Supplemental Digital Content is available in the text
Abstract
The issue of the clinical appropriateness of blood transfusion has become a focus of transfusion medicine worldwide. In China, irrational uses of blood have often been reported in recent years. However, to date there lacks a systematic review of the rational uses of blood.
This study aimed to determine the clinical appropriateness of blood transfusion in China.
We searched PubMed, Web of Science, the Cochrane Library, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database, WanFang Database, and Chinese BioMedical Literature Database, and the retrieval cut-off date was June 31, 2015. SPSS 17.0 and MetaAnalyst 3.13 were employed as the statistics tools in this review. A pooled rate of clinical inappropriateness of transfusion was analyzed by DerSimonian–Laird method.
In this study, a total of 39 observational studies were included, which related to 75,132 cases of blood transfusion. According to the meta-analysis results, the overall incidence of clinical inappropriateness of transfusion in China was estimated to be 37.3% (95% confidence interval [CI] [32.1, 42.8]). The subgroup analyses revealed that the pooled rates of clinical inappropriateness of transfusion of plasma, red blood cells (RBCs), cryoprecipitate, and platelets were 56.3% (95% CI [45.8, 66.2]), 30.9% (95% CI [27.1, 35.0]), 25.2% (95% CI [13.2, 42.7]), and 14.1% (95% CI [8.8, 21.9]), respectively. However, the pooled incidence of inappropriateness of transfusion in operative departments was 47.5% (95% CI [36.8, 58.3]), which was significantly higher than that in nonoperative departments, 25.8% (95% CI [18.7, 34.4], P < 0.05). The overall rates of inappropriate use were 36.7% (95% CI [30.2, 43.6]) in major cities and 37.5% (95% CI [31.2, 44.3]) in other cities, respectively; there was no statistically significant difference (P > 0.05).
In conclusion, China has suffered from a disadvantage in the clinical appropriateness of blood transfusion, especially in plasma and RBC use. In future, comprehensive measures should be implemented in order to improve the clinical appropriateness of blood transfusion.
INTRODUCTION
Blood transfusion has become an irreplaceable means of clinical treatment. However, transfusion related adverse events, such as immunologic reaction and infection, have been reported.1–11 Previous studies have shown restrictive transfusion strategies are superior to liberal transfusion strategies.12–20 According to a systematic review and meta-analysis published in 2015, compared with liberal strategies, restrictive transfusion strategies were associated with a reduction in the number of red blood cell (RBC) units transfused and number of patients being transfused, but disadvantageous outcomes seemed to be unaltered.19 Restrictive transfusion strategies are safe in most clinical settings, and liberal transfusion strategies have not been shown to convey any benefit to patients.19 However, another recent meta-analysis showed that, in patients with critical illness or bleed, restricting blood transfusions by using a hemoglobin trigger of <7 g/dL significantly reduces cardiac events, rebleeding, bacterial infections, and total mortality.20 The World Health Organization proposed the rational use of blood and blood products to reduce unnecessary transfusions and minimize the risks associated with transfusion.21 Many countries have developed national guidelines on the appropriate clinical use of blood.22–32 The Ministry of Health of the People's Republic of China formulated guidelines for blood transfusion in 200033 and the regulations for clinical management of blood use in medical institutions in 1999 and 2012.34,35 Moreover, in 2010, a national program for improvement of medical quality was launched by the Ministry of Health, which especially emphasized the rational use of blood and the conduct of relevant audits.36 In response, the problem of appropriate blood use has caused widespread concern among the Chinese, and irrational uses of blood have often been reported in recent years. However, considering the lack of a systematic review and meta-analysis of rational uses of blood in China, we performed the current study to clarify the relevant issues.
MATERIALS AND METHODS
Ethnic Statement
All analyses in this study were based on the previous published papers. Therefore, ethical approval and patient consent are not required.
Eligibility Criteria
The inclusion criteria were as follows: observational studies that reported the incidence of inappropriateness of blood transfusion; studies that used the Specifications and Guidelines of Clinical Blood Transfusion Technology33 as the standard to determine blood transfusion appropriateness; studies carried out in China; studies involved in clinical blood transfusion cases; studies that reported the total number of cases of blood use and the cases of blood inappropriate use; and design type was observational study. Exclusion criteria were as follows: the records were not related to this study; incorrect or inconsistent data were confirmed; and the records were confirmed to be repeated or indexed in different databases.
Information Sources
The following electronic databases were searched: PubMed, Web of Science, the Cochrane Library, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database, WanFang Database, and BioMedical Literature Chinese Database. Additional data and information were solicited from the authors of the included studies.
Search Strategy for Identification of Studies
The retrieval terms used were “blood transfusion,” “blood use,” “clinical,” “appropriateness,” “inappropriateness,” “China,” “blood product,” “blood component,” “irrational,” “irrationality” “rational,” “plasma,” “red blood cell,” “white blood cell,” “cryoprecipitate,” “granulocyte,” and related entry terms. The terms were searched in different combinations. Limits for language were not used in this study. The retrieval cut-off time was June 31, 2015.
Study Selection, and Quality and Bias of Studies
In the study, 2 independent reviewers (QYL and ZSG) independently screened the references in accordance with the inclusion and exclusion criteria and extracted the data according to a format table. Disagreements were resolved by consensus or by a third reviewer (CTZ).
Loney scoring system37 was employed as an analytic tool for assessing the methodological quality in this study. The Loney scoring system provides a checklist including 8 items (each item was assigned a score of 1 point, totaling 8 points): random sample or whole population, unbiased sampling frame, adequate sample size, standardized measures, outcomes measured by unbiased assessors, adequate response rate (at least 70%), and description of refusers, confidence interval (CI) and subgroup analysis, and all study subjects described.37,38 Scores of 0 to 4 indicated lower quality, while scores greater than 5 suggested higher quality.38
Statistical Analysis
Statistical calculations were performed using the Statistical Package for Social Sciences program (SPSS, Chicago, IL) version 17.0 and MetaAnalyst version 3.13 (Boston, MA), and the pooled rate with 95% CI was automatically calculated. The heterogeneity test was performed by homogeneity test. Heterogeneity among the studies was evaluated by the Cochran Chi-squared test (Cochran Q) and the I2 statistics. If there was heterogeneity among the included studies, a random-effect model (REM) to pool the incidences was used; otherwise, a fixed-effect model (FEM) was selected. In this study, the DerSimonian–Laird method was used for the REM.39 In addition, Begg funnel plot by MetaAnalyst version 3.13 was used for the observation of the potential publication bias.
RESULTS
Study Selection
A total of 4345 records were retrieved from the databases, 66 of which were selected for full-text assessment for eligibility. Finally, 39 observational studies involving 75,132 transfusion cases were included in this study.40–78 The process of database retrieval and literature screening is shown in Figure 1. The review protocols were in accordance with the Preferred Report Items for Systematic Reviews and Meta-analyses 2009 Checklist (Supplemental file 1).
FIGURE 1.
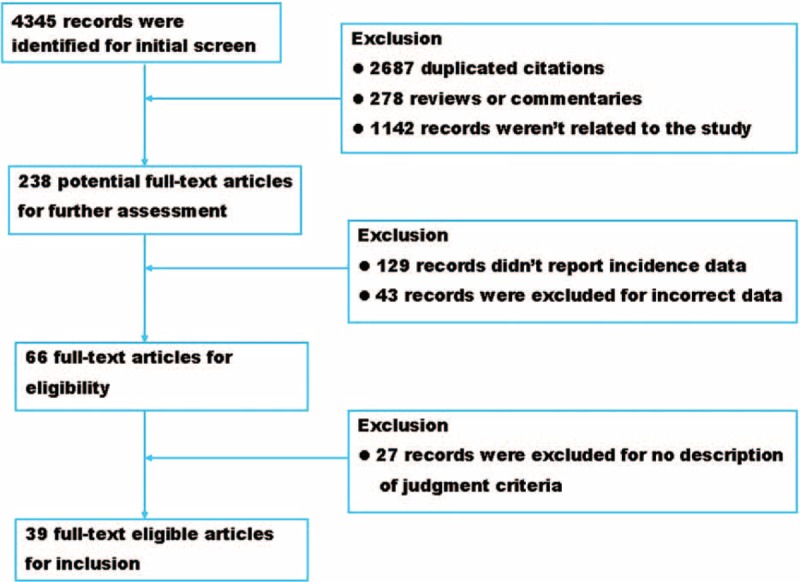
The flow chart of database retrieval and literature screening in this study.
Characteristics and Quality Evaluation of Included Studies
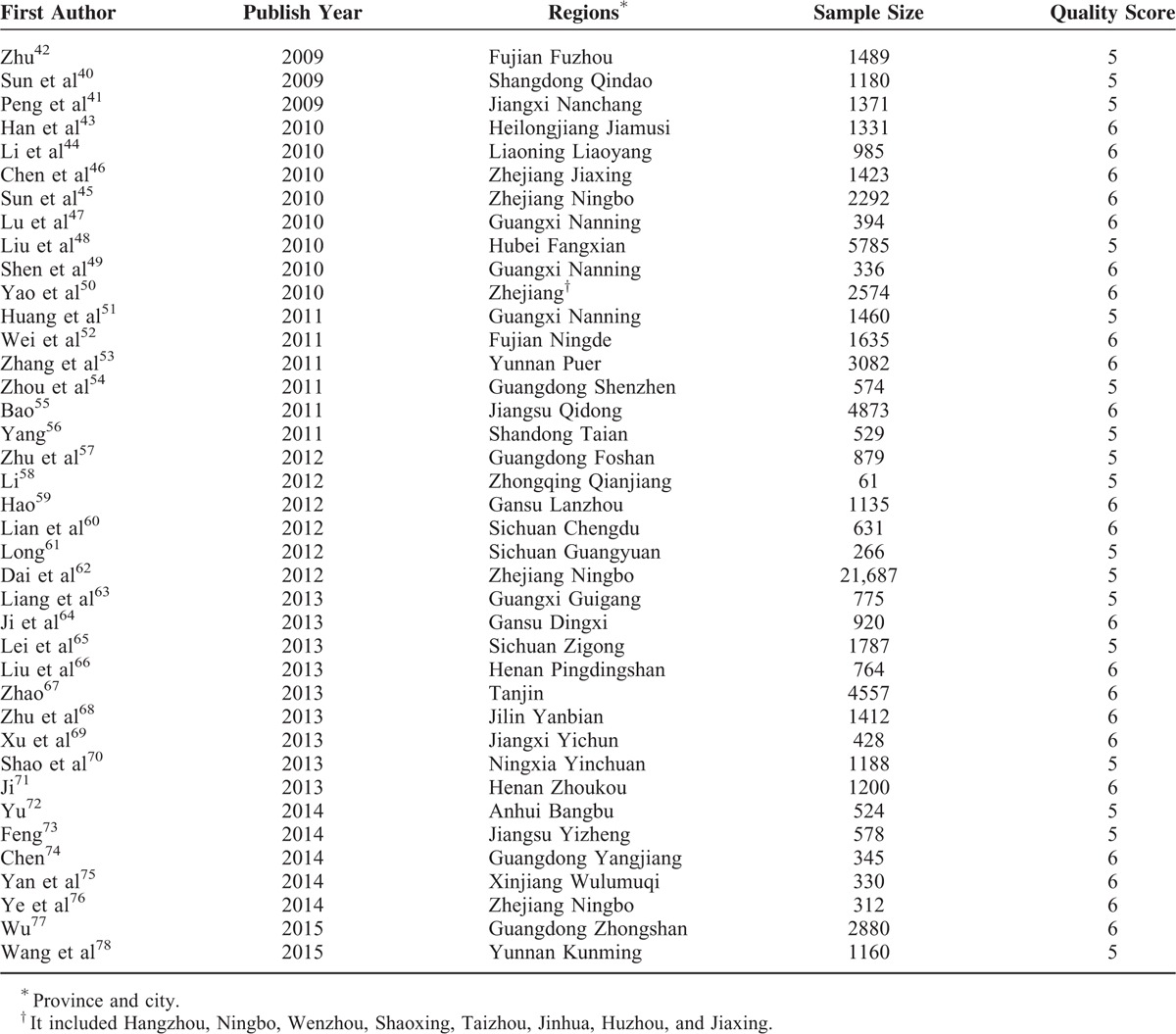
The studies included 75,132 cases of blood transfusion, performed in 44 cities under the jurisdiction of 17 provinces and 2 municipalities in China. As far as the geographical and population distribution were concerned, the sampling in this study was representative. In the study, the sample size ranged from 61 to 21,687 cases, a median of 1060; however, among subgroups, the sample size had great difference (Table 1). The Loney system score evaluation revealed that the range of the report quality scores was from 5 to 6 points and that the average quality score was 5.6 points. The overall quality of the literature was high. The characteristics and quality evaluation of included studies is shown in Table 2.
TABLE 1.
Sample Analysis in Each of Groups in This Study
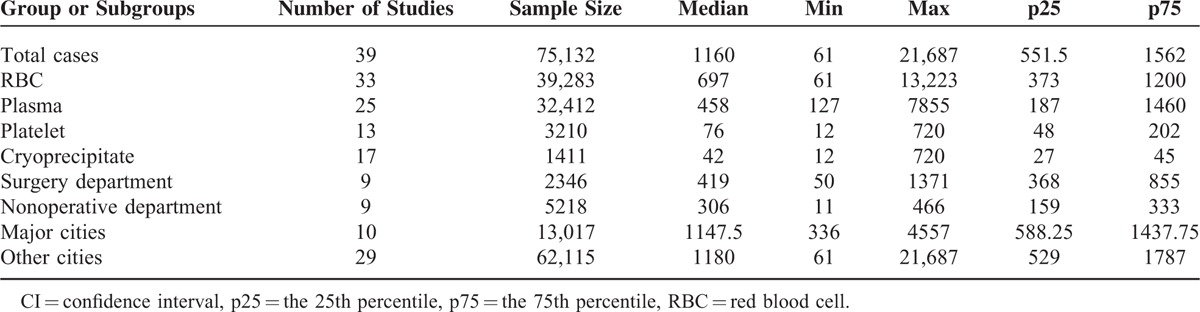
TABLE 2.
General Characteristics and Quality Evaluation of the Included Studies
Synthesis of Results
Heterogeneity tests and the main results of the meta-analysis are shown in Table 3. The results of the meta-analysis indicated that the overall rate of clinical irrational blood use was 37.3% (95% CI [32.1, 42.8], REM) (Fig. 2). Further subgroup analysis showed that the pooled rate of clinical irrational transfusion in RBC use was 30.9% (95% CI [27.1, 35.0], REM) (Fig. 3); in plasma use it was 56.3% (95% CI [45.8, 66.2], REM) (Fig. 4); in platelet use it was 14.1% (95% CI [8.8, 21.9], REM) (Fig. 5); and in cryoprecipitate use it was 25.2% (95% CI [13.2, 42.7], REM) (Fig. 6).
TABLE 3.
Heterogeneity Tests of Meta-Analysis in This Study
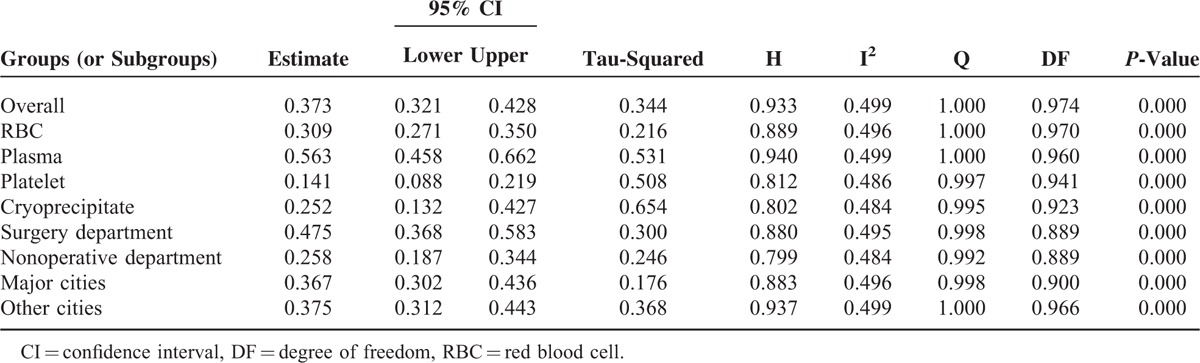
FIGURE 2.
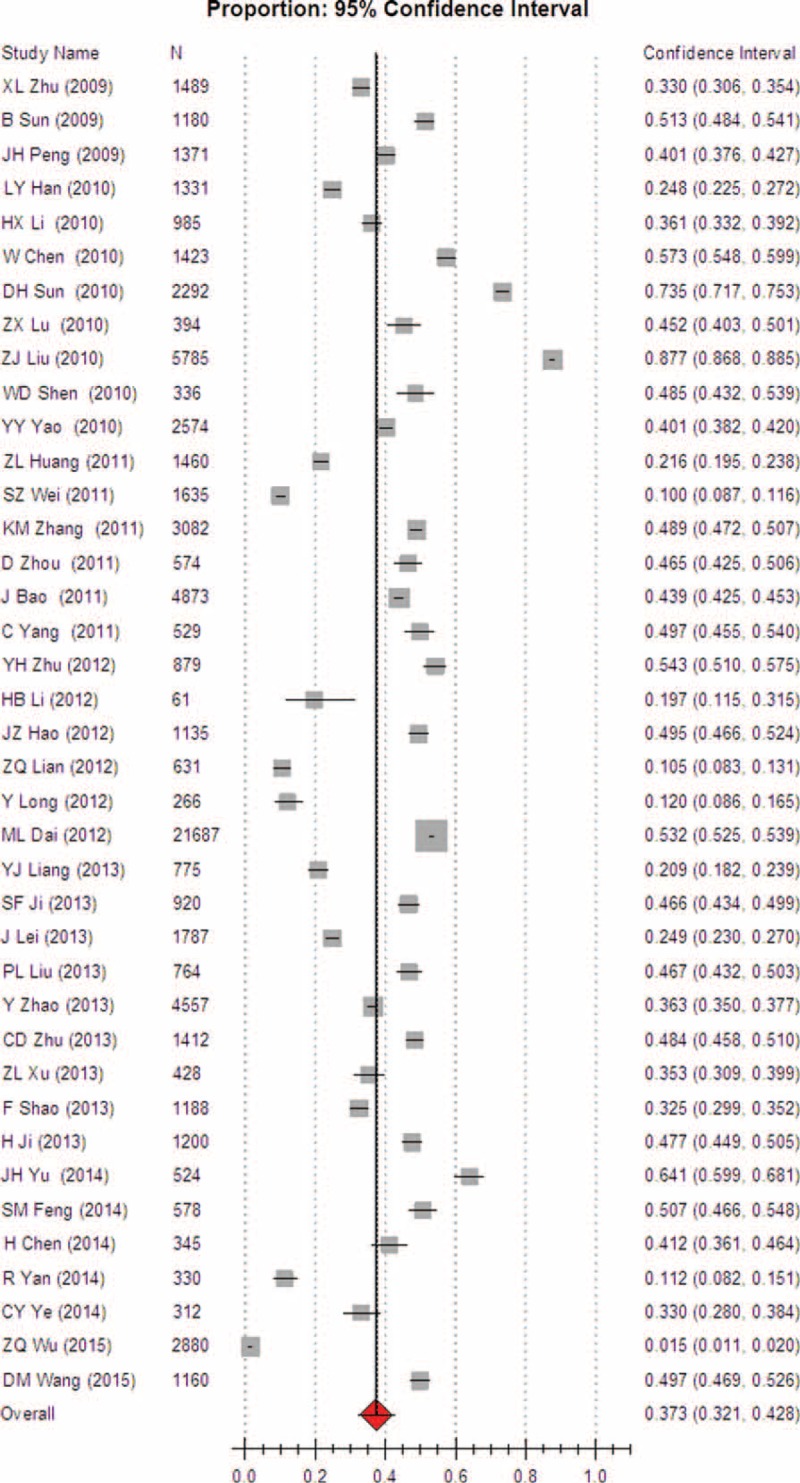
Meta-analysis of the overall rate of clinical irrational blood use.
FIGURE 3.
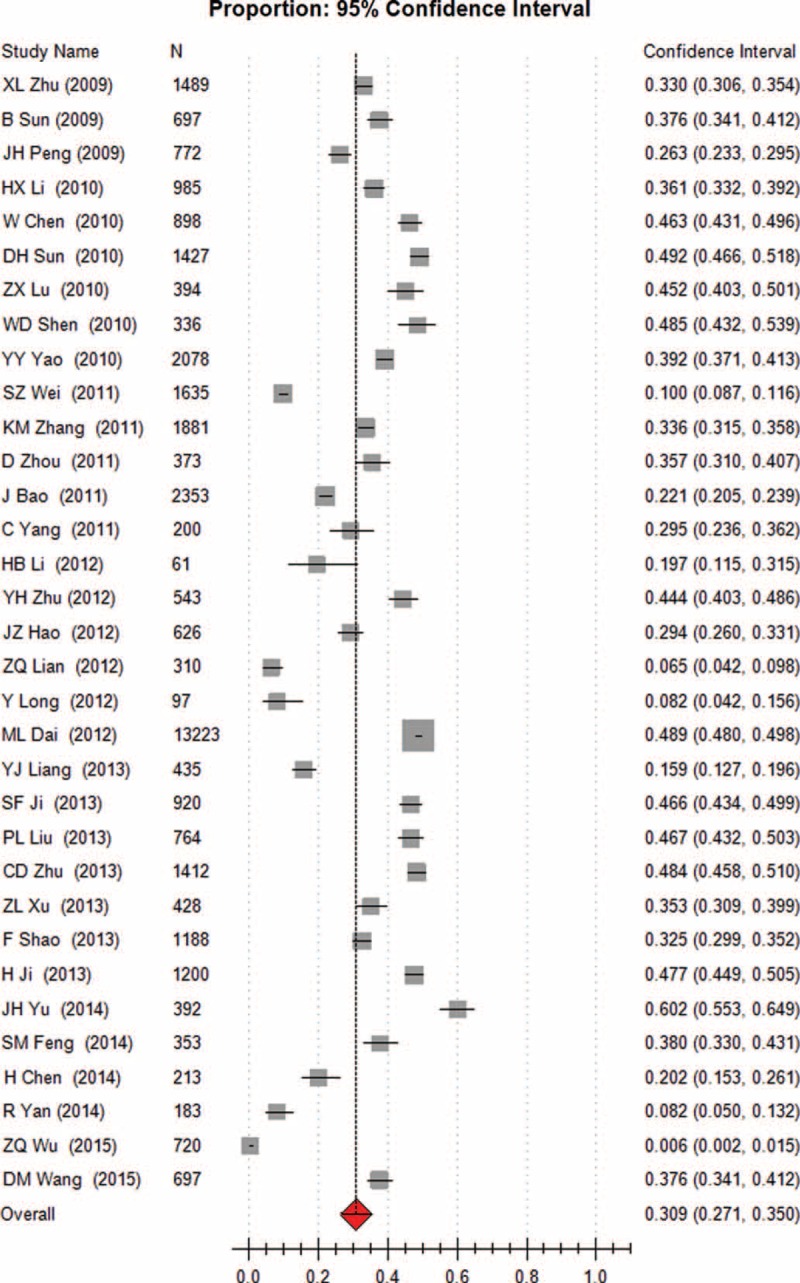
Meta-analysis of the pooled rate of clinical irrational use in red blood cell.
FIGURE 4.
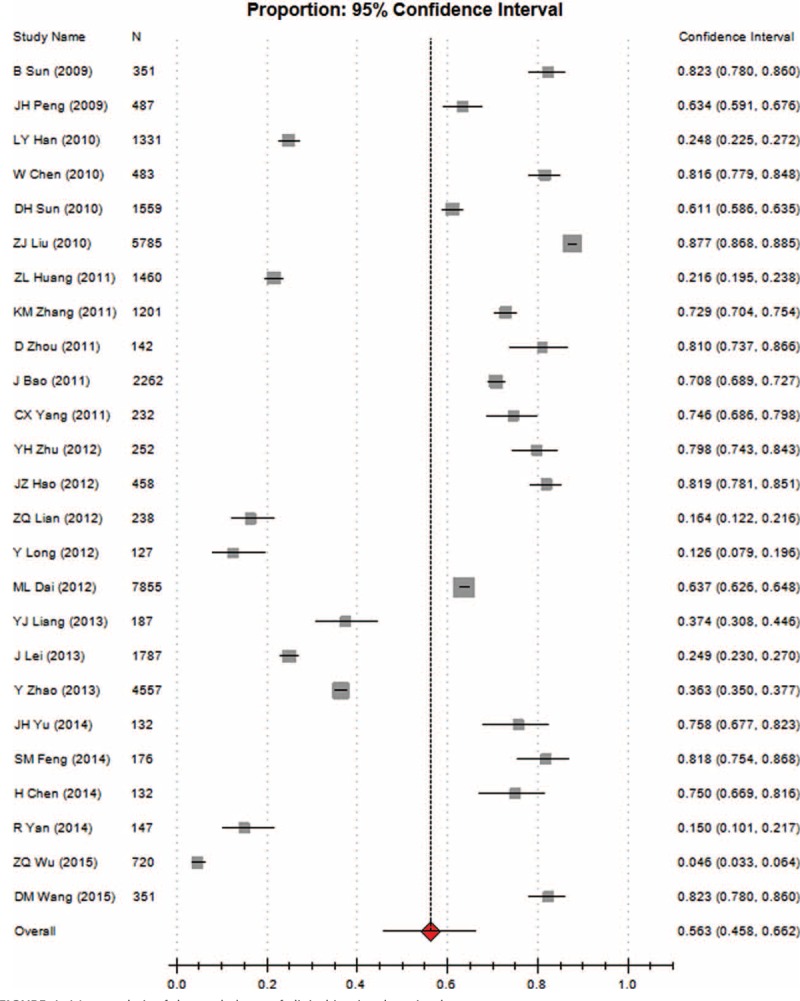
Meta-analysis of the pooled rate of clinical irrational use in plasma.
FIGURE 5.
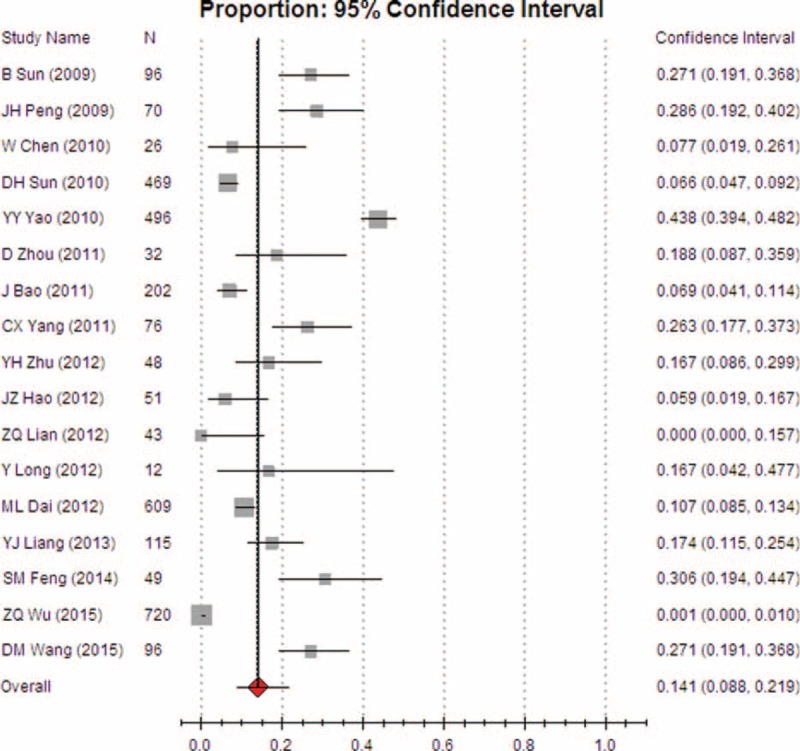
Meta-analysis of the pooled rate of clinical irrational use in platelet.
FIGURE 6.
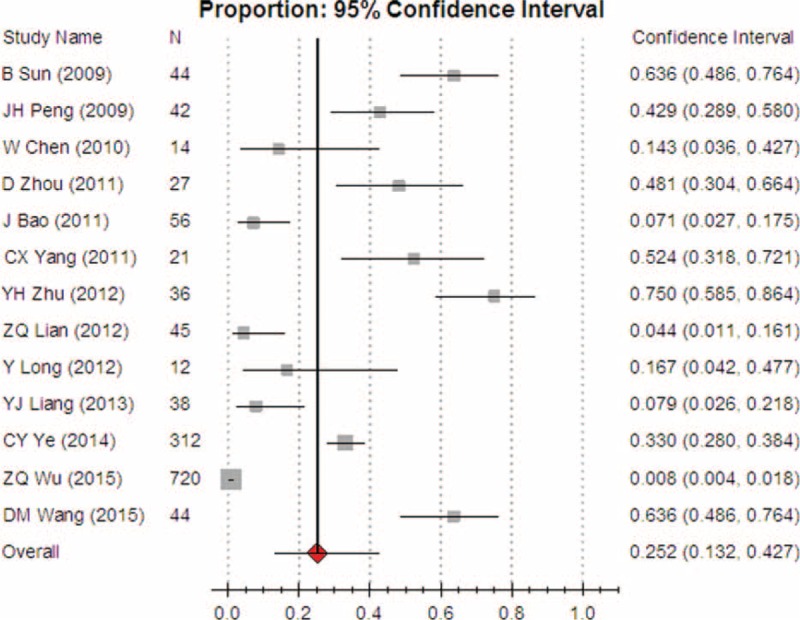
Meta-analysis of the pooled rate of clinical irrational use in cryoprecipitate.
Comparisons of subgroups showed that the pooled incidence of inappropriate transfusion in surgery departments was 47.5% (95% CI [36.8, 58.3], REM) (Fig. 7), while the incidence in nonoperative departments was 25.8% (95% CI [18.7, 34.4], REM) (Fig. 8). The pooled rate in surgery departments was significantly higher than that in nonoperative departments (P < 0.05).
FIGURE 7.
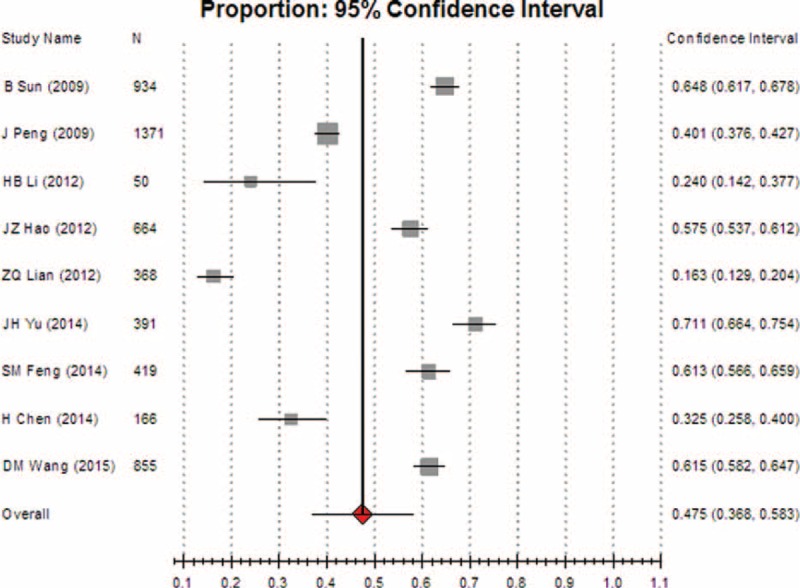
Meta-analysis of the pooled rate of clinical irrational use in operative departments.
FIGURE 8.
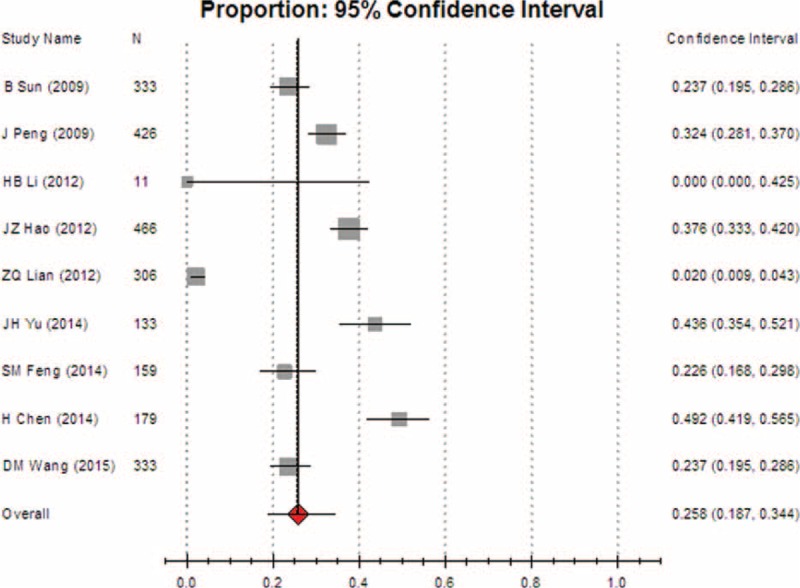
Meta-analysis of the pooled rate of clinical irrational use in nonoperative departments.
The overall rates of inappropriate use were 36.7% (95% CI [30.2, 43.6], REM) in major cities (including municipalities and provincial capitals) (Fig. 9) and 37.5% (95% CI [31.2, 44.3], REM) in other cities (Fig. 10), respectively. No statistically significant difference was found between them (P > 0.05).
FIGURE 9.
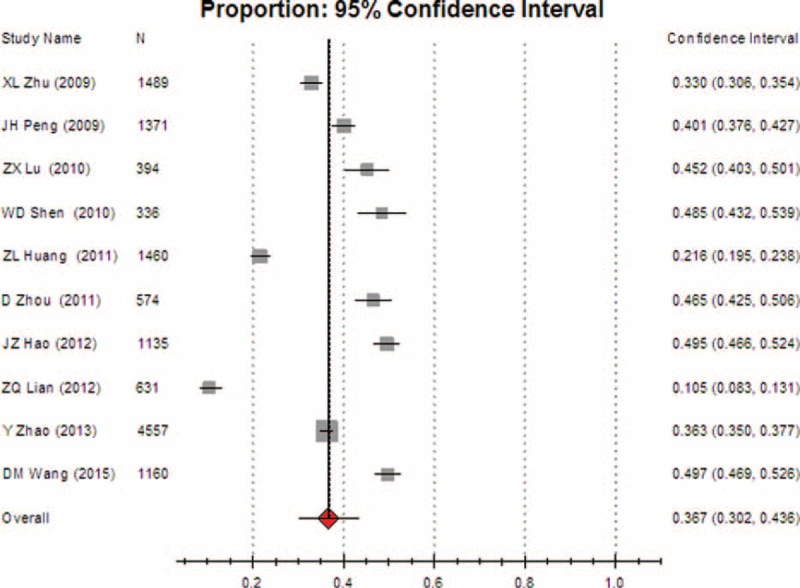
Meta-analysis of the pooled rate of clinical irrational use in major cities.
FIGURE 10.
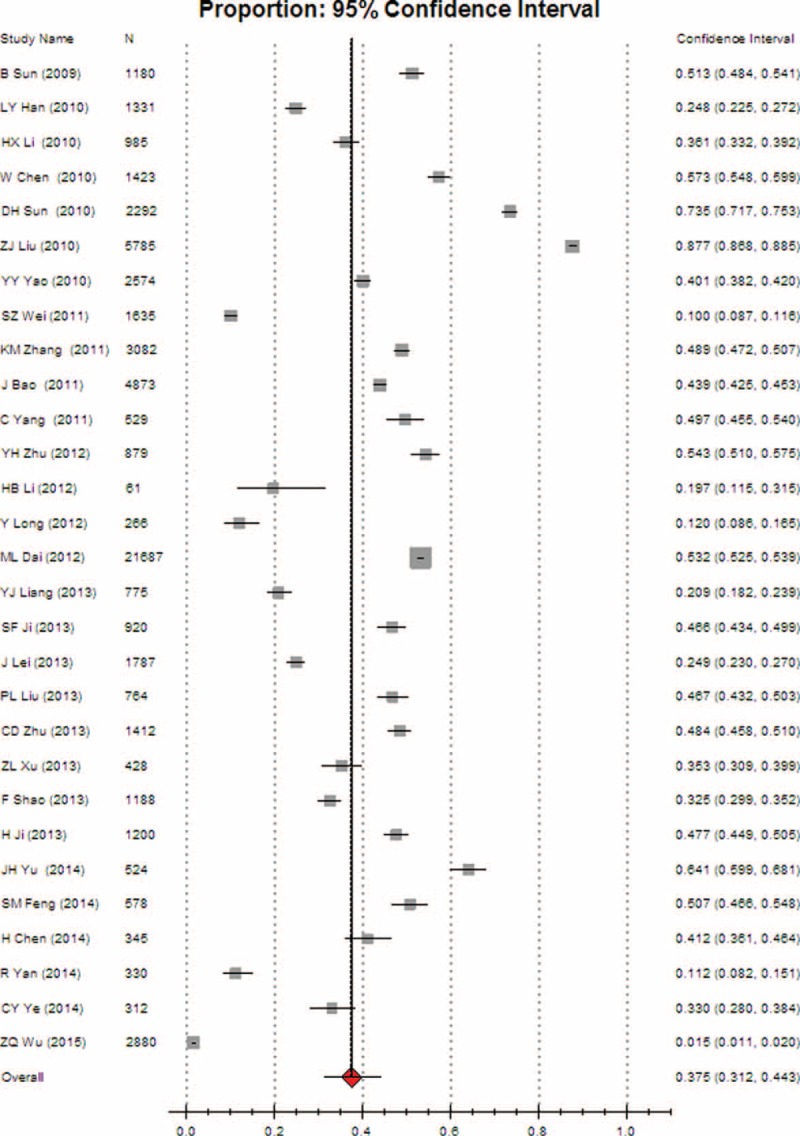
Meta-analysis of the pooled rate of clinical irrational use in other cities.
Publication Bias
The funnel plot was asymmetric (Fig. 11), suggesting that a publication bias occurred in this study.
FIGURE 11.
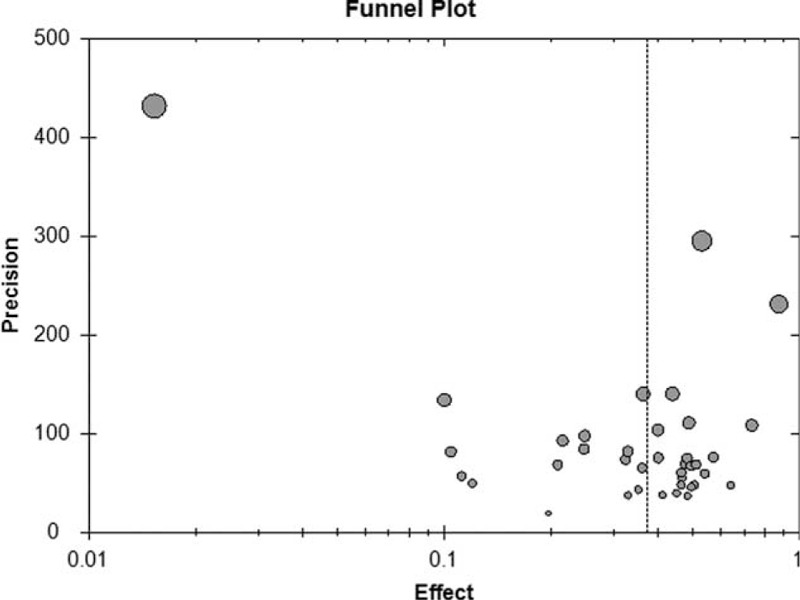
Funnel plot for the observation of the publication bias.
DISCUSSION
Blood and blood products are a limited resource and therefore should be used effectively. The irrational use of blood is not only a waste of precious medical resources, but also increases the blood transfusion risk and economic burden. Therefore, research into the rationality of clinical blood transfusion and subsequent management is warranted. In recent years, the issue of the appropriateness of blood use has become a focus.79–93 In 2012, 70% of countries had a national blood policy. Overall, 62% countries (81% high-income countries, 60% middle-income countries, and 44% low-income countries) had specific legislation covering the management of blood transfusion.20 Currently, most developed countries have implemented a national guideline for blood use. However, some studies have reported that there are still different degrees of inappropriate blood use in these countries.84–93
According to a previous report from Sydney, New South Wales, and Australia, a total of 1117 medical records from 10 major urban hospitals in 1998 and 1999 were audited in order to assess the appropriateness of RBC transfusions. The authors of the report found that about a 3rd of RBC transfusions were assessed as inappropriate, and more RBC transfusions were inappropriate in surgical patients than in those treated by other specialties.92
A recent study in a tertiary hospital in the United States reported that the implementation by blood bank staff of more rigorous prospective audits of orders for blood products resulted in a significant decrease of 9.1% and 10.3% in the use of fresh frozen plasma and platelets, respectively.93 In China, although most urban hospitals have established a Hospital Transfusion Committee as required by the relevant regulations since 2009, the current status of appropriate blood use seems to not be satisfactory, based on the published reports in recent years.
The above facts show that both developed and developing countries have issues with appropriate blood use. More attention should be paid to this issue worldwide. However, to date, there has not been a systematic review and meta-analysis of irrational uses of blood in China or in other countries. Therefore, we performed this study to clarify the relevant issues.
In this study, we included 39 reports involving 75,132 cases of blood transfusion in China. As far as geographical and population distribution was concerned, the sampling in this study was representative. In this study, the Loney system score evaluation revealed that the report quality ranged from 5 to 6 points and that the average quality score was 5.6 points, suggesting that the overall quality of the included studies was high.
Meta-analysis results revealed that the overall rate of clinical irrational blood use was 37.3% (95% CI [32.1, 42.8]), indicating that a considerable proportion of blood products have been irrationally used in China in recent years.
Further subgroup analysis showed that the pooled rates of clinical irrational transfusion of plasma, RBC, cryoprecipitate, and platelet were 56.3% (95% CI [45.8, 66.2]), 30.9% (95% CI [27.1, 35.0]), 25.2% (95% CI [13.2, 42.7]), and 14.1% (95% CI [8.8, 21.9]), respectively. These results suggested that among each of the blood components that we observed, the overall appropriateness was worrying.
According to the meta-analysis results, clinical appropriateness was the lowest for plasma transfusion, followed by RBC, cryoprecipitate, and platelet transfusion. The causes of the difference were complicated; however, we found that one of the most important factors may be linked to the supply shortage of these blood components. In China, the shortage of platelet supplies is most serious, followed by cryoprecipitate and RBC, while shortages of plasma supplies are relatively mild. We believe that the extent of the supply shortage of blood products plays an important role in determining the clinical practice of physicians, influencing the implementation of policies and management measures related to blood product use. For example, the pretransfusion audits of platelets are more rigorous than plasma use in clinical practice.
In reviewing the literature, we found that the main phenomena for irrational use of clinical blood were as follows: blood transfusion indications were incorrect; and the volume of blood transfusion was incorrect (beyond the limits recommended by the guidelines).32 Furthermore, we generalized that the main causes of irrational clinical blood use were: lack of transfusion knowledge amongst clinicians; low efficiency with respect to blood transfusion management; blood substitutes not available (the shortage of some plasma-derived medicinal products has often occurred in China); and unnecessary blood transfusion due to the worry of death of patients after operation. However, we also noted that the main reasons for irrational plasma use by clinicians were as follows: to improve immunity; to add up to nutrition; to increase circulating blood volume; and to supply blood albumin (plasma is more easily available and is cheaper compared to commercial albumin in China). Considering that the issue of irrational use was the most prominent for plasma, more rigorous measures should be taken to constrain and regulate the clinical use of plasma in the future.
We also found that the pooled rate of inappropriateness transfusion in surgery departments was 47.5% (95% CI [36.8, 58.3]), which was significantly higher than that in nonoperative departments, suggesting that a large difference in irrational use exists among different departments. Hence, more attention should be paid in the future to the problem of irrational transfusion in surgical patients.
Through reviewing the literature, we found that differences in the appropriateness of blood use in the different regions were closely associated with the implementation of the relevant regulations, the transfusion indicator audits, and the physicians’ compliance with the guidelines on blood transfusion. However, subgroup comparison revealed that there was no statistically significant difference in the overall rates of inappropriate use between major cities and other regions, suggesting that China has suffered from universal irrational blood uses.
None of the retrieved studies reported data for granulocyte transfusion and therefore, we did not assess the appropriateness of granulocyte transfusion. In addition, we also noted that whole blood had been rarely used and blood component transfusion had become a routine in clinical practice in recent years through the review of the literatures.
The limitations of this study were as follows:
The asymmetric funnel plot suggests that publication bias is affecting this study, indicating that studies with larger effects were more likely to be published and therefore included in the current review. In addition, the gray literature such as dissertations and unpublished data were not included in the current meta-analysis, which might exaggerate the effect sizes of the meta-analysis results.
Some studies did not report whether outcomes were measured by unbiased (blinded) assessors.
There was large variability in sample size and the incidence of inappropriate use among different studies, which conferred heterogeneity to this study.
The lack of data in some provinces might have affected the results.
Therefore, more powerful evidence and more scientific research are needed in future to clarify the rational use of blood.
At present, under the influence of cultural values and other factors, the rate of voluntary blood donation in the Chinese population is lower compared with developed countries, which has resulted in a shortage of clinical blood supply, and even in delayed operations. However, according to the above results, a considerable proportion of blood components were irrationally used, suggesting that some of the available blood resource in China was potentially wasted. Therefore, it is important that more effective measures are taken to improve the management of blood use in China. We recommend that the following methods be adopted in the future: implementation of mandatory pretransfusion approval programs such as using a computer as a guide to pretransfusion evaluation;82,94 improving the blood transfusion skills and evidence-based medicine knowledge of clinicians;80,95–99 conferring pretransfusion audit to transfusion appropriateness for blood bank staff or hematologists;100,101 and establishing more effective blood transfusion management systems, such as a national internet platform for monitoring the rational use of blood. In addition, we introduced an automatically computerized calculator for assessing the precise transfusion amount of RBC, plasma, and cryoprecipitate and the effectiveness of platelet transfusion (Supplemental file 2) in this study. By the useful software, the complicated calculation process for the pretransfusion evaluation becomes very simple; therefore, we recommend the original application software to the clinicians for the pretransfusion evaluation in clinical practice. We believe that introduction of these comprehensive measures will greatly improve the rational use of blood in China.
In conclusion, China has suffered from inappropriate clinical use of blood transfusions, especially plasma and RBC use. In future, comprehensive measures should be implemented to improve the clinical appropriateness of blood transfusion.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, RBC = red blood cell.
Changtai Zhu and Yulu Gao contributed equally to this work.
Funding: This work was supported by Research Grant from Shanghai Hospital Development Center (No. SHDC12015910), the Social Development Technology Projects of Kunshan City (No. KS1011) and the National Natural Science Foundation of China (No. 81372212).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Parker RI. Transfusion in critically III children: indications, risks, and challenges. Crit Care Med 2014; 42:675–690. [DOI] [PubMed] [Google Scholar]
- 2.Squires JE. Risks of transfusion. South Med J 2011; 1041:762–769. [DOI] [PubMed] [Google Scholar]
- 3.Berseus O, Boman K, Nessen SC, et al. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion 2013; 53:114s–123s. [DOI] [PubMed] [Google Scholar]
- 4.Patel SV, Kidane B, Klingel M, et al. Risks associated with red blood cell transfusion in the trauma population, a meta-analysis. Injury 2014; 45:1522–1533. [DOI] [PubMed] [Google Scholar]
- 5.Brick T, Peters MJ. Risks and benefits of transfusion for children with severe anemia in Africa. BMC Med 2014; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belyakova VV, Gukasyan IA, Donskaya OV, et al. Residual risks of transfusion transmissive transfer of HIV infection and viral hepatitis C in the Moscow region in laboratory screening of donor blood by Nat technologies. Gematol Transfuziol 2014; 59:15–18. [Google Scholar]
- 7.Wang JX, Liu J, Yao FZ, et al. Prevalence, incidence, and residual risks for transfusion-transmitted human immunodeficiency virus Types 1 and 2 infection among Chinese blood donors. Transfusion 2013; 53:1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pattison JW. Ensuring appropriate use of blood transfusion in anaemia. Nurs Times 2005; 101:30–33. [PubMed] [Google Scholar]
- 9.Laperche S, Lefrere JJ, Morel P, et al. Blood transfusion: control of infectious risks. Presse Med 2015; 44:189–199. [DOI] [PubMed] [Google Scholar]
- 10.Menis M, Forshee RA, McKean S, et al. Transfusion-related acute lung injury rali occurrence among inpatient medicaid beneficiaries, under 65 years of age, as recorded by large administrative databases during 2007–2010. Value Health 2015; 18:169. [Google Scholar]
- 11.Rocha BD. TRALI or ARDS or TDGE versus blood transfusion. Rev Bras Cir Cardiovasc 2015; 30:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLoughery TG. Review: restrictive RBC transfusion strategies reduce nosocomial infections compared with liberal strategies. Ann Intern Med 2014; 161:JC11. [DOI] [PubMed] [Google Scholar]
- 13.Jairath V, Kahan BC, Gray A, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding RIGGER: a pragmatic, open-label, cluster randomised feasibility trial. Lancet 2015; 6736:61999. [DOI] [PubMed] [Google Scholar]
- 14.Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion 2014; 540 (Pt 2:)2753–2759. [DOI] [PubMed] [Google Scholar]
- 15.Mirski MA, Frank SM, Kor DJ, et al. Restrictive and liberal red cell transfusion strategies in adult patients: reconciling clinical data with best practice. Crit Care 2015; 19:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boone JD, Kim KH, Marques M, et al. Compliance rates and outcomes associated with a restrictive transfusion policy in gynecologic oncology patients. Gynecol Oncol 2014; 132:227–230. [DOI] [PubMed] [Google Scholar]
- 17.Dunn A. ACP Journal Club A restrictive transfusion strategy reduced 45-day mortality in patients with acute upper GI bleeding. Ann Intern Med 2013; 19:158.JC6. [DOI] [PubMed] [Google Scholar]
- 18.Cuenca J, Garcia-Erce JA, Martinez F, et al. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol reduce the need for allogeneic blood after knee replacement surgery. Transfusion 2006; 46:1112–1119. [DOI] [PubMed] [Google Scholar]
- 19.Holst LB, Petersen MW, Haase N, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015; 350:h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014; 127:124–131. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Blood safety and availability. 2015. http://www.who.int/mediacentre/factsheets/fs279 (accessed August 10, 2015). [Google Scholar]
- 22.Koh BC, Chong LL, Goh LG, et al. Ministry of health clinical practice guidelines: clinical blood transfusion. Singapore Med J 2011; 52:209–218. [PubMed] [Google Scholar]
- 23.Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol 2012; 160:445–464. [DOI] [PubMed] [Google Scholar]
- 24.Lundh B. [Guidelines of blood transfusion.]. Lakartidningen 1972; 69 Suppl 4:55–58. [PubMed] [Google Scholar]
- 25.Hervig T, Flesland O, Svenningsen V, et al. Guidelines for transfusion in Norway. Transfus Apher Sci 2004; 31:181–184. [DOI] [PubMed] [Google Scholar]
- 26.Baele PL, Muylle L, Noens L, et al. Guidelines for the transfusion of red cells. Acta Clin Belg 2008; 63:301–312. [DOI] [PubMed] [Google Scholar]
- 27.Salazar M. [Guidelines for the transfusion of blood and its components]. Rev Panam Salud Publica 2003; 3–13.183-190. [DOI] [PubMed] [Google Scholar]
- 28.Joint Working Party of the Transfusion and Clinical Haematology Task Forces of the British Committee for Standards in Haematology. Guidelines for the clinical use of blood cell separators. Clin Lab Haematol 1998; 20:265–278. [PubMed] [Google Scholar]
- 29.Ohsaka A, Kikuta A, Ohto H, et al. Guidelines for safety management of granulocyte transfusion in Japan. Int J Hematol 2010; 91:201–208. [DOI] [PubMed] [Google Scholar]
- 30.Sagawa K. [Guidelines for safer and more appropriate use of blood transfusion systems in Japan]. Nihon Geka Gakkai Zasshi 2005; 106:7–12. [PubMed] [Google Scholar]
- 31.Milkins C, Berryman J, Cantwell C, et al. Guidelines for pre-transfusion compatibility procedures in blood transfusion laboratories. British Committee for Standards in Haematology. Transfus Med 2015; 23:3–35. [DOI] [PubMed] [Google Scholar]
- 32.Hessel EA, Levy JH. Guidelines for perioperative blood transfusion and conservation in cardiac surgery: lessons and challenges. Anesth Analg 2010; 111:1555–1559. [DOI] [PubMed] [Google Scholar]
- 33.The Ministry of Health of the People's Republic of China. Specifications and guidelines of clinical blood transfusion technology. 2000. http://www.moh.gov.cn/mohyzs/s3589/200804/18676.shtml (accessed August 10, 2015). [Google Scholar]
- 34.The Ministry of Health of the People's Republic of China. The Regulation of Clinical Management for Blood Use in Medical Institutions (Trial Version). 1999. http://china.findlaw.cn/yiliao/yiliaofagui/ylsgqltl/sifajieshi/14592.html (accessed August 10, 2015). [Google Scholar]
- 35.The Ministry of Health of the People's Republic of China. The Regulation of Clinical Management for Blood Use in Medical stitutions (Order No. 85th of the Ministry of Health). 2012. http://www.moh.gov.cn/mohzcfgs/s3576/201206/55072.shtml (accessed August 10, 2015). [Google Scholar]
- 36.The Ministry of Health of Republic of China. The Notification of the Launch of 2010 National Program of Improvement of Medical Quality. 2010. http://www.gov.cn/gzdt/2010-05/24/content_1612245.htm (accessed August 10, 2015). [Google Scholar]
- 37.Loney PL, Chambers LW, Bennett KJ, et al. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can 1998; 19:170–176. [PubMed] [Google Scholar]
- 38.Hertz JT, Reardon JM, Rodrigues CG, et al. Acute myocardial infarction in Sub-Saharan Africa: the need for data. PLoS One 2014; 9:e96688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 40.Sun B, Liu SZ, Chen C, et al. Cross-sectional study of clinical use transfusion. Journal of Qilu Medicine 2009; 06:531–532. [Google Scholar]
- 41.Peng JH, Zhang XQ, Ma HW, et al. Investigation of clinical blood use in Jiangxi Province. Journal of Jiangxi Medical College 2009; 10:107–109. [Google Scholar]
- 42.Zhu XL, Wang HY, Huang H, et al. Evaluation of the indications of perioperative red blood cell transfusion in tertiary hospitals Fujian Province in 2008. Chinese Journal of Blood Transfusion 2009; 11:914–916. [Google Scholar]
- 43.Han LY, Fan YL, Chi JQ, et al. Observation of 875 cases of patients with plasma tranfusion. Heilongjiang Medicine and Pharmacy 2010; 33:90–190. [Google Scholar]
- 44.Li HX, Liu JH. Observation of the transfusion appropriateness in 985 cases of patients with red blood cell. Medical Information 2010; 23:669. [Google Scholar]
- 45.Sun DH. Analysis of scientific appropriateness of 3460 cases of clinical blood transfusion. Modern Pratical Medicine 2010; 22:405–409. [Google Scholar]
- 46.Chen W, Sun JL, Xu LP, et al. Observation of 1043 cases of perioperative period transfusion. Zhe Jiang Medical Journal 2010; 32:1271–1274. [Google Scholar]
- 47.Lu ZX, Zhou JW. Observation of clinical blood transfusion in rural of Nanning, Guangxi province. Internal Medical Journal 2010; 5:505–506. [Google Scholar]
- 48.Liu ZJ, Tan DY, Rao R, et al. Analysis of causes and countermeasures of plasma irrational use in primary hospitals. International Journal of Medical Laboratory Medicine 2010; 311:1311–1313. [Google Scholar]
- 49.Shen DW, Zhong P, Li B, et al. Observation and analysis of clinical blood transfusion appropriateness. Chinese Journal of Health Quality Management 2010; 17:82–84. [Google Scholar]
- 50.Yao YY, Wang HY, Zhu SM, et al. Survey on clinical blood transfusion of the perioperative incidences in hospital settings in Zhejiang Province. Laboratory Medicine and Clinic 2010; 90:894–897. [Google Scholar]
- 51.Huang ZL. Clinical observation and clinical laboratory medicine. Clinical observation on 982 plasma infusions. Laboratory Medicine and Clinic 2011; 81:1306–1307. [Google Scholar]
- 52.Wei SZ, Huang ZR, Li XG, et al. Analysis of the appropriateness on 1488 red blood cell transfusions. Journal of Clinical Hematology 2011; 24:736–738. [Google Scholar]
- 53.Zhang KM, Lan S, Ou H, et al. Analysis of the appropriateness on 1942 patients with perioperative blood transfusion. Heilongjiang Medical Journal 2011; 35:145–146. [Google Scholar]
- 54.Zhou D, Yang BC, Zhu WG, et al. Observation on the current status of obstetric blood component transfusion. Journal of Clinical Transfusion and Laboratory 2011; 13:218–221. [Google Scholar]
- 55.Bao J. Analysis of appropriateness and safety in clinical use of blood transfusion. Medicine Information 2011; 24:1148–1149. [Google Scholar]
- 56.Yang CX. Observation of status of transfusion departments and clinical blood use in the secondary level hospitals or above Taian. Journal of Clinical Hematology 2011; 24:742–744. [Google Scholar]
- 57.Zhu YH, Ma CH, Lu J, et al. Observation of clinical blood use in Foshan, Guangdong. Guangxi Medicine 2012; 34:929–930. [Google Scholar]
- 58.Li HB. Analysis of clinical blood use in Qianjiang District. Inner Mongolia Journal of Traditional Chinese Medicine 2010; 23:90–91. [Google Scholar]
- 59.Hao JZ. Analysis and observation of appropriateness in clinical use of blood in Lanzhou. Gansu Science and Technology 2012; 28:139–140.101. [Google Scholar]
- 60.Lian ZQ, Wu WJ, Kang K, et al. Observation of clinical transfusion-related medical records in a tertiary first rate hospital. Chinese Journal of Blood transfusion 2012; 252:1306–1307. [Google Scholar]
- 61.Long Y. Observation and analysis of transfusion appropriateness in a hospital. Frontiers of Medicine 2012; 334:106–107. [Google Scholar]
- 62.Dai ML, Huang CS, Gui Y, et al. Observation of clinical perioperative blood use in those more than secondary level of hospitals in Ningbo. Zhejiang Medical Journal 2012; 34:107–109. [Google Scholar]
- 63.Liang YJ, Yang K. Analysis of the irrational transfusion in medical audit on the transfusion-related records in Guigang. The Chinese and Foreign Health Abstract 2013; 344:126–127. [Google Scholar]
- 64.Ji SF, Xu CJ. Analysis of the unreasonable transfusion in clinical settings. Health Vocational Education 2013; 314:151–152. [Google Scholar]
- 65.Lei J, Song ZL. Observation and analysis of plasma application in clinical settings. Laboratory Medicine and Clinic 2013; 10:612–613. [Google Scholar]
- 66.Liu PL, Dong KY, Xu GB, et al. Observation of the appropriateness of surgery blood transfusion in an area. Guide of China Medicine 2013; 121:141–142. [Google Scholar]
- 67.Zhao Y. Evaluation and intervention of transfusion department in reasonable transfusion of fresh frozen plasma for clinical use. Beijing Medicine 2013; 35:305–306. [Google Scholar]
- 68.Zhu CD, Ding XZ, Jin YS, et al. Observation and analysis of clinical red blood cell transfusion in Yanbian. Journal of Clinical Transfusion and Laboratory 2013; 15:270–271. [Google Scholar]
- 69.Xu ZL, Yi CX, Lu HP, et al. Retrospective analysis of appropriateness transfusion in hospital settings. Chinese Medical Innovation 2013; 104:89–91. [Google Scholar]
- 70.Shao F, Song LJ, Yang J, et al. Survey on clinical blood use in Yinchuan. Journal of Ningxia Medical University 2013; 35:78–80. [Google Scholar]
- 71.Ji H. Observation and analysis of clinical red blood cell use in Zhoukou. Chinese Modern Drug Application 2013; 78:253–254. [Google Scholar]
- 72.Yu JH. Appropriateness evaluation on the medical records in 339 cases of clinical blood transfusion in Bengbu. Huaihai Medicine 2014; 12:550–551. [Google Scholar]
- 73.Feng SM. Observation of Clinical blood transfusion in Yizheng People's Hospital, Jiangsu Province. Shanxi Medical Journal 2014; 43:636–638. [Google Scholar]
- 74.Chen H. Appropriateness observation and analysis of clinical blood use in Yangjiang. International Medicine & Health Guidance News 2014; 204:2158–2160. [Google Scholar]
- 75.Yan R, Meng FF, Liu YH, et al. Management analysis of perioperative transfusion in cases with tumor. China Oncology 2014; 241:857–860. [Google Scholar]
- 76.Ye CY, Yu Y, Chen WY, et al. Survey on cryoprecipitate use from 2005 to 2013 in hospital setting in Ningbo. Modern Practical Medicine 2014; 262:1578–1580. [Google Scholar]
- 77.Wu ZQ. Observation of clinical blood use in an area. Guide of China Medicine 2015; 134:96–97. [Google Scholar]
- 78.Wang DM, Ding SB, Tong L, et al. Appropriateness analysis of clinical blood transfusion in hospital settings. Journal of Kunming Medical University 2015; 36:171–172. [Google Scholar]
- 79.Desalu I, Kushimo OT, Bode CO, et al. Appropriateness of intra-operative blood transfusion in children at the Lagos University Teaching Hospital – an initial survey. Nig Q J Hosp Med 2009; 19:131–134. [DOI] [PubMed] [Google Scholar]
- 80.Liumbruno GM, Rafanelli D. Appropriateness of blood transfusion and physicians’ education: a continuous challenge for Hospital Transfusion Committees? Blood Transfus 2011; 10:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osei EN, Odoi AT, Owusu-Ofori S, Allain JP. Appropriateness of blood product transfusion in the Obstetrics and Gynaecology & G department of a tertiary hospital in West Africa. Transfus Med 2013; 23:160–166. [DOI] [PubMed] [Google Scholar]
- 82.Lin YC, Chang CS, Yeh CJ, et al. The appropriateness and physician compliance of platelet usage by a computerized transfusion decision support system in a medical center. Transfusion 2010; 50:2565–2570. [DOI] [PubMed] [Google Scholar]
- 83.Zielinski MD, Park MS, Jenkins D. Appropriate evidence-based practice guidelines for plasma transfusion would include a high ratio of plasma to red blood cells based on the available data. Transfusion 2010; 50:2762. [DOI] [PubMed] [Google Scholar]
- 84.Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev 2011; 25:232–246. [DOI] [PubMed] [Google Scholar]
- 85.Barr PJ, Donnelly M, Cardwell CR, et al. The appropriateness of red blood cell use and the extent of overtransfusion: right decision? Right amount? Transfusion 2011; 51:1684–1694. [DOI] [PubMed] [Google Scholar]
- 86.Murphy MF, Waters JH, Wood EM, Yazer MH. Transfusing blood safely and appropriately. BMJ 2013; 347:f4303. [DOI] [PubMed] [Google Scholar]
- 87.Leal-Noval SR, Arellano-Orden V, Maestre-Romero A, et al. Impact of national transfusion indicators on appropriate blood usage in critically ill patients. Transfusion 2011; 51:1957–1965. [DOI] [PubMed] [Google Scholar]
- 88.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340:409–417. [DOI] [PubMed] [Google Scholar]
- 89.So-Osman C, Cicilia J, Brand A, et al. Triggers and appropriateness of red blood cell transfusions in the postpartum patient--a retrospective audit. Vox Sang 2009; 98:65–69. [DOI] [PubMed] [Google Scholar]
- 90.Silverman JA, Barrett J, Callum JL. The appropriateness of red blood cell transfusions in the peripartum patient. Obstet Gynecol 2004; 104:1000–1004. [DOI] [PubMed] [Google Scholar]
- 91.Friedman MT, Ebrahim A. Adequacy of physician documentation of red blood cell transfusion and correlation with assessment of transfusion appropriateness. Arch Pathol Lab Med 2006; 130:474–479. [DOI] [PubMed] [Google Scholar]
- 92.Rubin GL, Schofield WN, Dean MG, et al. Appropriateness of red blood cell transfusions in major urban hospitals and effectiveness of an intervention. Med J Aust 2001; 175:354–358. [DOI] [PubMed] [Google Scholar]
- 93.Haldiman L, Zia H, Singh G. Improving appropriateness of blood utilization through prospective review of requests for blood products: the role of pathology residents as consultants. Lab Med 2014; 45:264–271. [DOI] [PubMed] [Google Scholar]
- 94.Chang CS, Lin YC, Wu YC, et al. The effects of a computerized transfusion decision support system on physician compliance and its appropriateness for fresh frozen plasma use in a medical center. Am J Clin Pathol 2011; 135:417–422. [DOI] [PubMed] [Google Scholar]
- 95.Haspel RL. Implementation and assessment of a resident curriculum in evidence-based transfusion medicine. Arch Pathol Lab Med 2010; 134:1054–1059. [DOI] [PubMed] [Google Scholar]
- 96.Rana R, Afessa B, Keegan MT, et al. Evidence-based red cell transfusion in the critically ill: quality improvement using computerized physician order entry. Crit Care Med 2006; 34:1892–1897. [DOI] [PubMed] [Google Scholar]
- 97.Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program 2007; 1:172–178. [DOI] [PubMed] [Google Scholar]
- 98.Roback JD. Evidence-based guidelines for blood transfusion. J Infus Nurs 2015; 35:187–190. [DOI] [PubMed] [Google Scholar]
- 99.Heddle NM. Evidence-based decision making in transfusion medicine. Vox Sang 2006; 91:214–220. [DOI] [PubMed] [Google Scholar]
- 100.Luk C, Eckert KM, Barr RM, et al. Prospective audit of the use of fresh-frozen plasma, based on Canadian Medical Association transfusion guidelines. CMAJ 2002; 1662:1539–1540. [PMC free article] [PubMed] [Google Scholar]
- 101.Hawkins TE, JCarter M, Hunter PM. Can mandatory pretransfusion approval programmes be improved? Transfus Med 1994; 4:45–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


