Abstract
Pheochromocytoma and paraganglioma (PPG) are rare and late-diagnosed catecholamine secreting tumors, which may be associated with unrecognized and/or severe cardiomyopathies.
We performed a computer-assisted systematic search of the electronic Medline databases using the MESH terms “myocarditis,” “myocardial infarction,” “Takotsubo,” “stress cardiomyopathy,” “cardiogenic shock”, or “dilated cardiomyopathy,” and “pheochromocytoma” or “paraganglioma” from 1961 to August 2012. All detailed case reports of cardiomyopathy due to a PPG, without coronary stenosis, and revealed by acute symptoms were included and analyzed.
A total of 145 cases reports were collected (49 Takotsubo Cardiomyopathies [TTC] and 96 other Catecholamine Cardiomyopathies [CC]). At initial presentation, prevalence of high blood pressure (87.7%), chest pain (49.0%), headaches (47.6%), palpitations (46.9%), sweating (39.3%), and shock (51.0%) were comparable between CC and TTC. Acute pulmonary edema (58.3% vs 38.8%, P = 0.03) was more frequent in CC. There was no difference in proportion of patients with severe left ventricular systolic dysfunction (LV Ejection Fraction [LVEF] < 30%) at initial presentation between both groups (P = 0.15). LVEF recovery before (64.9% vs 40.8%, P = 0.005) and after surgical resection (97.7% vs73.3%, P = 0.001) was higher in the TTC group. Death occurred in 11 cases (7.6%). In multivariate analysis, only TTC was associated with a better LV recovery (0.15 [0.03–0.67], P = 0.03).
Pheochromocytoma and paraganglioma can lead to different cardiomyopathies with the same brutal and life-threatening initial clinical presentation but with a different recovery rate. Diagnosis of unexplained dilated cardiomyopathy or TTC should lead clinicians to a specific search for PPG.
INTRODUCTION
Pheochromocytoma is a rare neuroendocrine tumor developed in the adrenal medulla with the ability to synthesize catecholamines.1,2 Paragangliomas are also neuroendocrine tumors, but developed in the extra-adrenal sympathetic and parasympathetic nervous systems3 with a lower incidence (<1 per 300,000 inhabitants per year).4 These tumors may occur sporadically or secondarily to germline mutations of several tumor-susceptibility genes.12
The clinical presentation of pheochromocytoma and paraganglioma (PPG) is heterogeneous. Patients can be asymptomatic but may also present multiple and nonspecific symptoms. Furthermore, life-threatening complications may occur.5 Among these, the prevalence of cardiovascular complications reaches 20% in PPG.6 It has been proposed that chronic or acute catecholamine intoxication may lead to these structural myocardial alterations.7–11 Several cases reports and rare series have investigated cardiomyopathies due to PPG.12–15 As a result, different types of PPG-induced cardiomyopathies have been described, both chronic and acute, among which Takotsubo Cardiomyopathies (TTC) are increasingly reported. However, clinical presentation, management, and prognosis of such cardiomyopathies with frequent severe forms remain little known. Moreover, prospective studies have not been performed due to its low incidence.
The aim of the present study was to conduct a systematic literature review to describe acute initial clinical presentation and prognosis of nonischemic cardiomyopathies induced by PPG.
METHODS
Study Design
A National Library of Medicine MEDLINE search was performed using the following MESH terms (“dilated cardiomyopathy” or “myocarditis” or “acute myocardial infarction” or “Takotsubo cardiomyopathy” or “cardiogenic shock” or “stress cardiomyopathy”) and (“pheochromocytoma” or “paraganglioma”) for articles published during the period 1961 to 2012 and undertaken in August 2012. Only English and French language publications were considered. Articles were included according to abstracts or title descriptions with the diagnosis of secondary cardiomyopathy due to catecholaminergic secreting tumors. All included manuscripts were reviewed. Only articles (including case reports, series of case reports, literature review, meta-analysis, and letters to the editor) with diagnosis of cardiomyopathy due to a confirmed catecholaminergic secreting tumor (pheochromocytoma or paraganglioma) revealed by acute symptoms were retained.
In each retained article, clinical, biological, echocardiographic, coronary angiographic, and survival data were available and reportable.
Articles were excluded if cases were not described individually, if the information provided was insufficiently detailed, or if the diagnosis of cardiomyopathy was not possible retrospectively with the available data. Each case without described left ventricle morphology or kinetics, either by echocardiography or coronary angiography, or left ventricle morphology at autopsy examination were also excluded. Cases with significant coronary artery stenosis, described in either angiography or autopsy report, which could explain the clinical presentation as well as obstructive hypertrophic cardiomyopathies were excluded. Reports were screened for duplication and duplicated data were excluded.
Each article was blindly and successively read by 2 endocrinologists and 2 cardiologists in order to complete the dataset. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines16 were used to guide this review.
This study did not involve human subjects, so informed consent was not required. In addition, no approval was required from an institutional review board.
Cardiomyopathy Classification
For each case report, a diagnosis was retrospectively assessed according to the current literature, regardless of the authors’ diagnosis. Thereafter, cases were classified into 2 types of cardiomyopathies: those called Takotsubo Cardiomyopathy (TTC) and meeting the John Hopkins criteria9,17 (except for the recovery item, which was used as an endpoint data in the analysis), and those that did not meet this description, termed herein Catecholamine Cardiomyopathy (CC). According to current Mayo Clinic criteria, pheochromocytoma had to be excluded before TCC diagnosis was made.19 Left ventricular ejection fraction (LVEF) and kinetic abnormalities, described on echocardiography and angiography reports, were used to assess diagnosis. Initial LV systolic dysfunction was classified as severe when LVEF was measured <30%.
Data Assessment
The following data were assessed at acute initial presentation in this analysis: clinical data (age, sex, gender, cardiovascular risk factors [diabetes, cardiovascular family history, hypertension, smoking status, dyslipidemia, duration of hypertension], pregnancy, onset symptoms [dyspnea, chest pain, digestive symptoms, fever, sweating, headaches, palpitations, blood pressure]), paraclinical data (chest X-Ray, electrocardiogram [ECG] [heart rate, ventricular or supra-ventricular arrhythmias, changes in repolarization], echocardiography [left ventricle global and segmental kinetics, valvular diseases], angiocoronarography data [presence of coronary artery stenosis, LV morphology and LVEF], biological data [biological parameters were converted to fold normal values {fold N} to standardize the results]), initial management (oral drugs, vasopressive drugs, extracorporeal membrane oxygenation life support and intra-aortic balloon pump) as well as endocrinological parameters (tumor size, abdominal or thoracic localization, multiple, bilateral, metastatic, genetic forms, postsurgery urinary derivatives from epinephrine and norepinephrine concentrations). Also assessed were time from first symptoms to diagnosis and time to last available LVEF. Bilateral or metastatic forms were characterized from MIBG scans or adrenal tomography results when available.
LVEF recovery was defined as complete when LVEF was measured ≥60% or reported as normal by authors.
Statistical Analysis
Statistical analysis was performed using Stata software, version 13 (StataCorp, College Station). The tests were 2-sided, with a type I error set at α=0.05. Means and standard deviations (SD) or medians and interquartile ranges are presented for continuous variables according to statistical distribution, whereas frequencies and associated percentages were calculated for categorical parameters. Comparisons between the independent groups TTC and CC, presence of the items “deaths” and “recovery,” were performed using the chi square test or Fisher's exact test for categorical variables and with Student's t test or the Mann–Whitney test for quantitative variables (with normality verified by Shapiro–Wilk test and homoscedasticity by the Fisher–Snedecor test). Given that the distribution of TTC and CC pathologies varied over time, it was deemed relevant to consider an adjustment based on the year of publication using regression random-effects models taking into account between- and within-study variability. Finally, multivariate analyses (logistic regression model with deaths and recovery as dependent variables) were performed to study the effect of TCC/CC on outcomes adjusted on fixed parameters according to (1) clinical relevance and (2) univariate results presented in supplemental data. Between-factor interactions were tested and results expressed as odds-ratios (OR) and 95% confidence intervals (95% CI).
RESULTS
The number of cases that met the inclusion criteria for this systematic review was 145 (Fig. 1) of which 49 TTC (33.8%) and 96 CC (66.2%) were identified. Eighty-three (57.2%) reported patients were women. There was no difference in the mean age between both groups (44.6 ± 16.1 years in TTC vs 40.5 ± 14.2 years in CC, P = 0.12). The reported age ranged from 8 to 86 years. The year of publication of the case reports is presented in Figure 2.
FIGURE 1.
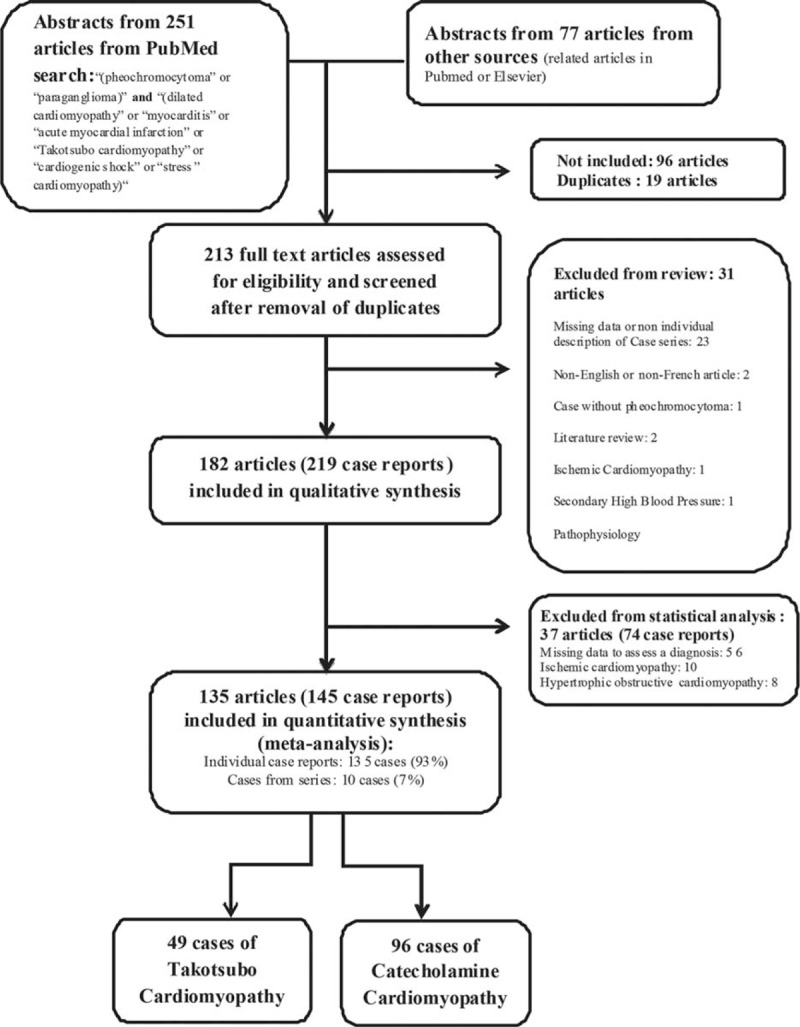
Flowchart of searched articles and case reports.
FIGURE 2.
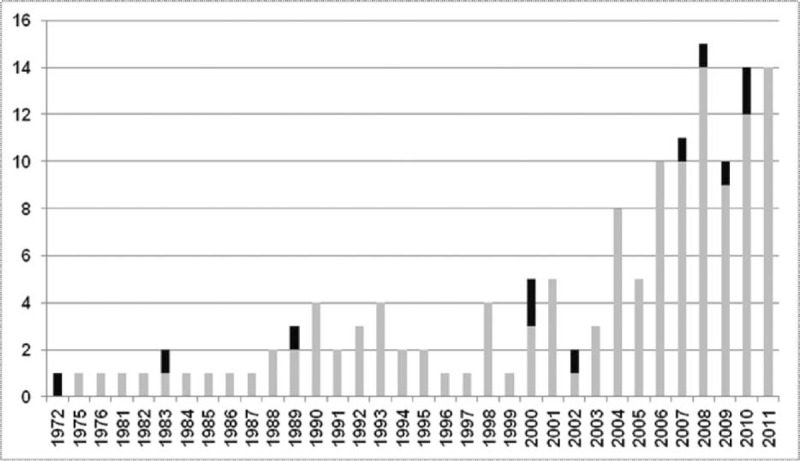
Annual distribution of case reports included in the analysis. The 145 case reports included in the analysis are shown according to their year of publication. Among these, the 11 deaths reported are depicted in black (stacked bars) of the published cases by year. The median year of published case reports studied is 2006. Six deaths of 78 cases (7.7%) were reported between 1972 and 2006, and 5/67 (7.5%) from 2007 to 2012 (P = 0.96). The year 2012 is not represented as the collection of case reports ended in July 2012 (3 cases reports studied, no deaths).
Among the 145 cases, 1 case of CC (0.7%) was diagnosed after autopsy and 3 cases of CC (2.1%) after heart transplantation.
There were no observed differences in the delay of diagnosis (n = 89 cases) between the TTC group and CC group (105 [21–1095] days vs 180 [30–730] days, P = 0.78).
Characteristics of Catecholamine-Secreting Tumors
In the majority of cases (131/145, 90.3%), an adrenal pheochromocytoma was reported (89.8% of TTC vs 90.6% of CC, P = 1.00). Only 14 (9.7%) paraganglioma were observed, located in the abdomen in 12 cases (8.3%) and in the neck in 2 cases (1.4%). Only 4 (2.8%) metastatic and 8 (5.5%) bilateral forms were documented. Five (10.2%) abdominal paraganglioma were observed in the TTC group vs 7 (7.3%) in the CC group (P = 0.54). The 2 observed cervical paraganglioma were in the CC group.
With regard to genetic presentation, 7 patients (4.8%) were found in the setting of type 2 multiple endocrine neoplasia, 3 (2.1%) in neurofibromatosis, and 1 (0.7%) in von Hippel Lindau syndrome. There were 3 (2.1%) recurrent forms, 1 (2.0%) among TTC, and 2 (2.1%) among CC (P = 1.00). No difference in terms of tumor size was observed between both groups (5.8 ± 2.5 cm for TTC vs 6.1 ± 2.7 cm for CC, P = 0.62).
Clinical Characteristics at Initial Presentation
A history of hypertension was reported in 48 (33.1%) patients (34.7% in TTC vs 32.3% in CC, P = 0.77). There was no difference between the groups with regard to cardiovascular risk factors. Their global prevalence was 12.4% for diabetes, 7.6% for active smoking, 6.2% for dyslipidemia, and 6.2% for history of familial cardio-vascular event. One TTC and 3 CC were found in the setting of pregnancy.
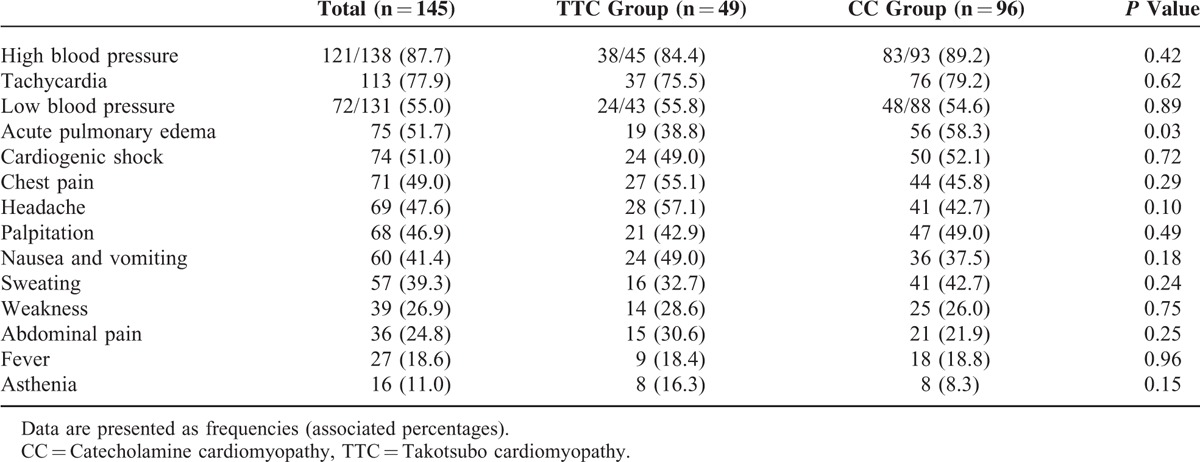
Clinical features of cardiomyopathy at initial presentation are reported in Table 1. There was no difference between both groups regarding clinical features except for the fact that acute pulmonary edema was more frequent in CC (56 [58.3%] vs 19 [38.8%], P = 0.03). High blood pressure at initial presentation was reported in 38/45 TTC (84.4%) and in 83/93 CC (89.2%), P = 0.42. Cardiogenic shock was observed in 74 cases (51.0%), without any difference between the 2 groups (24 (49.0%) in TTC vs 50 (52.1%) in CC, P = 0.72).
TABLE 1.
Clinical Characteristics of the Study Groups at Initial Presentation
Paraclinical Features
Electrocardiogram and Chest X-Ray
Electrocardiogram data were available in 141 cases (98.0% of TTC and 96.9% of CC) and are described in Table 2. The most frequent features were sinus tachycardia (63.8%), ST elevation (37.6%) and T wave inversion (32.6%). ST segment abnormalities were more frequent in the TTC group (ST elevation 54.2% vs 29.0%, P = 0.004; ST depression 37.5% vs 15.1%, P = 0.003). In addition, a trend toward a higher incidence of atrial fibrillation was observed in the CC group [12 (12.9%) in CC vs 1 (2.1%) in TTC, P = 0.06].
TABLE 2.
Electrocardiographic, Chest X-Ray, and Echocardiographic Data
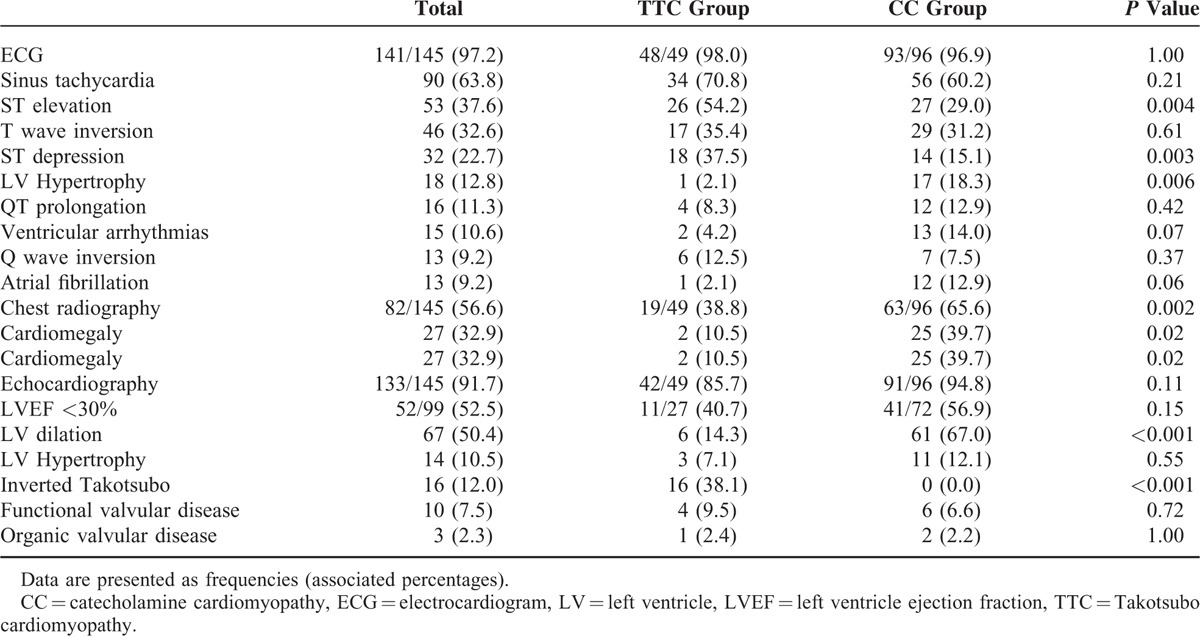
Chest radiography data were documented in 82 (56.6%) cases. Cardiomegaly (10.5% vs 39.7%, P = 0.02) was more often observed in the CC group.
Transthoracic Echocardiography
Echocardiographic data were available in 133 cases (91.7%): 42 (85.7%) in the TTC group and 91 (94.8%) in the CC group (P = 0.11) (Table 2).
In the total population, left ventricle dilation (50.4%) was more frequent than hypertrophic cardiopathy (10.5%). LV dilation was observed more frequently in the CC group (14.3% vs 67.0%, P < 0.001).
Left ventricular ejection fraction at initial presentation was described in 99 cases (68.3%): median LVEF was similar in both groups: 30.0% (23.0–35.0) in the TTC group vs 26.0% (20.0–35.5) in the CC group (P = 0.47). Furthermore, no difference in proportion of patients with a severe LV systolic dysfunction (LV ejection fraction < 30%) was observed between the 2 groups (40.7% in TTC vs 56.9% in CC, P = 0.15).
Coronary Artery and LV Angiography
Coronary artery and LV angiography were documented in 89 cases: 38 (77.6%) of TTC and 51 (53.1%) of CC (P = 0.004). Nonsignificant atheroma was found in only 3 (7.9%) TTC and in 6 (11.8%) CC (P = 0.73). Furthermore, as described previously, all patients with a suspected ischemic cardiomyopathy (by angiocoronarography and/or autopsy) were excluded from the present analysis.
Histological Data
Myocardial histological data were available in 22 cases (15.2%): 5 (10.2%) in the TTC group vs 17 (17.7%) in the CC group (P = 0.23).
Fibrosis (0 [0.0%] vs 9 [52.9%], P = 0.05) was more often reported in the CC group, contrasting with myocardial inflammation (0 [0.0%] vs 8 [47.1%], P = 0.12) and necrosis (2 [40.0%] vs 11 [64.7%], P = 0.61).
Management and Outcome
Management
Preoperative medical and invasive treatment was similar in both groups (Table 3).
TABLE 3.
Preoperative Management of the Study Population
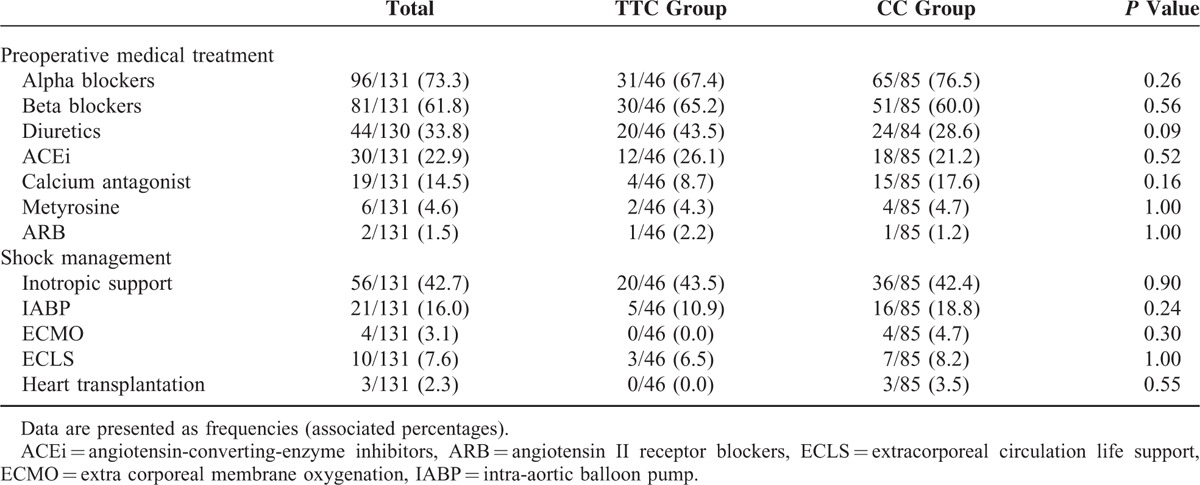
Furthermore, a complete surgical catecholamine-secreting tumor resection was reported in 127/129 (98.4%) cases: 44/44 (100.0%) of TTC and 83/85 (97.6%) of CC (P = 0.55).
Outcome
Eleven in-hospital deaths (7.6%) were observed: 1 (2.0%) in the TTC group vs 10 (10.4%) in the CC group (P = 0.10). Under medical treatment, half of the patients (56/112 [50.0%]) had recovered their LV systolic function before surgery. Patients in the TTC group displayed a higher rate of LV recovery before surgery compared to the CC group (25/36 [69.4%] in the TTC group vs 31/76 [40.8%] in CC group, P = 0.005). After tumor resection, a complete recovery of LV systolic function was found in 97/118 cases (82.2%), and was more pronounced in the TTC group (42/43 [97.7%] vs 55/75 [73.3%] for CC group, P = 0.001). No difference was observed in median recovery time of LV systolic function (14 [7–24] days for TTC vs 30 [10–90] days for CC, P = 0.13) and cardiac episode recurrence between both groups (9 TTC [18.4%] vs 15 CC [15.6%], P = 0.67).
In multivariate analysis, only TTC was associated with a better LV recovery (0.15 [0.03– 0.67], P = 0.03), although none of the 2 groups was associated with death (5.58 [0.69–44.93], P = 0.15 for the CC group) (Supplementary data, Online Tables 1 and 2).
DISCUSSION
The present review is, to our knowledge, the largest case series dealing with cardiomyopathies complicating catecholamine-secreting tumors.6,12,14,20–22 Knowledge of these pathologies is currently limited by difficulties in diagnosing PPG and the variability in clinical manifestations. This results in a lack of prospective studies and the publication of mainly isolated case reports. This review may hence help clinicians to better understand this pathology and to identify patients in whom diagnosis of PPG needs to be established rapidly.
Clinical and Paraclinical Presentation
Similar prevalences of both cardiological (dyspnea, chest pain, or weakness) and specific pheochromocytoma symptoms (headaches, palpitations, sweating, and digestive signs) were observed at clinical onset in both identified TTC and CC populations (Table 1). These prevalences were comparable to those described in the literature regarding Takotsubo Cardiomyopathy (TTC)8,10,11,23,24,25 and PPG.1 However, high blood pressure at initial presentation (83.8% of total population) is not usually described during TTC and in heart failure with reduced left ventricular ejection fraction.23 Thus, the presence of a PPG had to be eliminated after the diagnosis of a TTC or unexplained dilated cardiomyopathy, especially in the presence of hypertension at clinical onset. More frequent acute pulmonary edema and electric ventricular hypertrophy were observed in the CC group. A more important LV dilation was also observed in the CC group at baseline.
Prognosis
The present review confirms the severity of these cardiomyopathies with a 7.6% in-hospital mortality rate and a 51.0% cardiogenic shock rate at initial presentation, as previously described,12 versus only 6% in all-cause TTC.26,27,24,28
Of importance, this literature review enabled us to identify 2 different types of cardiomyopathies due to PPG having similar initial acute presentations but different evolutions and prognosis, namely (i) Takotsubo Cardiomyopathy, also called “stress cardiomyopathy,”18 with a higher rate of LV function recovery and (ii) more chronic catecholaminergic cardiomyopathies, including dilated cardiomyopathies revealed by acute heart failure, with a lower recovery rate. Indeed, this study nonetheless highlights certain key differences between these 2 populations, which must be interpreted cautiously. At initial presentation, the proportion of patients with LVEF < 30% was similar in both groups. The proportion of LV systolic function recovery was only 50% via medical treatment while reaching 82.2% after surgery. However, there was a quicker and better recovery of LV systolic dysfunction in the TTC group before and after surgery. End of catecholamine exposure due to surgical resection was systematically associated with an improvement in LV systolic function in both groups. Evolution of CC was likely longer due to chronic exposure even if the acute initial presentation was quite similar.
The CC group probably constituted a more heterogeneous group which included mainly dilated or nonobstructive hypertrophic cardiomyopathies. This latter observation nevertheless emphasizes that a large segment of this group likely suffered from a more chronic and long-standing catecholaminergic intoxication, leading to structural myocardial alterations (fibrosis and inflammation). This chronic exposure may explain the lower level of LVEF recovery in this group.
Pathophysiology
These 2 types of PPG-induced cardiomyopathies appear to have different pathophysiological pathways. Adrenergic cardiac toxicity is now well-documented.29 An acute catecholaminergic stress in TTC (physical or emotional stress) inundates myocardial β receptors leading to a stunning of the left ventricle7,8,10,11,30 and slight histological apoptosis.31 In the case of chronic adrenergic exposure, the heart is able to adapt. Desensitization is mediated by adrenergic receptors phosphorylation via G-protein coupled receptor kinase (GRKs) followed by binding to β-arrestin promoting the receptor internalization.32 Moreover, recent studies demonstrate that secretion of catecholamin in the adrenal medulla is regulated by GRK2 and β-arrestin suggesting a GRK2/β-arrestin-mediated cross talk between adrenal gland and the heart.33 In the same way, protein kinase A (PKA) is able to promote desensitization and downregulation of β-adrenergic receptors in cardiomyocytes through PIP3 and cAMP signaling modulation.34 In spite of this regulation, chronic catecholamin administration causes interstitial fibrosis, promotes cardiac apoptosis, and induces contractile dysfunction through left ventricular dilatation.29 In the CC population, repetitive myocardial aggression due to longer catecholaminergic exposure could explain LV hypertrophy, extended histological fibrosis, and extracellular matrix collagen turnover35–37,5,38 all of which lead to a lower recovery rate. This is all the more significant as myocardial fibrosis has been associated with a poorer prognosis.39 Such delay in diagnosis may lead to a deterioration in systolic and diastolic functions thereby influencing the recovery rate, and ultimately, the type of cardiomyopathy.
The Importance of Time of Diagnosis
In the present study, diagnosis time was estimated at about 2 years after the first report of PPG signs. This delay is classically described at 3 years for pheochromocytoma1 and even 5 years for paraganglioma.40 Moreover, we found several cases with recurrence of cardiac episodes (15.5%). This highlights the necessity of an early diagnosis of PPG. Furthermore, favorable evolution after complete surgery should lead clinicians to conduct a systematic biochemical screening when faced with these PPG-suggestive situations.
Moreover, we found that larger tumor sizes, recurrent tumors as well as head and neck locations were associated with a poorer prognosis (supplementary data Table 1). Accordingly, tumor size has been previously demonstrated as a predictive factor of malignancy, tumor recurrence, and a poorer prognosis in PPG.41,42,43
Clinical Implications
Clinical consequences for clinicians and especially cardiologists are particularly important: discovery of any Takotsubo Cardiomyopathy or unexplained dilated cardiomyopathy without coronary significant lesion should lead to an active search for a PPG. Indeed, association of headaches, sweating, or palpitations episodes classically evokes the presence of a PPG. Initial hypertension, sinus tachycardia, electrical left ventricular hypertrophy are strong supplemental arguments in driving the search for PPG. In the case of TTC, an emotional stress must not discount the suspicion, because of the possible emotional trigger in a PPG crisis.7 Indeed, a triggering factor, whether physical or emotional, has been reported in ∼10 % of the cases studied.39,44,45,12 The currently recommended tests to detect PPG are sampling of urinary methoxylated metabolites and plasma-free metanephrines.1
Concomitantly for the endocrinologist, the diagnosis of a PPG should invariably lead to cardiac imagery (echocardiography or Magnetic Resonance Imaging) in order to detect a potential silent chronic cardiomyopathy. Such an approach could improve perioperative management and shorten the delay in introducing heart failure-specific drugs.
Study Limitations
In spite of the exhaustive collection of all biological values, their interpretation has not been possible. Indeed, there is currently no international standardization procedure regarding urinary or plasma catecholamine metabolite assays, or troponin or Brain Natriuretic Peptides and its precursors, nor with regard to their normal values, units, or dosage techniques, even if such values would have been highly informative.46 For the oldest articles, one could expect that certain Takotsubo cardiomyopathies, not described before 1990, may have been designated as a nonspecific cardiomyopathy therefore precluding their inclusion in the TTC group herein. Nevertheless, this situation involves a minority of articles of which some may have indeed been included in the Takotsubo group owing to a precise description of the systolic dysfunction.
This systematic review also suffers from an inherent bias usually associated with retrospective studies. The descriptive quality of case reports was heterogeneous which rendered the classification and analysis interpretation somewhat difficult for some articles. The wide chronological range also leads to heterogeneous knowledge and therapeutic techniques, all of which may influence prognosis interpretation.
Nevertheless, this review enabled us to collect the largest series of case reports dealing with cardiomyopathies associated with PPG. This is a major point, given the rarity of these tumors, the difficulties with regard to their diagnosis as well as poor prognosis, all of which do not allow prospective studies.
CONCLUSION
Pheochromocytoma and paraganglioma can lead to chronic and acute cardiomyopathies with the same acute and life-threatening clinical onset. Takotsubo cardiomyopathy displayed a better LV systolic function recovery. Diagnosis of an unexplained dilated cardiomyopathy without coronary stenosis or any TTC should encourage clinicians to eliminate the presence of PPG.
Acknowledgments
We thank Mr. Pierre Pothier for the editing of this manuscript.
Footnotes
Abbreviations: CC = cathecholamie cardiomyopathy, ECG = electrocardiogram, GRK = G-protein coupled receptor kinase, LV = left ventricular, LVEF = left ventricular systolic dysfunction, PKA = protein kinase A, PPG = pheochromocytoma and paraganglioma, SD = standard derivation, TTC = Takotsubo cardiomyopathy.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Lenders JWM, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet 2005; 366:665–675. [DOI] [PubMed] [Google Scholar]
- 2.Amar L, Servais A, Gimenez-Roqueplo A-P, et al. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab 2005; 90:2110–2116. [DOI] [PubMed] [Google Scholar]
- 3.Lee JA, Duh Q-Y. Sporadic paraganglioma. World J Surg 2008; 32:683–687. [DOI] [PubMed] [Google Scholar]
- 4.Gjuric M, Gleeson M. Consensus statement and guidelines on the management of paragangliomas of the head and neck. Skull Base 2009; 19:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luca F, Holl N, Vinzio S, et al. Manifestations cardiaques des phéochromocytomes. Ann Endocrinol 2009; 70:43–47. [DOI] [PubMed] [Google Scholar]
- 6.Zelinka T, Petrák O, Turková H, et al. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res 2012; 44:379–384. [DOI] [PubMed] [Google Scholar]
- 7.Coupez E, Eschalier R, Pereira B, et al. A single pathophysiological pathway in Takotsubo cardiomyopathy: catecholaminergic stress. Arch Cardiovasc Dis 2014; 107:245–252. [DOI] [PubMed] [Google Scholar]
- 8.Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352:539–548. [DOI] [PubMed] [Google Scholar]
- 9.Wittstein IS. Stress cardiomyopathy: a syndrome of catecholamine-mediated myocardial stunning? Cell Mol Neurobiol 2012; 32:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol 2008; 124:283–292. [DOI] [PubMed] [Google Scholar]
- 11.Milinis K, Fisher M. Takotsubo cardiomyopathy: pathophysiology and treatment. Postgrad Med J 2012; 88:530–538. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal V, Kant G, Hans N, et al. Takotsubo-like cardiomyopathy in pheochromocytoma. Int J Cardiol 2011; 153:241–248. [DOI] [PubMed] [Google Scholar]
- 13.Park J-H, Kim KS, Sul J-Y, et al. Prevalence and patterns of left ventricular dysfunction in patients with pheochromocytoma. J Cardiovasc Ultrasound 2011; 19:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giavarini A, Chedid A, Bobrie G, et al. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart Br Card Soc 2013; 99:1438–1444. [DOI] [PubMed] [Google Scholar]
- 15.Galetta F, Franzoni F, Bernini G, et al. Cardiovascular complications in patients with pheochromocytoma: a mini-review. Biomed Pharmacother 2010; 64:505–509. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madias JE. Why the current diagnostic criteria of Takotsubo syndrome are outmoded: a proposal for new criteria. Int J Cardiol 2014; 174:468–470. [DOI] [PubMed] [Google Scholar]
- 18.Sinning C, Keller T, Abegunewardene N, et al. Tako-Tsubo syndrome: dying of a broken heart? Clin Res Cardiol 2010; 99:771–780. [DOI] [PubMed] [Google Scholar]
- 19.Kawai S, Kitabatake A, Tomoike H. Takotsubo Cardiomyopathy Group. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J 2007; 71:990–992. [DOI] [PubMed] [Google Scholar]
- 20.Liao WB, Liu CF, Chiang CW, et al. Cardiovascular manifestations of pheochromocytoma. Am J Emerg Med 2000; 18:622–625. [DOI] [PubMed] [Google Scholar]
- 21.Van Vliet PD, Burchell HB, Titus JL. Focal myocarditis associated with pheochromocytoma. N Engl J Med 1966; 274:1102–1108. [DOI] [PubMed] [Google Scholar]
- 22.Wiswell JG, Crago RM. Reversible cardiomyopathy with pheochromocytoma. Trans Am Clin Climatol Assoc 1969; 80:185–195. [PMC free article] [PubMed] [Google Scholar]
- 23.El-Sayed AM, Brinjikji W, Salka S. Demographic and co-morbid predictors of stress (takotsubo) cardiomyopathy. Am J Cardiol 2012; 110:1368–1372. [DOI] [PubMed] [Google Scholar]
- 24.Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 2010; 55:333–341. [DOI] [PubMed] [Google Scholar]
- 25.Redfors B, Ali A, Shao Y, et al. Different catecholamines induce different patterns of takotsubo-like cardiac dysfunction in an apparently afterload dependent manner. Int J Cardiol 2014; 174:330–336. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S, Ungprasert P, Ratanapo S, et al. Clinical characteristics of takotsubo cardiomyopathy in North America. North Am J Med Sci 2013; 5:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh NK, Rumman S, Mikell FL, et al. Stress cardiomyopathy: clinical and ventriculographic characteristics in 107 North American subjects. Int J Cardiol 2010; 141:297–303. [DOI] [PubMed] [Google Scholar]
- 28.Ionescu CN, Aguilar-Lopez CA, Sakr AE, et al. Long-term outcome of Tako-tsubo cardiomyopathy. Heart Lung Circ 2010; 19:601–605. [DOI] [PubMed] [Google Scholar]
- 29.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 2013; 113:739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elesber A, Lerman A, Bybee KA, et al. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J 2006; 152:469.e9–469.e13. [DOI] [PubMed] [Google Scholar]
- 31.Lyon AR, Rees PSC, Prasad S, et al. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 2008; 5:22–29. [DOI] [PubMed] [Google Scholar]
- 32.Santulli G, Campanile A, Spinelli L, et al. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol 2011; 107:1125–1130. [DOI] [PubMed] [Google Scholar]
- 33.Santulli G. Adrenal signaling in heart failure: something more than a distant ship's smoke on the horizon. Hypertension 2014; 63:215–216. [DOI] [PubMed] [Google Scholar]
- 34.Perino A, Ghigo A, Ferrero E, et al. Integrating cardiac PIP3 and cAMP signaling through a PKA anchoring function of p110 γ. Mol Cell 2011; 42:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kassim TA, Clarke DD, Mai VQ, et al. Catecholamine-induced cardiomyopathy. Endocr Pract 2008; 14:1137–1149. [DOI] [PubMed] [Google Scholar]
- 36.W M, M I, S F, et al. Irreversible dilated cardiomyopathy after surgical resection of pheochromocytomas associated with von Hippel–Lindau disease. Int J Cardiol 2009; e95–e96. [DOI] [PubMed] [Google Scholar]
- 37.Dalby MC, Burke M, Radley-Smith R, et al. Pheochromocytoma presenting after cardiac transplantation for dilated cardiomyopathy. J Heart Lung Transplant 2001; 20:773–775. [DOI] [PubMed] [Google Scholar]
- 38.Wilkenfeld C, Cohen M, Lansman SL, et al. Heart transplantation for end-stage cardiomyopathy caused by an occult pheochromocytoma. J Heart Lung Transplant 1992; 11:363–366. [PubMed] [Google Scholar]
- 39.Marcovitz PA, Czako P, Rosenblatt S, et al. Pheochromocytoma presenting with Takotsubo syndrome. J Intervent Cardiol 2010; 23:437–442. [DOI] [PubMed] [Google Scholar]
- 40.Saddoud N, Turki I, Chammakhi R, et al. Malignant paraganglioma with vertebral and skull metastasis. J Radiol 2006; 87:1887–1890. [DOI] [PubMed] [Google Scholar]
- 41.Plouin P-F, Chatellier G, Fofol I, et al. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension 1997; 29:1133–1139. [DOI] [PubMed] [Google Scholar]
- 42.Scholten A, Vriens MR, Cromheecke GJE, et al. Hemodynamic instability during resection of pheochromocytoma in MEN versus non-MEN patients. Eur J Endocrinol 2011; 165:91–96. [DOI] [PubMed] [Google Scholar]
- 43.Miskulin J, Shulkin BL, Doherty GM, et al. Is preoperative iodine 123 meta-iodobenzylguanidine scintigraphy routinely necessary before initial adrenalectomy for pheochromocytoma? Surgery 2003; 134:918–922. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Yu A, Filippone LA, et al. Inverted-takotsubo pattern cardiomyopathy secondary to pheochromocytoma: a clinical case and literature review. Clin Cardiol 2010; 33:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi AP, Bing-You RG, Thomas LR. Recurrent takotsubo cardiomyopathy associated with pheochromocytoma. Endocr Pract 2009; 15:560–562. [DOI] [PubMed] [Google Scholar]
- 46.Frhlich GM, Schoch B, Schmid F, et al. Takotsubo cardiomyopathy has a unique cardiac biomarker profile: NT-proBNP/myoglobin and NT-proBNP/troponin T ratios for the differential diagnosis of acute coronary syndromes and stress induced cardiomyopathy. Int J Cardiol 2012; 154:328–332. [DOI] [PubMed] [Google Scholar]


