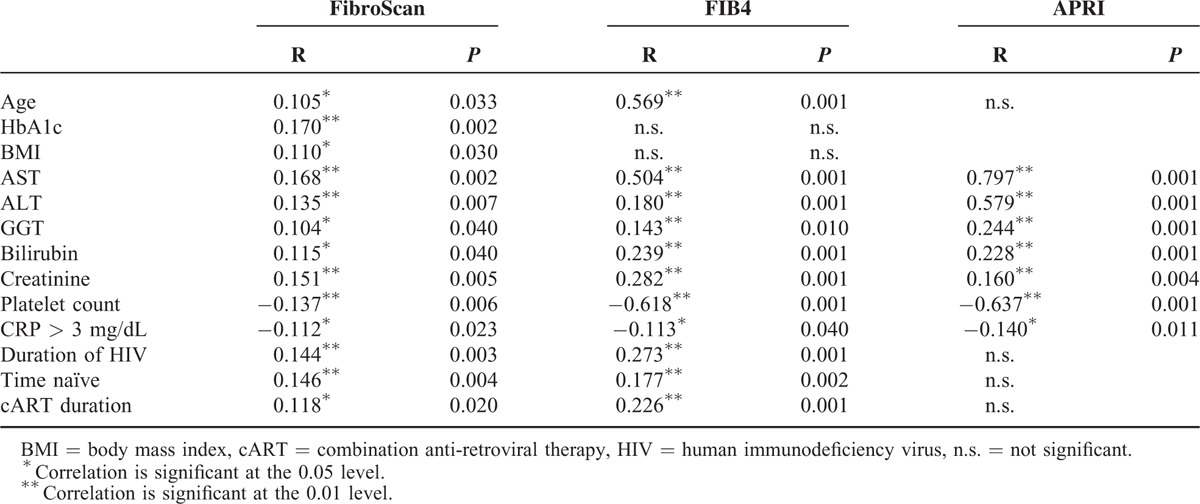
FibroScan values, APRI score, and FIB4 score correlated with each other highly significant (R = 0.218–0.754; P < 0.001). Metabolic conditions (HbA1c, BMI), markers of liver injury (AST, ALT, GGT, and bilirubin), renal function (creatinine), and age correlated positively with LS, whereas CRP and platelets showed an inverse correlation. Interestingly, the total duration of HIV infection, the time the patient was treatment naive, and the duration of cART showed all positive correlations with LS values. FIB4 and APRI merely showed a trend toward these variables (Table 2).
TABLE 2.
Correlations of Patient Characteristics and Clinical Parameters With Liver Fibrosis Assessed by LS, FIB4, and APRI
In the HIV mono-infected population significant differences between the patients with fibrosis compared with patients with normal LS values was observed with regard to diabetes (P < 0.001) and the cumulative duration of HIV infection (P = 0.01), obesity showed a trend toward statistical significance (Supplemental Table 1). Figure 1(E–H) shows that DM and BMI higher 25 kg/m2 as well as HIV infection longer 10 years and ART naive time greater 2 years are associated independently with higher LSM values in HIV mono-infected subjects. In the co-infected patients, these variables do not reach statistical significance and their effect on fibrosis development might be exceeded by the much stronger profibrogenic effect of viral hepatitis co-infection.
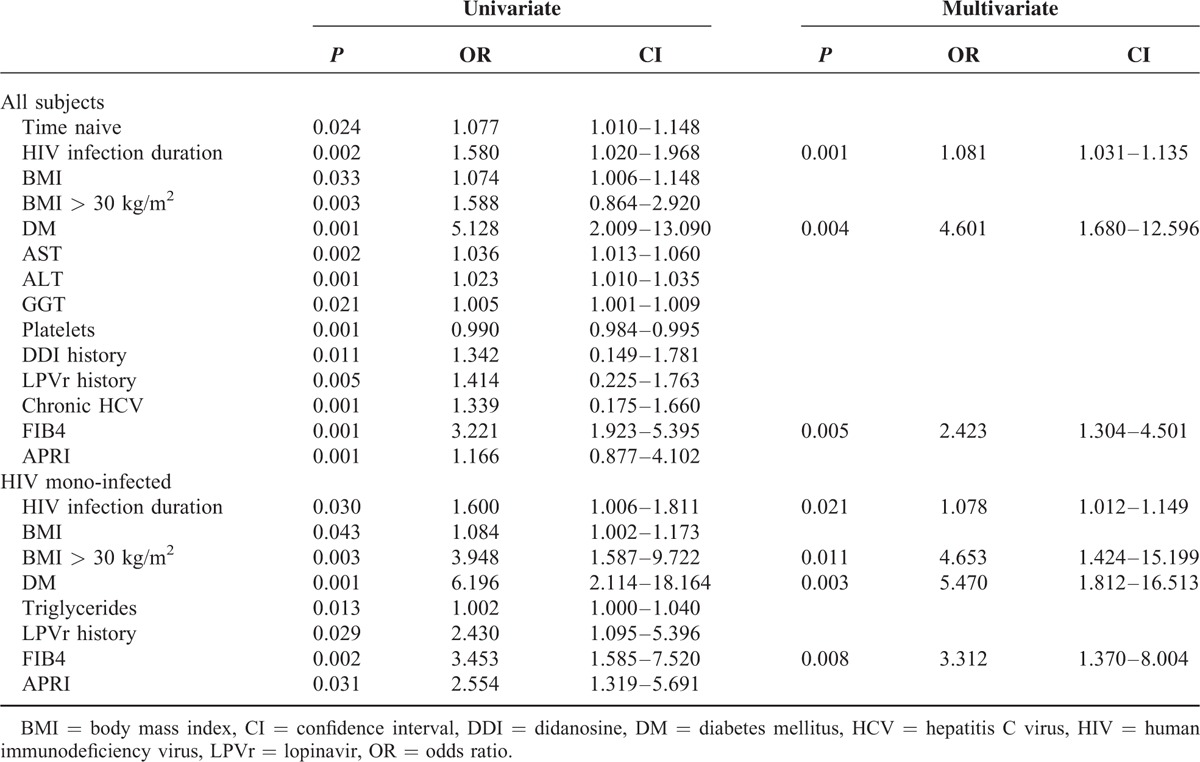
On univariate logistic regression analysis in HIV mono-infected patients the duration of HIV infection, obesity, serum triglyceride levels, diabetes, and the history of ART regimes containing LPVr had a significant impact on abnormal LS values. If taking into account the whole study population, serum aminotransferases, platelet count, exposure to DDI, ART naive duration, and chronic hepatitis C additionally showed significant influence. On subsequent multivariate logistic regression analysis cumulative HIV duration (odds ratio [OR]: 1.078; 95% CI: 1.012–1.149), obesity (OR: 4.653; 95% CI: 1.424–15.199), and diabetes mellitus (OR: 5.470; 95% CI: 1.812–16.513) were selected as independent predictors accounting for abnormal LS values in HIV mono-infected subjects (Table 3), whereas HIV viral load was not associated with fibrosis development.
TABLE 3.
Evaluating Risk Factors for Increased Liver Stiffness by Logistic Regression: Uni- and Multivariate Analysis of the Cohort and of HIV Mono-Infected Patients
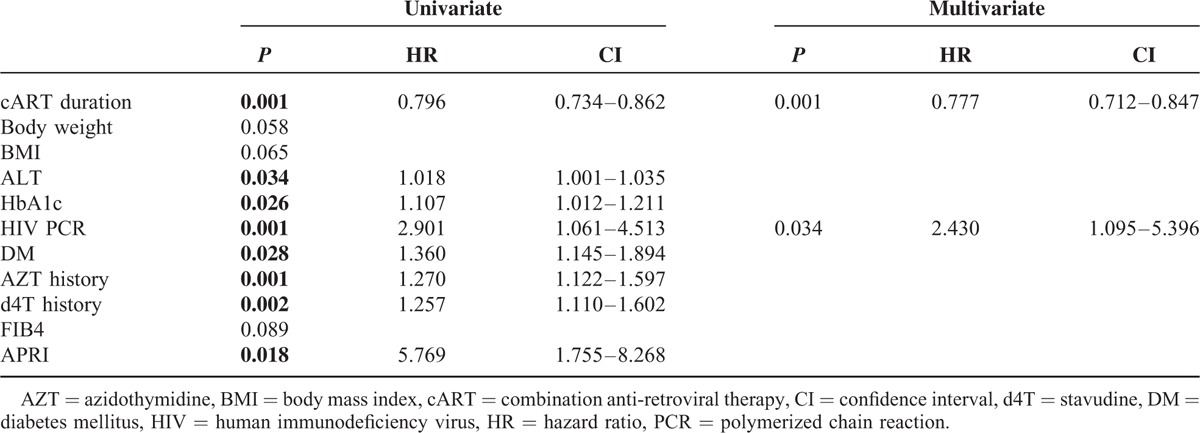
Per treatment naive year the risk of developing significant fibrosis was found to rise by about 12% (OR: 1.12; 95% CI: 1.00–1.24). In a multivariate Cox regression analysis, taking into account the total duration of HIV infection, the cumulative cART duration was demonstrated to have a protective effect on liver fibrosis development (hazard ratio [HR]: 0.777; 95% CI: 0.712–0.847) and uncontrolled HIV viral replication, assessed by HIV viral load, was clearly associated with the risk of developing significant liver fibrosis (HR: 2.430; 95% CI: 1.095–5.396; Table 4).
TABLE 4.
Cox Regression (Time Since HIV Diagnosis): Uni- and Multivariate Analysis in HIV Mono-Infected Patients
DISCUSSION
This study demonstrated that presence of DM, obesity, as well as HIV viral load were associated with presence of significant fibrosis in HIV mono-infected patients. Also, HIV infection is known to accelerate HBV and HCV-related liver injury15–17 and is said to act independently as a profibrogenic factor. However, previously published data largely rely on studies using serum biomarkers17–22 for the assessment of liver fibrosis and are likely to underestimate the magnitude of liver disease. It is known that liver enzyme elevations are poor markers of liver fibrosis or cirrhosis, especially in HIV-infected patients.23,24 Moreover, studies using TE to assess fibrosis in HIV mono-infected subjects were conducted with rather small sample sizes.25–28 Our prospective study is the first in a large cohort of HIV patients using TE and demonstrated a high rate of moderate and severe fibrosis in the HIV-infected population with a 10% prevalence of abnormal LS among HIV mono-infected individuals and a markedly higher prevalence of fibrosis in viral hepatitis co-infected patients.
Our findings strongly indicate that HIV may also have a direct effect on fibrogenesis, since HIV viral load was an independent predictor in the time dependent Cox regression analysis. Importantly, the time since HIV infection was independently associated with higher fibrosis risk. The total duration of HIV infection includes both, the ART naive period—consecutively the potential damaging effect of uncontrolled HIV replication—and the time of antiretroviral treatment (with possible hepatotoxic effects). Cumulative ART suggested a protective effect on liver fibrosis development and HIV viral replication was associated with a higher risk of liver fibrosis. This goes along with in vitro data showing that HIV infects human stellate cell lines, promotes proinflammatory cytokine secretion, and favors fibrogenesis.5–7 More importantly, markers of liver fibrosis remodeling were significantly elevated in HIV patients compared with healthy controls and correlated to HIV viral loads and inversely to cART duration.29 Consistent with our and other studies, the virological control offered by cART is likely to protect against liver fibrosis.16,30–32 Our findings suggest that successful cART and complete suppression of HIV replication beneficially influences and protects liver fibrosis development. However, our study with the vast majority of patients receiving antiretrovirals was not designed to prove a beneficial impact of cART. With regard to exposure to individual antiretrovirals, we found a direct relationship between LS and exposure to regimens containing AZT, d4T, or DDI, although this was not confirmed by multivariate analysis. Importantly, cumulative cART exposure was not associated with significant fibrosis. Our findings are consistent with a recent report demonstrating that modern cART has a negligible effect on liver fibrosis progression in HIV mono-infected patients.33 While older antiretroviral drugs such as AZT, d4T, and DDI might have increased the risk of liver damage through hepatotoxicity, this effect might be counterbalanced by a beneficial impact of newer and currently recommended first-line regimens, which were applied in our collective.
Besides that, known metabolic risk factors such as insulin resistance (IR) are increasingly observed in patients infected with HIV.34 It is unclear whether this is a direct effect of HIV infection alone or a complication of antiretroviral drugs (eg. protease inhibitors). Hyperglycemia and insulin stimulate HSC,35 the key cells involved in the progression of fibrosis.11 Recent liver biopsy data confirm that the main cause of liver damage of uncertain origin in HIV-infected patients was associated with metabolic disorders; increase in BMI and homeostasis model assessment—insulin resistance (HOMA-IR) values higher than 2.6 were identified as the main risk factors, while NASH was diagnosed as the main cause of liver damage of uncertain origin.36 Interestingly, in our large prospective study we could confirm that obesity and DM are risk factors for presence of significant fibrosis in HIV patients with and without HBV or HCV co-infection.
Our findings, together with other recently published data in this field, might raise the question of whether the HIV-infected population should be considered a high risk group for NAFLD and should be screened as recommended by the AASLD in the recent practice guidelines.37 An adequate monitoring of liver disease and development of management algorithms are clearly needed to optimize outcome and care of the aging HIV population. The European AIDS Clinical Society guidelines recommend regular determination of liver enzymes even in mono-infected patients and when persistently elevated, after ruling out other causes, annual noninvasive fibrosis stage assessment or liver biopsy.38
The main limitations of this study are the cross-sectional design, which limits the ability to evaluate change in variables of interest over time, and the presence of a nonrandomized study population. Alcohol consumption was self-reported by the patient and this value may have been underestimated. Moreover, rare causes of liver disease (eg. hemochromatosis and Wilson disease) were not specifically excluded. Finally, liver biopsy remains the gold standard for the assessment of liver fibrosis, but the acceptability and risk of biopsy make its application difficult for a large study population. TE is noninvasive and has been found to be accurate and reproducible in determining the stage of liver fibrosis. New reliability criteria for TE to increase the number of accurate measurements were recently published, but the applied criteria were classified as reliable and accurate for the diagnostic performance of significant fibrosis.39
In conclusion, the correlation between HIV-related virological status and LS suggests that HIV itself together with the metabolic profile might promote liver fibrosis. These findings underscore that in aging with HIV the interplay and additive effects of different factors might be crucial for the development of liver disease. Modern cART regimes beneficially influence liver fibrosis. However, further long-term prospective studies are needed. A regular assessment of liver fibrosis in HIV mono-infected patients seems to be advisable.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Arite Eickert, Nadine Owsiany, Gudrun Hack, and Silke Bellinghausen for excellent assistance.
Footnotes
Abbreviations: ART = antiretroviral therapy, AZT = azidothymidine, BMI = body mass index, cART = combination anti-retroviral therapy, CI = confidence interval, d4T = stavudine, DDI = didanosine, DM = diabetes mellitus, HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus, HOMA-IR = homeostasis model assessment—insulin resistance, HR = hazard ratio, HSC = hepatic stellate cells, IQR = interquartile range, LPVr = lopinavir, LS = liver stiffness, MSM = men who have sex with men, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, OR = odds ratio, SVR = sustained virologic response, TE = transient elastography.
RM: Designed the original study, wrote the first draft of the article, and performed the analyses. RS: Wrote the first draft of the article and performed the analyses. CS-Z: Blood collection. CB: Blood collection. J-CW: Blood collection. JT: Designed the original study, supervised the analyses, and co-ordinated the study. JKR: Designed the original study, supervised the analyses, and co-ordinated the study.
JT and JKR shared last authorship.
The study was supported by German Center for Infection Research (DZIF to JKR), H.W. & J. Hector Stiftung (M60.2 to JT), and Deutsche Forschungsgemeinschaft (SFB TRR57 P18 to JT).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–248. [DOI] [PubMed] [Google Scholar]
- 3.Hernando V, Perez-Cachafeiro S, Lewden C, et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J Hepatol 2012; 57:743–751. [DOI] [PubMed] [Google Scholar]
- 4.Alejos B, Hernando V, López-Aldeguer J, et al. Overall and cause-specific mortality in HIV-positive subjects compared to the general population. J Int AIDS Soc 2014; 17:19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–1641. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908–943. [DOI] [PubMed] [Google Scholar]
- 7.Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010; 50:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuyama AC, Hong F, Saiman Y, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 2010; 52:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong F, Saiman Y, Si C, et al. X4 human immunodeficiency virus type 1 gp120 promotes human hepatic stellate cell activation and collagen I expression through interactions with CXCR4. PLoS ONE 2012; 7:e33659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno R, Galastri S, Sacchi P, et al. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut 2010; 59:513–520. [DOI] [PubMed] [Google Scholar]
- 11.Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 2013; 4:2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockstroh JK, Mohr R, Behrens G, et al. Liver fibrosis in HIV: which role does HIV itself, long-term drug toxicities and metabolic changes play? Curr Opin HIV AIDS 2014; 9:365–370. [DOI] [PubMed] [Google Scholar]
- 13.Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006; 55:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008; 48:835–847. [DOI] [PubMed] [Google Scholar]
- 15.Salmon-Ceron D, Lewden C, Morlat P, et al. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol 2005; 42:799–805. [DOI] [PubMed] [Google Scholar]
- 16.Thio CL, Seaberg EC, Skolasky R, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–1926. [DOI] [PubMed] [Google Scholar]
- 17.Al-Mohri H, Murphy T, Lu Y, et al. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr 2007; 44:463–469. [DOI] [PubMed] [Google Scholar]
- 18.Blackard JT, Welge JA, Taylor LE, et al. HIV mono-infection is associated with FIB-4—a noninvasive index of liver fibrosis—in women. Clin Infect Dis 2011; 52:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendeni M, Focà E, Gotti D, et al. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis 2011; 52:1164–1173. [DOI] [PubMed] [Google Scholar]
- 20.Tahiri M, Sodqi M, Lahdami FEZ, et al. Risk factors for liver fibrosis among human immunodeficiency virus monoinfected patients using the FIB4 index in Morocco. World J Hepatol 2013; 5:584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price JC, Seaberg EC, Badri S, et al. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis 2012; 205:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DallaPiazza M, Amorosa VK, Localio R, et al. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis 2010; 10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Carbonero L, de Ledinghen V, Moreno A, et al. Liver fibrosis in patients with chronic hepatitis C and persistently normal liver enzymes: influence of HIV infection. J Viral Hepat 2009; 16:790–795. [DOI] [PubMed] [Google Scholar]
- 24.Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999; 29:1306–1310. [DOI] [PubMed] [Google Scholar]
- 25.Bailony MR, Scherzer R, Huhn G, et al. Association of HIV infection, hepatitis C virus infection, and metabolic factors with liver stiffness measured by transient elastography. J Infect Dis 2013; 208:1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brescini L, Orsetti E, Gesuita R, et al. Evaluating liver fibrosis by transient elastometry in patients with HIV-HCV coinfection and monoinfection. Hepat Mon 2014; 14:e15426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han SH, Kim SU, Kim CO, et al. Abnormal liver stiffness assessed using transient elastography (FibroScan® in HIV-infected patients without HBV/HCV coinfection receiving combined antiretroviral treatment. PLoS ONE 2013; 8:e52720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasson H, Merli M, Galli L, et al. Non-invasive fibrosis biomarkers—APRI and Forns—are associated with liver stiffness in HIV-monoinfected patients receiving antiretroviral drugs. Liver Int 2013; 33:1113–1120. [DOI] [PubMed] [Google Scholar]
- 29.Leeming DJ, Anadol E, Schierwagen R, et al. Combined antiretroviral therapy attenuates hepatic extracellular matrix remodeling in HIV patients assessed by novel protein fingerprint markers. AIDS 2014; 28:2081–2090. [DOI] [PubMed] [Google Scholar]
- 30.Qurishi N, Kreuzberg C, Lüchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 2003; 362:1708–1713. [DOI] [PubMed] [Google Scholar]
- 31.Benhamou Y, Di Martino V, Bochet M, et al. Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology 2001; 34:283–287. [DOI] [PubMed] [Google Scholar]
- 32.Bräu N, Salvatore M, Ríos-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol 2006; 44:47–55. [DOI] [PubMed] [Google Scholar]
- 33.Moodie EEM, Pant Pai N, Klein MB. Is antiretroviral therapy causing long-term liver damage? A comparative analysis of HIV-mono-infected and HIV/hepatitis C co-infected cohorts. PLoS ONE 2009; 4:e4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999; 30:1356–1362. [DOI] [PubMed] [Google Scholar]
- 35.Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 2001; 34:738–744. [DOI] [PubMed] [Google Scholar]
- 36.Rivero-Juárez A, Camacho A, Merchante N, et al. Incidence of liver damage of uncertain origin in HIV patients not co-infected with HCV/HBV. PLoS ONE 2013; 8:e68953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142:1592–1609. [DOI] [PubMed] [Google Scholar]
- 38.European AIDS Clinical Society (EACS). EACS Guidelines 2014. 2014. [Google Scholar]
- 39.Schwabl P, Bota S, Salzl P, et al. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int 2015; 35:381–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


