Abstract
Effects of vitamin D on acute exacerbation, lung function, and fraction of exhaled nitric oxide (FeNO) in patients with asthma are controversial. We aim to further evaluate the roles of vitamin D supplementation in addition to asthma controllers in asthmatics.
From 1946 to July 2015, we searched the PubMed, Embase, Medline, Cochrane Central Register of Controlled Trials, and ISI Web of Science using “Vitamin D,” “Vit D,” or “VitD” and “asthma,” and manually reviewed the references listed in the identified articles. Randomized controlled trials which reported rate of asthma exacerbations and adverse events, forced expiratory volume in 1 s (FEV1, % of predicted value), FeNO, asthma control test (ACT), and serum 25-hydroxyvitamin D levels were eligible. We conducted the heterogeneities test and sensitivity analysis of the enrolled studies, and random-effects or fixed-effects model was applied to calculate risk ratio (RR) and mean difference for dichotomous and continuous data, respectively. Cochrane systematic review software Review Manager (RevMan) was used to test the hypothesis by Mann–Whitney U test, which were displayed in Forest plots.
Seven trials with a total of 903 patients with asthma were pooled in our final studies. Except for asthma exacerbations (I2 = 81%, χ2 = 10.28, P = 0.006), we did not find statistical heterogeneity in outcome measures. The pooled RR of asthma exacerbation was 0.66 (95% confidence interval: 0.32–1.37), but without significant difference (z = 1.12, P = 0.26), neither was in FEV1 (z = 0.30, P = 0.77), FeNO (z = 0.28, P = 0.78), or ACT (z = 0.92, P = 0.36), although serum 25-hydroxyvitamin D was significantly increased (z = 6.16, P < 0.001).
Vitamin D supplementation in addition to asthma controllers cannot decrease asthma exacerbation and FeNO, nor improve lung function and asthma symptoms, although it can be safely applied to increase serum 25-hydroxyvitamin D levels.
INTRODUCTION
Asthma is a chronic inflammatory disorder of the airways in which many cells and cellular elements involved, such as eosinophils, mast cells, and T helper (Th) lymphocytes.1 It is estimated an annual worldwide deaths from asthma is as high as 250,000, and acute exacerbations are major causes of morbidity and mortality.2 A variety of factors may cause asthma exacerbations, including allergens, pollutants, and poor inherence to the controllers, while viral upper respiratory infections are predominant reasons. In order to improve asthma clinical outcomes, it is essential to seek strategies to decrease asthma exacerbations.
Vitamin D were reported to prevent adverse influence of corticosteroids, which are the core controllers for asthma due to the potent anti-inflammation effects, such as bone mass density decrease and fractures.3,4 Observational studies have demonstrated that lower serum 25-hydroxyvitamin D levels were associated with worse lung function, worse corticosteroid responsiveness, greater exacerbation frequency, as well as more susceptibility to upper respiratory infections in patients with asthma.5–7 Arshi et al8 enrolled 130 patients with moderate persistent asthma in an open-label randomized controlled trial (RCT) and they found that vitamin D supplementation significantly improved forced expiratory volume in 1 s (FEV1) and ratio of FEV1 to forced vital capacity (FEV1/FVC). However, Castro et al9 recently illustrated that treatment with vitamin D had no effect on lung function (FEV1: −0.07 [95% confidence interval (CI): −0.14 to 0.01] vs −0.04 [95% CI: −0.11 to 0.03], P = 0.64), and did not reduce the rate of first asthma exacerbation (13% [95% CI: 8–18%] vs 19% [95% CI: 13–24%], P = 0.21). Therefore, the effects of vitamin D supplementation in addition to asthma controllers on acute exacerbation and lung function in patients with asthma are still controversial thus necessitating further evaluations.
In this study, we conducted a meta-analysis of all published RCTs to further clarify the efficacy and safety of vitamin D in patients with asthma.
METHODS
The Institutional Ethical Committee for Clinical and Biomedical Research of West China Hospital (Sichuan, China) approved our study protocol. Each enrolled trial included approved protocol by respective institutional review board, and all participants provided written informed consent.
Search Strategies
We conducted a comprehensive computer search, from 1946 to July 2015, in PubMed, Embase, Medline, Cochrane Central Register of Controlled Trials (CENTRAL), and ISI Web of Science using “Vitamin D,” “Vit D,” or “VitD” and “asthma.” Publication type of RCTs was limited. A review of references listed in the identified articles and a manual search of the related articles were performed to identify all relevant and eligible studies and minimize publication bias.
Inclusion and Exclusion Criteria
Eligible clinical trials were defined based on the following criteria: study design was RCT; asthma was diagnosed by physicians according to the Global Initiative for Asthma (GINA) or relevant guidelines before randomization; intervention treatment was vitamin D, regardless of the drug names, doses, and administration routines; outcome measures included rate of asthma exacerbations and adverse events, lung functions such as FEV1 (% of predicted value), fraction of exhaled nitric oxide (FeNO), asthma symptom scores such as asthma control test (ACT), and serum 25-hydroxyvitamin D levels. We did not include trials that were nonrandomized controlled or observational, neither were cohort or case–control studies.
Study Selection
Two investigators conducted study selection independently in 2 phases. First, they discarded duplicated and nonrandomized controlled studies by screening titles and abstracts. Second, eligible studies were extracted by reviewing full texts according to the study inclusion criteria. Any disagreement was solved by mutual consensus in the presence of a third investigator.
Data Extraction
Independently, 2 data collectors extracted and recorded desirable information of each enrolled study in a standard form recommended by Cochrane,10 which included authors, publication year, country, study design, participants and population, demographic characteristics (age, gender, body mass index [BMI], etc.), baseline characteristics (FEV1, FeNO, serum 25-hydroxyvitamin D levels, etc.), details of intervention treatment (drug name, dose, administration routine, and duration), follow-up period, and outcome measures and study results. For any missing data information, corresponding authors were contacted via email to request the full original data. Different opinions between the 2 collectors were determined by mutual consensus in the presence of a third investigator.
Quality Assessment
We used the Cochrane risk of bias tool to assess the quality of methods conducted and outcomes reported by each eligible study.10 The studies were considered as good quality if they meet the following criteria: random sequence generation (low selection bias); allocation concealment (low selection bias); blinding of participants and personnel (low performance bias); blinding of related outcomes assessment (low detection bias); incomplete outcome data (low attrition bias); selective reporting (low reporting bias); and without other bias. Two independent assessors performed the quality assessment, and any divergence was resolved by reaching a consensus or consulting a third investigator.
Statistical Analysis
An independent statistician performed the statistical analysis using Cochrane systematic review software Review Manager (RevMan; Version 5.3.5., The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014). Mann–Whitney U test was used for hypothesis test with statistical significance rendered as z-value and P value < 0.05, and the results were displayed in Forest plots.
Dichotomous variables were reported as frequency and proportion, while continuous variables were showed as mean and standard derivation (SD). An initial test for clinical, methodological, and statistical heterogeneities was conducted, and we used the χ2 test with P < 0.1 and I2 > 50% indicating significance. We also performed the sensitivity analysis to substitute alternative decisions or ranges of values for decisions that were arbitrary or unclear.
Random-effects model was applied in the presence of statistical heterogeneity; otherwise fixed-effects model was used. For dichotomous data we calculated risk ratio (RR) and 95% CI, while for continuous data we calculated mean difference and SD. Furthermore, in terms of FEV1, FeNO, ACT, and serum 25-hydroxyvitamin D levels, we separately conducted sub-analysis at different follow-up time points.
RESULTS
Primordially we identified 90 records in the electronic databases and extracted another 2 records from the reference lists. After screening the titles and abstracts, 83 studies were discarded, of which 25 studies were duplicated, 36 studies were not RCTs, 17 studies did not enroll asthmatic patients, and 5 studies did not prescribe vitamin D as intervention treatment. The remaining 9 studies were searched for full-text articles, and eventually we enrolled 7 trials3,4,9,11–14 in the final analysis, because the 2 studies discarded did not report our predefined outcome measures (Fig. 1).
FIGURE 1.
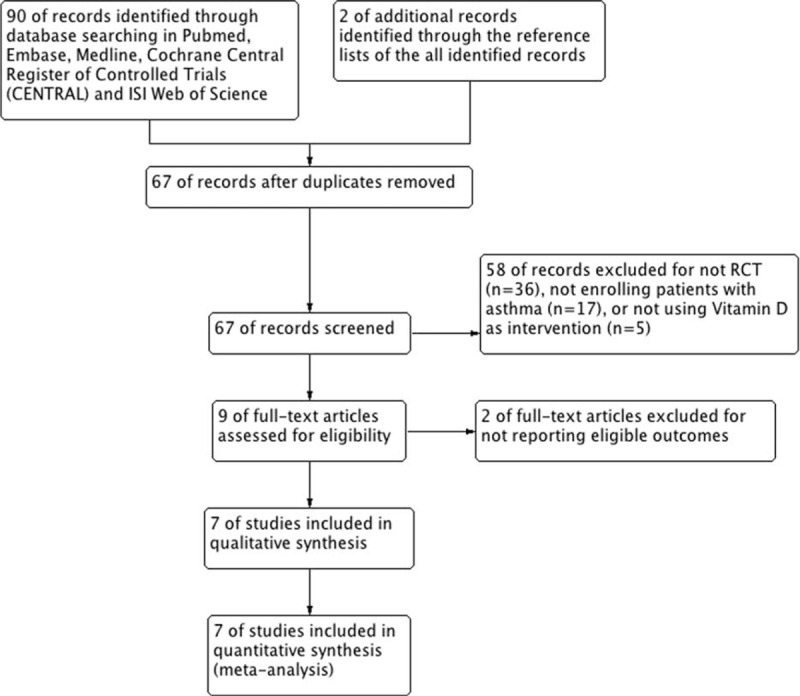
Study flow diagram. RCT = randomized controlled trial.
Study Description
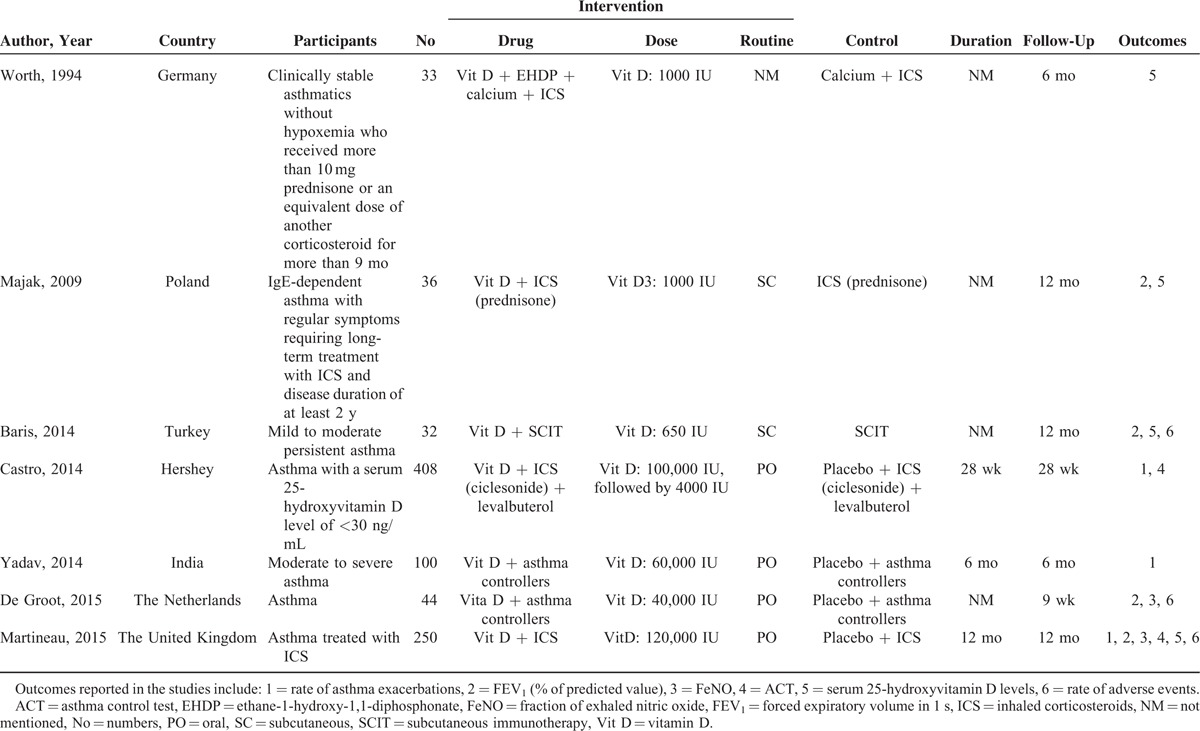
Seven enrolled trials were conducted separately in different countries, that is, Germany, Poland, Turkey, Hershey, India, The Netherlands, and The United Kingdom. Three studies were conducted in children,4,11,12 while 4 studies were in adults.3,9,13,14 Patients with clinically stable asthma, persistent asthma, and IgE-dependent asthma were, respectively, enrolled in 1 study,3,4,11 while the other 4 studies did not report the clinical features of the enrolled patients. Two studies applied identical dose (1000 IU) of vitamin D,3,4 and 2 studies4,11 and 4 studies,9,12–14 respectively, administered vitamin D subcutaneously and orally. Treatment duration were reported in 3 studies,9,12,14 and all studies reported the follow-up period, 1 in 9 weeks,13 1 in 28 weeks,9 2 in 6 months,3,12 and 3 in 12 months.4,11,14 In terms of the outcome measurements, 3 studies reported asthma exacerbation rate,9,12,14 4 studies reported change of FEV1 (% of predicted value),4,11,13,14 2 studies reported change of FeNO,13,14 2 studies provided data on change of ACT,9,14 4 studies provided change levels of 25-hydroxyvitamin D,3,4,11,14 and 3 studies provided rate of adverse events.11,13,14 Details of patients’ characteristics, intervention strategies, and outcomes were summarized in Table 1.
TABLE 1.
Details of the 7 Studies Reviewed
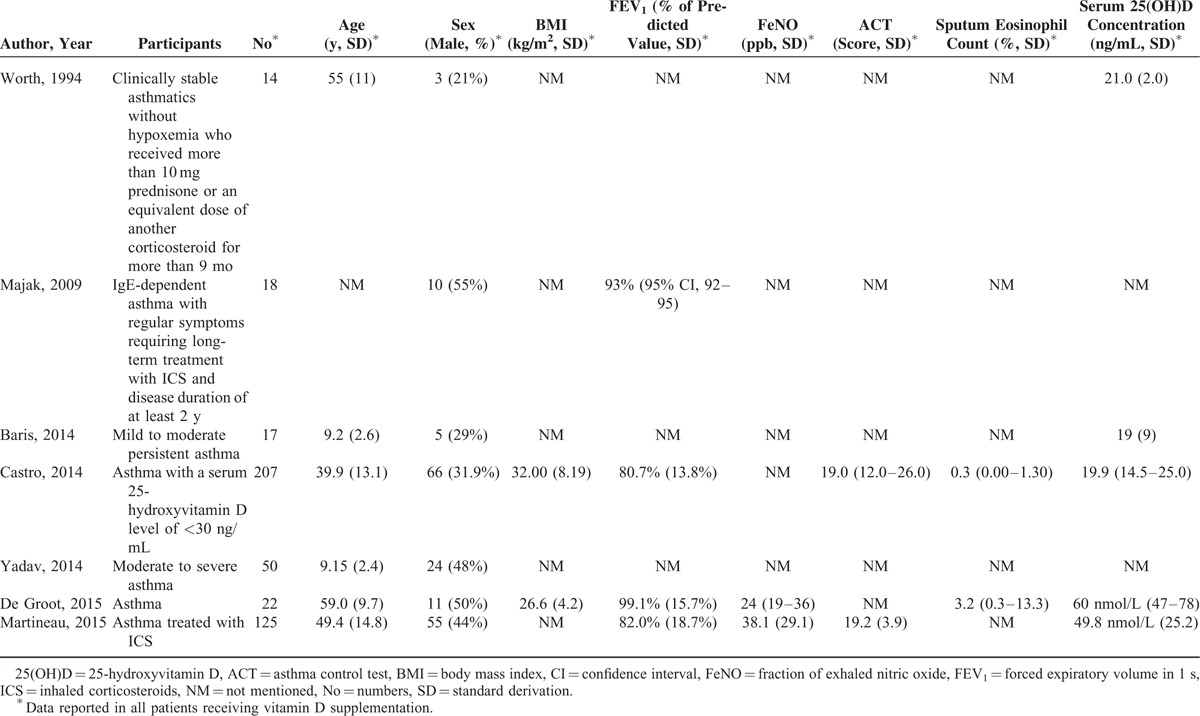
A total of 903 patients with asthma were pooled in our final studies, of which, 453 (50.2%) received vitamin D supplementation in addition to common asthma controllers. Baseline characteristics of the patients in the 7 enrolled studies were summarized in Table 2, all studies showed the data of sex proportion, but not for other demographic measures, such as age, BMI, FEV1, FeNO, as well as ACT. Baseline serum 25-hydroxyvitamin D levels in patients receiving vitamin D supplementation ranged from 19.0 to 21.0 ng/mL (49.8–60.0 nmol/L), which were also included in Table 2.
TABLE 2.
Baseline Characteristics of Patients in the 7 Studies Included
In patients with administration of vitamin D, 3 trials11,13,14 reported serious adverse events such as malignant cancer, acute appendicitis, internal fixation and upper gastrointestinal bleeding, and nonserious adverse events; for example, local urticarial plaques at the injection sites, acute respiratory infection, allergy symptoms, and psychiatric events. However, hypercalcemia was not observed in any of the patients.
Quality assessment of the 7 enrolled studies showed no bias in selection, attribution, or reporting, although 2 studies3,11 did not blind participants and personnel as well as outcome assessment (Figs. 2 and 3). We did sensitivity analysis, which resulted in no studies excluding for low quality or dubious decisions.
FIGURE 2.
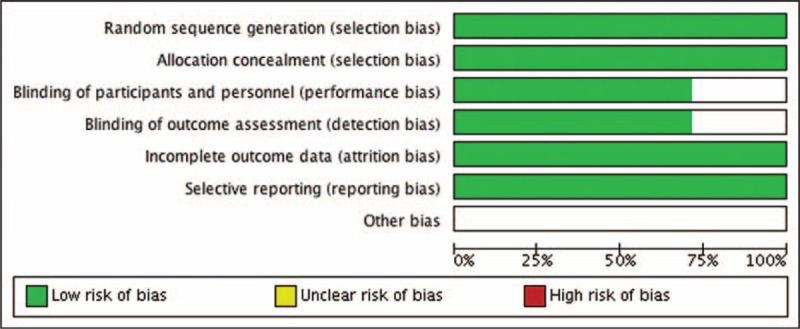
Risk of bias graph.
FIGURE 3.
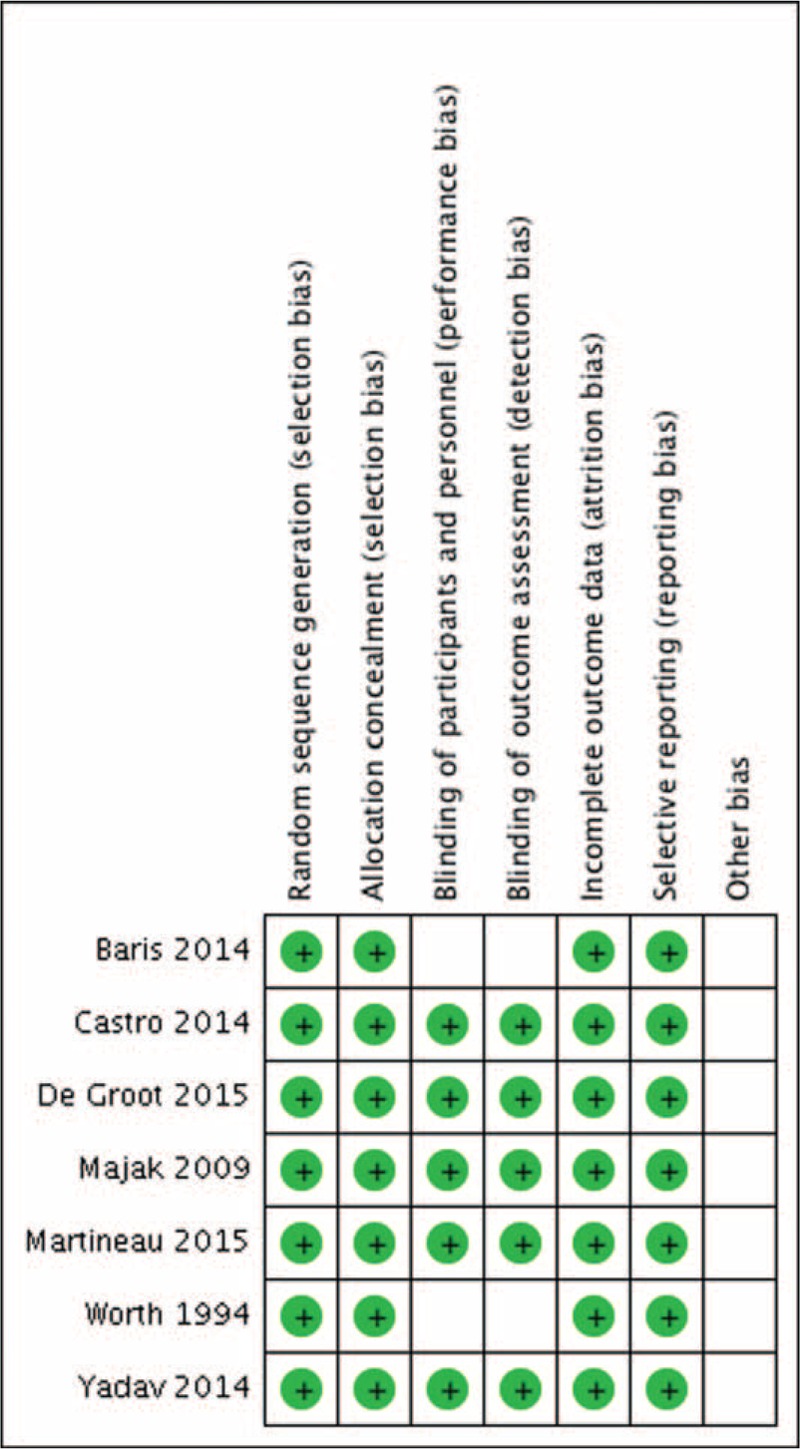
Risk of bias summary.
Heterogeneity
For all outcome measures, except for asthma exacerbations (I2 = 81%, χ2 = 10.28, P = 0.006), we did not find statistical heterogeneity (Figs. 4–9), thus we used fixed-effects model to analyze the effects of vitamin D on FEV1, FeNO, ACT, and serum 25-hydroxyvitamin D levels.
FIGURE 4.
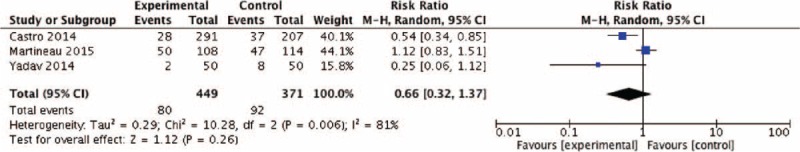
Effect of vitamin D versus placebo on asthma exacerbation. CI = confidence interval, M-H = Mantel-Haenszel.
FIGURE 9.
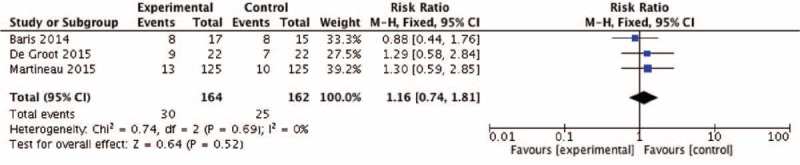
Effect of vitamin D versus placebo on adverse events. CI = confidence interval, M-H = Mantel-Haenszel.
Outcomes
The pooled RR of asthma exacerbation and adverse events rate was 0.66 (95% CI: 0.32–1.37) and 1.16 (95% CI: 0.74–1.81), but both data analysis did not show any significant differences (z = 1.12, P = 0.26 and z = 0.64, P = 0.52, respectively) (Figs. 4 and 9). Figure 5 showed that the pooled SD of FEV1 change at the 2-month and 12-month follow-up point from baseline were −0.03 (95% CI: −0.26 to 0.20) and 0.01 (95% CI: −0.21 to 0.23), but they did not show significant differences (z = 0.23, P = 0.82 and z = 0.07, P = 0.94, respectively), neither did the overall effect (z = 0.30, P = 0.77). Figures 6 and 7, respectively, presented the pooled effects of vitamin D on FeNO and ACT; however, we did not find significant differences at different follow-up points or in the overall effects.
FIGURE 5.
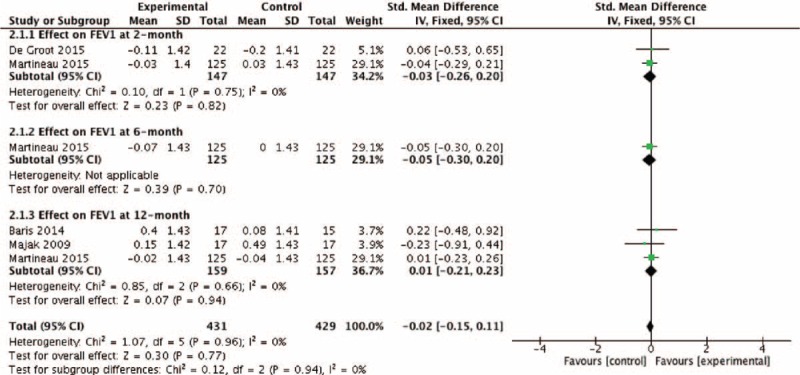
Effect of vitamin D versus placebo on FEV1 (% of predicted value). CI = confidence interval, FEV1 = forced expiratory volume in 1 s, SD = standard derivation.
FIGURE 6.
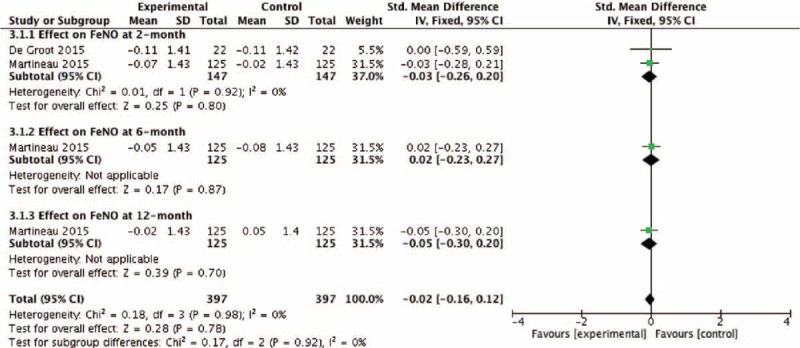
Effect of vitamin D versus placebo on FeNO. CI = confidence interval, FeNO = fraction of exhaled nitric oxide, SD = standard derivation.
FIGURE 7.
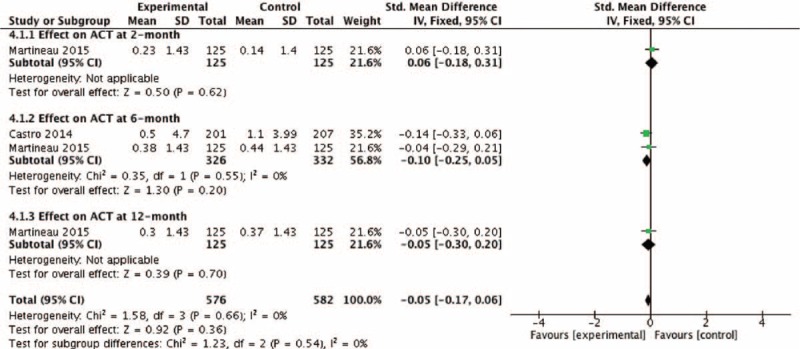
Effect of vitamin D versus placebo on ACT. ACT = asthma control test. CI = confidence interval, SD = standard derivation.
Four studies reported the effects of vitamin D on serum 25-hydroxyvitamin D, of which 2 studies reported the effects at 12-month after enrollment (Fig. 8). The pooled SD of serum 25-hydroxyvitamin D change at 12-month follow-up point from baseline and the overall effect was 0.67 (95% CI: 0.43–0.91) and 0.51 (95% CI: 0.35–0.68), and both analyses show vitamin D supplementation could significantly increase serum 25-hydroxyvitamin D levels (z = 5.50, P < 0.001 and z = 6.16, P < 0.001).
FIGURE 8.
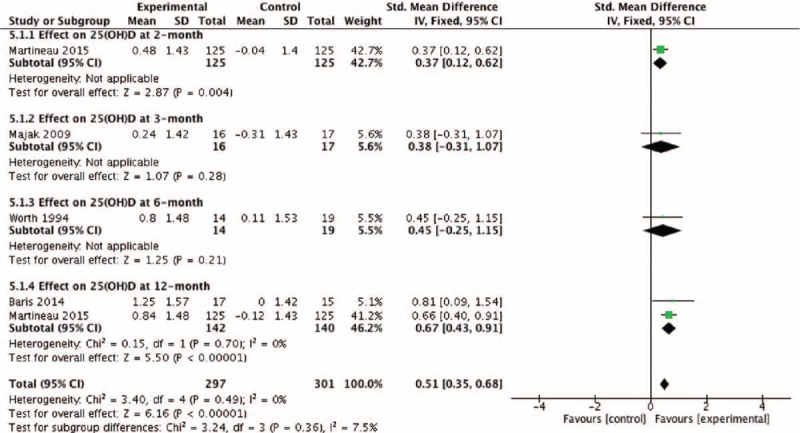
Effect of vitamin D versus placebo on serum 25(OH)D levels. 25(OH)D = 25-hydroxyvitamin D, CI = confidence interval, SD = standard derivation.
DISCUSSION
This meta-analysis included 7 studies, and the data analysis showed that vitamin D supplementation in addition to asthma controllers could not decrease asthma exacerbation and FeNO, nor improve lung function (FEV1, % of predicted value) and asthma symptoms (ACT), although it could significantly increase serum 25-hydroxyvitamin D without inducing higher adverse events frequency.
Recently, Kerley et al15,16 reviewed the potential mechanisms underlying the influence of vitamin D on asthmatic disease, and summarized the current human literature involving the possible consequences of vitamin D deficiency (VDD) for asthma. The interaction between vitamin D and the pathogenesis of asthma is complex, but the known mechanisms raise the potential for vitamin D repletion as adjunct therapy which include: effects on lung development (structural effects), VDD could alter lung structure and create deficits in lung function thus leading to permanent susceptibility to poorer respiratory outcomes; anti-inflammatory effects, vitamin D could inhibit the production of pro-inflammatory cytokines and vitamin D receptor (VDR) could suppress NF-κB activation and signaling; immunomodulation, addition of vitamin D to human monocytes could inhibits the expression of the toll-like receptors 2 and 4 resulting in reduced production of tumor necrosis factor alpha (TNF-α), but the role of vitamin D on Th2 cells is not consistent; airway smooth muscle (ASM) modulation, ASM cells possess the enzymatic machinery to form 1,25-dihydroxyvitamin D from 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D could in turn suppress ASM proliferation and decrease expression of inflammatory chemokines; altering the effect of anti-asthmatic therapy, VDD may lead to down-regulation of corticosteroids pathways and vitamin D could improve corticosteroids response by increasing mitogen-activated protein kinase 1 (MKP-1), which is a protein involved in directing cellular responses to a diverse array of stimuli. However, the consequences from human studies were inconsistent with some demonstrating benefit from increased vitamin D intake, while others suggesting inferior asthmatic outcomes associated with higher vitamin D, thus activating our interests in further evaluating the effects of vitamin D supplementation in patients with asthma.
Poorly controlled airway inflammation and hyperresponsiveness are pathological and clinical hallmarks underlying asthma exacerbations, which can be induced and enhanced by interleukins (ILs) released by Th lymphocytes via activation of arginase activity.17,18 Zhang et al found that the production of IL-6 and TNF-α could be suppressed by vitamin D via the inhibition of p38 mitogen-activated protein (MAP) kinase in monocytes,19 and several studies have also shown that higher levels of IL-17A and IL-22 were synthesized in asthmatic patients which could not be inhibited by glucocorticoids but vitamin D could significantly reduce both.20,21 However, our pooled data analysis showed that treatment of vitamin D did not significantly decrease asthma exacerbations, which we supposed to be caused by the multiple factors associated with development of exacerbations, such as indoor and outdoor allergens, pollutants, specific drugs, poorly adherent to asthma controllers, as well as viral infections. Martineau et al14 conducted a trial to evaluate the effect of vitamin D on upper respiratory infections in 250 patients with asthma, but the results showed no influence (adjusted hazard ratio [HR] 0.87, 95% CI: 0.64–1.16, P = 0.34). Moreover, asthma is considered mainly as a Th2-mediated disease, but the reported effects of vitamin D on Th2 cells were not identical.15 Kuo et al22 and Mathieu et al23 suggested a direct signaling effect of vitamin D on naïve CD4+ T cells toward Th2 differentiation or maintenance, which was further demonstrated in murine experiment that vitamin D could shift the Th1–Th2 cytokine balance toward Th2, thus potentially increasing risk of asthma. While a recent animal model study reported that perinatal VDD in mice leaded to Th2 skewing and reduced IL-10-secreting regulatory T cells, and vitamin D supplementation was associated with reduced serum IgE levels, pulmonary eosinophilia and peri-bronchiolar collagen deposition.24 On the other hand, interpretation of our result should be cautious, because significant statistical heterogeneity existed in the pooled data of enrolled studies, and we did not analyze the serum ILs levels as well as upper respiratory infection rate due to the incomplete data report.
Observational studies have associated lower vitamin D status with decreased lung function, which was further demonstrated by Arshi and Thuesen,5,8,25,26 but a recent cohort study did not find such an association (adjusted odds ratio [OR] 0.996, 95% CI: 0.990–1.002, P = 0.171),27 thus making the conclusions of relation between vitamin D and lung function illusive. Moreover, exacerbations are characterized by decreases in expiratory airflow that can be quantified by measurement of FEV1, and the degree of symptoms is a sensitive measure of the onset of an exacerbation.28,29 Our data analysis showed that treatment of vitamin D could not improve FEV1 and ACT no matter in short or long terms, although serum 25-hydroxyvitamin D was significantly increased, which we think may result from the variable factors involving vitamin D status. As explained by Kerley's review, exposure to ultraviolet-B, not through diet, is the major source of vitamin D; therefore, vitamin D status varies markedly depending on local weather conditions and individual sun behaviors.16 Due to the variable and changeable human behavior and season, both asthma and vitamin D outcomes are often influenced. In addition, although our study showed a significant elevation in serum 25-hydroxyvitamin D after administration of vitamin D, but not all patients reached the sufficiency level of 75 to 250 nmol/L released by the Endocrine Society,30 thus may offset the possible effect of vitamin D on improvement in lung function and symptoms.
Nitric oxide (NO) in respiratory tract can be synthesized from l-arginine by NO synthase (NOS) in a wide variety of cell types including inflammatory cells, epithelial cells, and airway nerves, and large amount of NO generated by inducible NOS in asthma can be detected in exhaled breath, that is, FeNO, which is regarded as a potential noninvasive method to reflect the degree of airway inflammation and diagnose asthma.31,32 In theory, vitamin D could decrease FeNO levels in asthma through the mechanism of anti-inflammation by reducing ILs mentioned above; however, de Groot et al13 did not find such an effect of vitamin D, and what is more, they further demonstrated that vitamin D did not decrease sputum eosinophils and neutrophils. Our pooled data analysis showed that treatment with vitamin D could not significantly decrease FeNO levels in patients with asthma, which was in line with the findings reported with de Groot, but we should interpret it cautiously because of the relatively limited studies pooled in the final analysis and the absence in the comparisons of biomarkers associated with FeNO such as sputum eosinophils, ILs, TNF-α, and interferon-γ (INF-γ).
Limitations of our meta-analysis are as follows: First, the name, dose, administration routine, and duration of the intervention drugs were not identical in the enrolled studies, which may result in performance biases. Second, the baseline characteristics of the patients were not completely provided, and we included all trials conducted in adults and children, which may lead to selection biases. Third, the total number of studies and patients enrolled was relatively small. And finally, these trials relied on a single measurement of vitamin D status or intake with asthmatic outcomes assessed up to 31 years later; therefore, it is impossible to discount variables other than vitamin D contributing to the development of asthma.
CONCLUSIONS
Vitamin D supplementation in addition to asthma controllers cannot decrease asthma exacerbation and FeNO, nor improve lung function and asthma symptoms, although it can be safely applied to increase serum 25-hydroxyvitamin D levels. More large double-blind RCTs are needed, particularly in identical medication doses and administration routines and durations, to further determine the role of vitamin D, including the cost-effectiveness, in patients with asthma.
ACKNOWLEDGMENTS
We thank Professor Dongtao Lin (College of Foreign Languages, Sichuan University), who is specialized in biomedical writing and editing, for copyediting this manuscript.
Footnotes
Abbreviations: ACTa = sthma control test, ASM = airway smooth muscle, BMI = body mass index, CENTRAL = Cochrane Central Register of Controlled Trials, CI = confidence interval, FeNO = fraction of exhaled nitric oxide, FEV1 = forced expiratory volume in 1 s, FEV1/FVC = ratio of FEV1 to forced vital capacity, GINA = the Global Initiative for Asthma, HR = hazard ratio, ILs = interleukins, INF-γ = interferon-γ, MAP = mitogen-activated protein, MKP-1 = mitogen-activated protein kinase 1, NO = nitric oxide, NOS = nitric oxide synthase, OR = odds ratio, RCT = randomized controlled trial, RR = risk ratio, SD = standard derivation, Th lymphocytes = T helper lymphocytes, TNF-α = tumor necrosis factor alpha, VDD = vitamin D deficiency, VDR = vitamin D receptor.
JL and DL have contributed equally to this work.
JL and DL designed the study, conducted the literature searching and data analysis, and prepared the manuscript; C-TL reviewed the manuscript and made the decision to submit the report for publication.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA), 2012. www.ginasthma.org Accessed March 13, 2012. [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–478. [DOI] [PubMed] [Google Scholar]
- 3.Worth H, Stammen D, Keck E. Therapy of steroid-induced bone loss in adult asthmatics with calcium, vitamin D, and a diphosphonate. Am J Respir Crit Care Med 1994; 150:394–397. [DOI] [PubMed] [Google Scholar]
- 4.Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clin Exp Allergy 2009; 39:1830–1841. [DOI] [PubMed] [Google Scholar]
- 5.Wu AC, Tantisira K, Li L, et al. Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med 2012; 186:508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010; 126:52–58.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 2009; 169:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshi S, Fallahpour M, Nabavi M, et al. The effects of vitamin D supplementation on airway functions in mild to moderate persistent asthma. Ann Allergy Asthma Immunol 2014; 113:404–409. [DOI] [PubMed] [Google Scholar]
- 9.Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA 2014; 311:2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Oxford: The Cochrane Collaboration, 2011. Updated March 2011. www.cochrane-handbook.org Accessed March 20, 2011. [Google Scholar]
- 11.Baris S, Kiykim A, Ozen A, et al. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy 2014; 69:246–253. [DOI] [PubMed] [Google Scholar]
- 12.Yadav M, Mittal K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian J Pediatr 2014; 81:650–654. [DOI] [PubMed] [Google Scholar]
- 13.de Groot JC, van Roon EN, Storm H, et al. Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. J Allergy Clin Immunol 2015; 135:670–675.e3. [DOI] [PubMed] [Google Scholar]
- 14.Martineau AR, MacLaughlin BD, Hooper RL, et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015; 70:451–457. [DOI] [PubMed] [Google Scholar]
- 15.Kerley CP, Elnazir B, Faul J, et al. Vitamin D as an adjunctive therapy in asthma. Part 1: a review of potential mechanisms. Pulm Pharmacol Ther 2015; 32:60–74. [DOI] [PubMed] [Google Scholar]
- 16.Kerley CP, Elnazir B, Faul J, et al. Vitamin D as an adjunctive therapy in asthma. Part 2: a review of human studies. Pulm Pharmacol Ther 2015; 32:75–92. [DOI] [PubMed] [Google Scholar]
- 17.Erdely A, Kepka-Lenhart D, Clark M, et al. Inhibition of phosphodiesterase 4 amplifies cytokine-dependent induction of arginase in macrophages. Am J Physiol Lung Cell Mol Physiol 2006; 290:L534–L539. [DOI] [PubMed] [Google Scholar]
- 18.Yang M, Rangasamy D, Matthaei KI, et al. Inhibition of arginase I activity by RNA interference attenuates IL-13-induced airways hyperresponsiveness. J Immunol 2006; 177:5595–5603. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012; 188:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi S, Pantalena LC, Liu XK, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol 2011; 31:3653–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanzer AM, Chambers ES, Ryanna K, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol 2013; 132:297–304.e3. [DOI] [PubMed] [Google Scholar]
- 22.Kuo YT, Kuo CH, Lam KP, et al. Effects of vitamin D3 on expression of tumor necrosis factor-alpha and chemokines by monocytes. J Food Sci 2010; 75:H200–H204. [DOI] [PubMed] [Google Scholar]
- 23.Mathieu C, Casteels K, Waer M, et al. Prevention of diabetes recurrence after syngeneic islet transplantation in NOD mice by analogues of 1,25(OH)2D3 in combination with cyclosporin A: mechanism of action involves an immune shift from Th1 to Th2. Transplant Proc 1998; 30:541. [DOI] [PubMed] [Google Scholar]
- 24.Vasiliou JE, Lui S, Walker SA, et al. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy 2014; 69:1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange NE, Sparrow D, Vokonas P, et al. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am J Respir Crit Care Med 2012; 186:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thuesen BH, Skaaby T, Husemoen LL, et al. The association of serum 25-OH vitamin D with atopy, asthma, and lung function in a prospective study of Danish adults. Clin Exp Allergy 2015; 45:265–272. [DOI] [PubMed] [Google Scholar]
- 27.Thuesen BH, Heede NG, Tang L, et al. No association between vitamin D and atopy, asthma, lung function or atopic dermatitis: a prospective study in adults. Allergy 2015; 70:1501–1504.doi: 101111/all12704 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.FizGerald JM, Grunfeld A. Status asthmaticus. In: Lichtenstein LM, Fauci AS, eds. Current Therapy in Allergy, Immunology, and Rheumatology. 5th ed. St. Louis, MO: Mosby; 1996:63–67. [Google Scholar]
- 29.Chan-Yeung M, Chang JH, Manfreda J, et al. Changes in peak flow, symptom score, and the use of medications during acute exacerbations of asthma. Am J Respir Crit Care Med 1996; 154:889–893. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 31.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax 2003; 58:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011; 184:602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]


