Abstract
Since the use of tumor necrosis factor (TNF) inhibitor therapy is becoming wider, the effects of concurrent intervention with exercises and stabilized TNF inhibitors therapy in patients with ankylosing spondylitis (AS) are different. The study aimed to objectively evaluate whether concurrent intervention with exercises and stabilized TNF inhibitors can reduce the disease activity in patients with AS.
A search from PubMed, Web of Science, EMBASE, and the Cochrane Library was electronically performed to collect studies which compared concurrent intervention with exercise and TNF inhibitor to conventional approach in terms of disease activity in patients with AS published from their inception to June 2015. Studies that measured the Bath Ankylosing Spondylitis Functional Index (BASFI), the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the Bath Ankylosing Spondylitis Metrology Index (BASMI), and chest expansion as outcomes were included. Two independent investigators screened the identified articles, extracted the data, and assessed the methodological quality of the included studies. Quantitative analysis was performed with Review Manager (RevMan) software (version 5.3.0).
A total of 5 studies comprising 221 participants were included in the study. Meta-analyses showed that concurrent intervention with exercises and stabilized TNF inhibitors therapy significantly reduced the BASMI scores (MD, −0.99; 95% CI, −1.61 to −0.38) and BASDAI scores (MD, −0.58; 95% CI, −1.10 to −0.06), but the BASFI scores (MD, −0.31; 95% CI, −0.76 to 0.15) was not reduced, and chest expansion (MD, 0.80; 95% CI, −0.18 to 1.78) was not increased.
Concurrent intervention with exercises and stabilized TNF inhibitors therapy can reduce the disease activity in patients with AS. More randomized controlled trials (RCTs) with high-quality, large-scale, and appropriate follow-up are warranted to further establish the benefit of concurrent intervention with exercises and TNF inhibitors for this given population due to some limitations impaired the power of our study.
INTRODUCTION
Ankylosing spondylitis (AS) is a chronic, progressive rheumatic disease, which characterized by inflammation, ankylosis of the axial skeleton and especially sacroiliitis.1 Previous studies indicated a strong correlation of AS and genetic marker HLA-B27.2 Rather than functional impairment, there is involvement of entheses, peripheral joints and extra-articular organs, reduction of health-related quality of life (HRQoL).3 Stiffness, pain, progressive loss of spine mobility may be key contributors to physical limitations for AS patients.4 These symptoms may decrease the functional status and increase disease activity of AS patients. As a result, AS is an important factor to cause work disability and serious socioeconomic burden.5
At the present, nonsteroidal antiinflammatory drugs (NSAIDs) and disease-modified antirheumatic drugs (DMARDs) always play an important role in pharmacological therapy for AS, but spinal mobility only moderately benefited from this option according to data issued previously.6 Tumor necrosis factor (TNF) inhibitor, which includes infliximab, etanercept, adalimumab, and golimumab, was adopted to improve signs, symptoms, function, and spinal mobility of AS patients in short-term and up to 5 years.7–9
Despite significant progress has been occurred in pharmacological therapy of AS, the recent Assessments of SpondyloArthritis international Society and the European League Against Rheumatism (ASAS/EULAR) recommendations emphasize that combination of pharmacological and nonpharmacological treatments should be the optimal management for it.10 A recent meta-analysis of the literature has confirmed that home-based interventions are an important part of a global therapy strategy for AS patients,11 and reviews also emphasized the significance of adopting physical therapy and exercise to manage AS patients.12,13
The ASAS/EULAR working group suggested that nonpharmacological therapy could be comprised of education, exercise, and physiotherapy, which was recommended to maintain function in patients with AS.14 Exercises seem to play an important role in management AS patients, particularly when performed in an outpatient group supervised by a physiotherapist or intensively in inpatients who showed a short-term improvement.15,16 Nowadays, all kinds of forms of exercises such as educational sessions, supervised exercise, home-based exercise, spa, swimming, the Global Posture Reeducation method, Tai Chi, McKenzie, Heckscher, and Pilates methods,17 yoga2 and so on had been embedded in rehabilitation program for AS patients.11 Some studies evaluated the impact of exercises on AS patients who did not receive prescribed TNF inhibitors17–19 and obtained consistent result that exercises improved the clinical outcomes of AS patients. TNF inhibitors have been widely used in AS patients, and some studies reported the importance of exercises plus stabilized TNF inhibitors therapy in AS patients.20,21 However, only scarce studies have been performed to investigate the effects of regime of exercises plus TNF inhibitors on clinical outcomes of AS patients. Therefore, this meta-analysis aimed to systematically investigate the effects of regime of exercises plus stabilized TNF inhibitors therapy on AS patients.
MATERIALS AND METHODS
Search Strategy
We aimed to explore the effects of regime of exercises plus stabilized TNF inhibitors therapy in AS patients. The research team searched 4 electronic databases including PubMed, Web of Science, EMBASE, and the Cochrane Library using combinations of the terms exercise, education and exercise, Incentive Spirometer Exercise (ISE), spa therapy and rehabilitation; infliximab, etanercept, adalimumab, golimumab, TNF inhibitors, and AS. We performed all analyses based on the published studies previously, and thus no ethical approval and informed consent were required.
Eligibility Criteria
Papers, which population inclusion criteria gathered adult patients with AS diagnosed by a rheumatologist were chosen. Randomized controlled trials (RCTs) or controlled clinical trials (CCTs), in which at least 1 of the groups received the regime of exercises plus TNF inhibitors were included. Participants aged less than 18 years old or with juvenile-onset of AS were excluded. Review articles, observational studies without controls, case reports, cross-sectional studies, self-controlled studies, and commentaries were also excluded. Finally, studies that the data type was skewed distribution and the sample sizes were small and that data could not be converted were excluded.
Information Sources and Study Selection
Studies were retrieved by searching electronic databases (PubMed, Web of Science, EMBASE, and the Cochrane Library) from their inception to June 2015. Search terms were adapted for use with each database. Common keywords and medical subject headings (MeSH) consisted of 2 components: the condition (AS), the intervention (regime of exercise plus stabilized TNF inhibitors therapy). Search restrictions (English language) were imposed. Finally, a hand search of the reference lists of included studies and topic-related review were also conducted.
Two authors (Hui Liang and Wenrong Li) independently screened titles and abstracts to identify studies that potentially met the eligibility criteria. Full texts of searching were retrieved and independently assessed for eligibility by the same 2 authors (Hui Liang and Wenrong Li).
Data Extraction and Quality Assessment
Searches were conducted and data were extracted by 2 independent researchers. Each study identified in the search was evaluated for design, eligibility criteria for participants, and outcome measures. Any disagreements concerning inclusion were resolved by discussion until a consensus was achieved, and for failing agreement, a third reviewer was consulted. Duplicate studies and records were eliminated by using EndNote software and screening the titles and abstracts. All remaining were screened by examining the full text. The quality of the trials included in this study was assessed by 2 independent researchers (Hui Liang and Wenrong Li) according to the Risk of Bias tool released by Cochrane Collaboration.22
Outcome Measures
The outcome measures of interest were BASMI, BASFI, BASDAI, and chest expansion. The characteristics of the outcome measures were showed in the study.23
Statistical Analysis
Outcome measures were compared between participants treated with regime of exercises plus stabilized TNF inhibitors therapy group and stabilized TNF inhibitors therapy alone group within each study. The homogeneity among trials was evaluated using P-value, and we used the fixed-effects model if there was no evidence of heterogeneity (P > 0.1), otherwise a random-effects model was used. Pooled differences in ratios or means were calculated and a 2-tailed P-value < 0.05 was considered to indicate statistical significance. All analyses were performed using Review Manger (RevMan) statistical software, version 5.3.0 (Cochrane Collaboration, Copenhagen, Denmark). Owing to the limited number (below 10) of studies included in each analysis, publication bias was not assessed.
RESULTS
Study Selection and Characteristics
A total of 121 trials were identified after initially literature searched (Fig. 1). Of these, because of the data type of 2 articles24,25 was an interquartile range and the sample sizes were small, we excluded them, finally, 5 trials including 221 participants were deemed eligible for inclusion in further analyses (Table 1). Three of the eligible studies were conducted in the Italy,26–28 1 in Turkey29 and 1 in Korea.20 Exercise interventions employed in these studies included home-based exercise program (HEP), education and home-based exercise, ISE, and spa therapy and rehabilitation. Participants use TNF inhibitors including Infliximab, Etanercept, Adalimumab, Golimumad for at least 3 months and they did not administrate of NSAIDs. The duration of the interventional programs ranged from 2 to 48 weeks.
FIGURE 1.
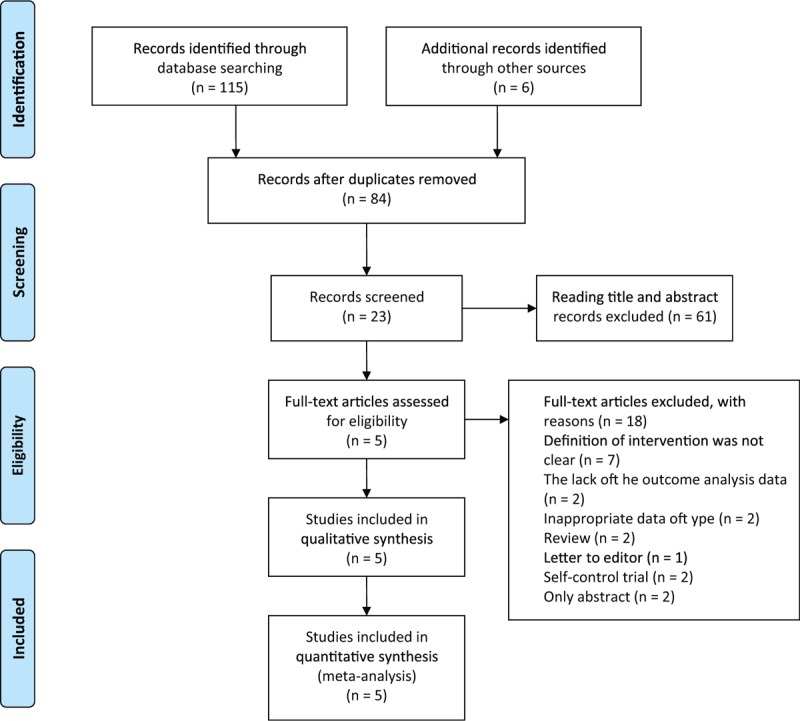
Flow chart of literature retrieval and trial selection.
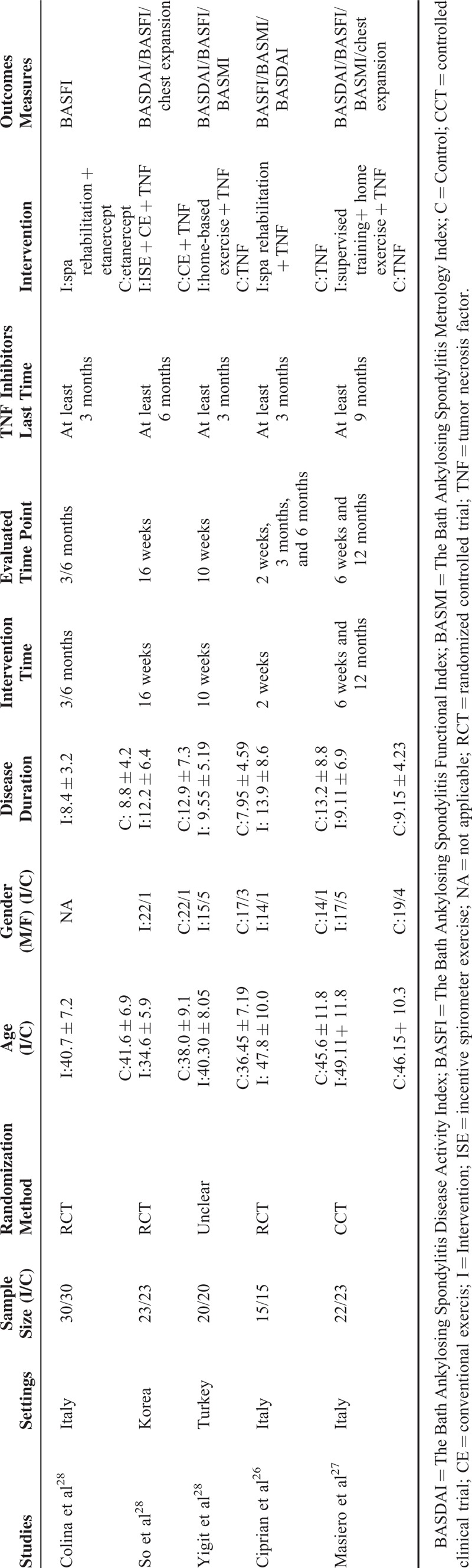
TABLE 1.
Characteristics of Included Studies
BASMI
Three studies26,27,29 involving 115 AS patients reported the BASMI after the interventions were initiated. There was no significant heterogeneity between the regime of exercises plus stabilized TNF inhibitors therapy groups and stabilized TNF inhibitors therapy alone groups in BASMI (P = 0.86, I2 = 0%). A statistically significant difference was observed (MD, −0.99; 95% CI, −1.61 to −0.38), which indicated that the regime of exercises plus stabilized TNF inhibitors therapy interventions reduced the BASMI scores compared to the stabilized TNF inhibitors therapy alone (Fig. 2A).
FIGURE 2.
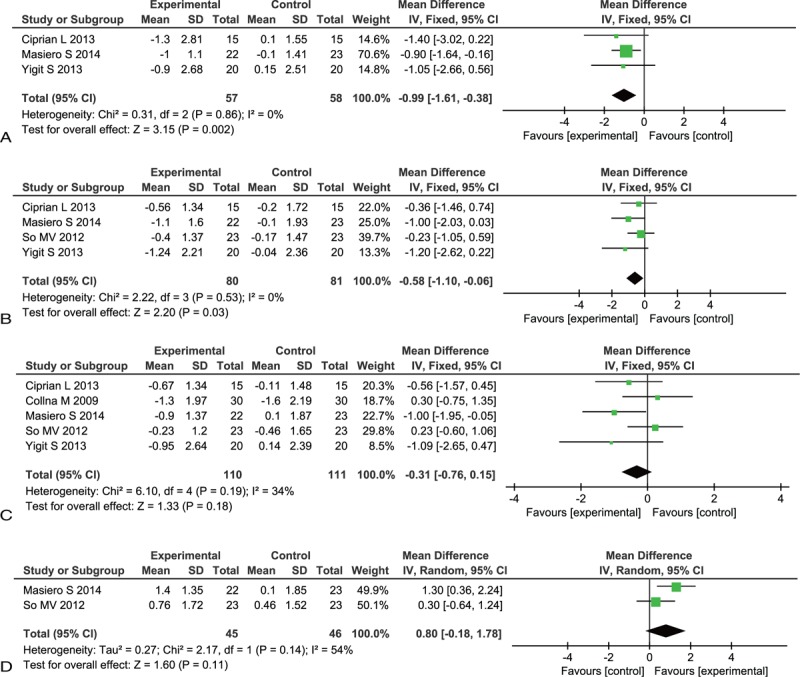
Meta-analyses of outcomes scores between combining exercises with TNF inhibitors and the only TNF group. (A) BASMI scores. (B) BASDAI scores. (C) BASFI scores. (D) Chest expansion.
BASDAI
Four studies20,26,27,29 involving 161 AS patients reported BASDAI after the interventions were initiated. A fixed-effects model was applied because of no significant heterogeneity between the regime of exercises plus stabilized TNF inhibitors therapy and stabilized TNF inhibitors therapy alone groups in BASDAI (P = 0.53, I2 = 0%). A statistically significant difference was observed (MD, −0.58; 95% CI, −1.10 to −0.06), which indicated that the regime of exercises plus stabilized TNF inhibitors therapy interventions reduced the BASDAI scores relative to the stabilized TNF inhibitors therapy alone (Fig. 2B).
BASFI
Five studies20,26–29 which enrolled 221 AS patients reported BASFI after the interventions were initiated. A fixed-effects model was applied because of moderate heterogeneity between the regime of exercises plus stabilized TNF inhibitors therapy and stabilized TNF inhibitors therapy alone groups in BASFI (P = 0.19, I2 = 34%). However, no significant difference was observed (MD, −0.31; 95% CI, −0.76 to 0.15), which indicated that the regime of exercises plus TNF inhibitors interventions were not-superior to the TNF inhibitors alone on reducing the BASFI scores (Fig. 2C).
Chest Expansion
Two studies20,27 involving 91 AS patients reported chest expansion after the interventions were initiated. A random-effects model was applied because of moderate heterogeneity between the regime of exercise plus stabilized TNF inhibitors therapy and stabilized TNF inhibitors therapy alone groups in chest expansion (P = 0.14, I2 = 54%). No statistically difference was observed (MD, 0.80; 95% CI, −0.18 to 1.78). There was no statistically significant difference between the regime of exercises plus stabilized TNF inhibitors therapy interventions and the stabilized TNF inhibitors therapy alone groups on increasing the chest expansion (Fig. 2D).
Quality Assessment
No studies included in this study could be identified as having adequate sequence generation, 3 studies20,26,28 illustrated the RCTs, 1 unclear29 and 1 CCTs27; allocation concealment and blinding were unclear and only 1 study illustrated the blinding of outcome assessment20; the 3 studies20,27,29 addressed incomplete outcome data. Furthermore, the baselines were comparable in all the studies. The quality assessment outcome was summarized in Figures 3 and 4.
FIGURE 3.
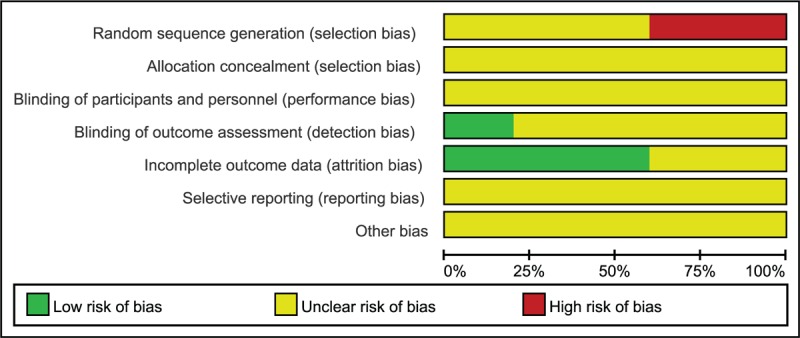
Percentage of risk of bias: authors’ judgments about percentages of each risk of bias item in all included studies.
FIGURE 4.
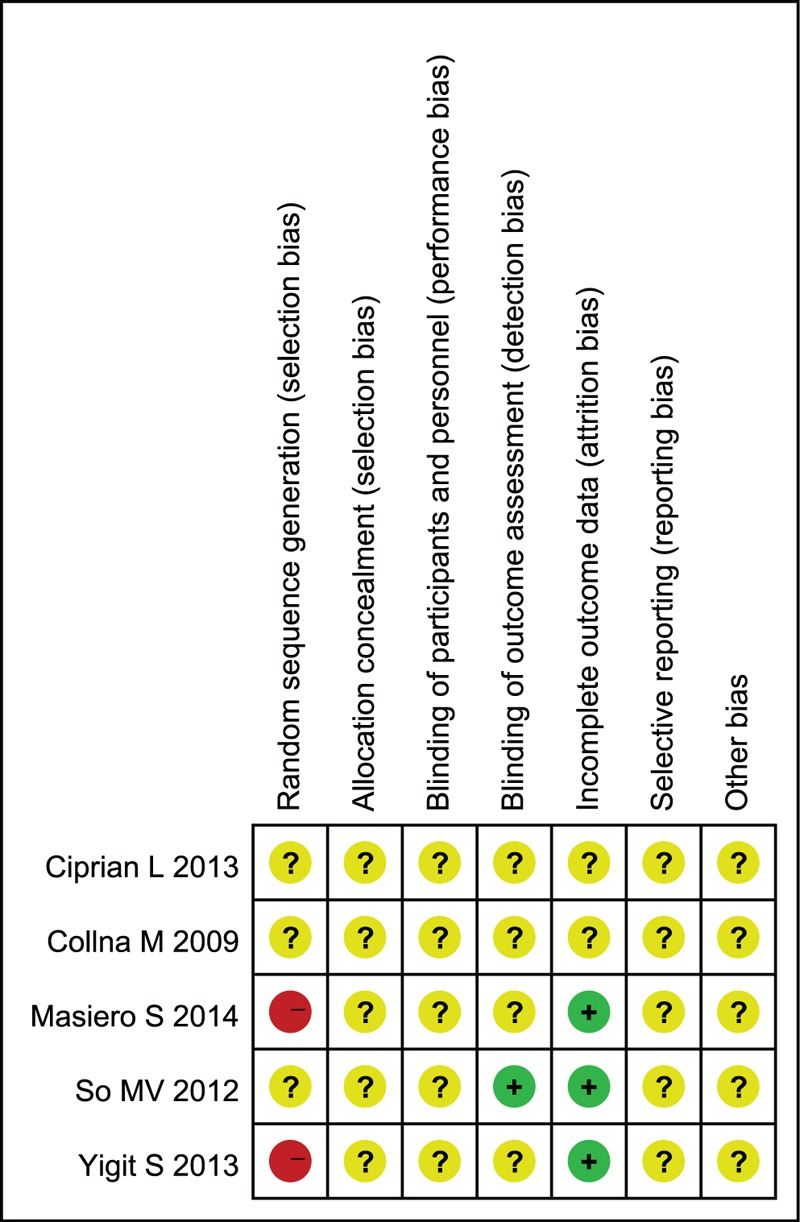
Risk of bias summary: authors’ judgments about each risk of bias item for each included study.
DISCUSSION
This meta-analysis found evidence that the regime of exercises combined with stabilized TNF inhibitors therapy can benefit on reducing BASMI, BASDAI and not increasing chest expansion in AS patients compared to stabilized TNF inhibitors therapy alone, For BASFI scores, the total pooled existing moderate heterogeneity (P = 0.19, I2 = 34%), and there was no significant effect of the regime of exercises plus stabilized TNF inhibitors therapy (MD, −0.31; 95% CI, −0.76 to 0.15). We found that there were 2 studies27,28 suggested the intervention group was superior to the control on BASFI scores. Masiero et al27 found at 6 weeks and 12 months, compared with the other 2 groups, the supervised training and home-based rehabilitation group exhibited a significant reduction in BASFI. Colina et al's study28 showed a statistically significant difference in favor of combining etanercept with an intensive rehabilitation. Clinical and methodology homogeneity are key factors to rational pooled results of meta-analysis. Clinical homogeneity refers to participants and methodology homogeneity refers to research protocol design, statistical methods design, and interventions.30 A comprehensive analysis of the studies found that the exercise intervention methods are different, especially for the one study,20 hence, methodological heterogeneity might exist in the trial.
Five studies are exercises and stabilized TNF inhibitors therapy, but the exercise interventions still were a little bit different. For example, 2 studies were home-based exercises, in which 1 study was that the intervention group (included educational-behavioral meetings and exercise-training part developed by the interdisciplinary team), while the control group did not take part in any exercise training, only continued standard biological therapy27; for the regime of exercises of the other study according to the study by Uhrin et al,31 patients following HEP (exercise group) were compared with those exercising less than exercise group (control group),29 and the subjects of the 2 studies were eligible to participate in the program that they were receiving TNF-α inhibitors at least 9 months and 3 months, respectively. Two studies used spa rehabilitation exercises, in 1 study the intensive rehabilitation program was conducted in a thermal baths center28; the other study the patients in the study group underwent 10 sessions of spa therapy and rehabilitation over a 2 weeks period.26 In one study the intervention group combined incentive spirometer and conventional exercises (ISE and CE), the control group patients used the CE regimen consisted of 20 exercises for 30 minutes once a day.20 Therefore, future studies should focus on the same exercise intervention method.
The primary aim of exercise treatment is to avoid stiffening in a flexed position and to maintain or improve functional capacity and reduce the disease activity. The long-term goal is the maintenance of a good posture.32 In the trials analyzed, the exercise was associated with a statistically significant improvement on spinal mobility (BASMI) and daily activities (BASDAI) in our study. A statistically significant improvement in quality of life (QoL) expressed by Short Form 36(SF-36),29 Health Assessment Questionnaire,26 and EuroQol-5Dimensions28 was found in our included studies. In addition, our included studies evaluated the pain results using VAS in one study,26 fatigue results using Multidimensional Assessment of Fatigue Scale in 1 study,29 the level of morning stiffness in 1 study,27 the pulmonary function test, 6-minute walk distance and finger to floor distance.20 Symptoms such as pain, fatigue, depression, and stiffness would influence the QoL of AS patients. Therefore, exercises have positive effects in improving QoL, which should always be included between the outcomes of exercise treatment,4,33 so future studies should increase the outcomes of exercise.
The success obtained with TNF inhibitors does not excluded the need for physical therapy or exercise,34 which plays an important role in AS patients’ management and in the era of biological drugs.10 Since this association seemed to be more effective than a single exercise program, a synergistic role between biological drugs and exercise might in fact be hypothesized, although the mechanisms of the combination treatment function are not clear yet. We found that a combination treatment including rehabilitation and etanercept versus rehabilitation only, the combination treatment seems to improve function, disability, and QoL in patients with active AS.35 Therefore, TNF inhibitors therapy may be also important for AS patients.
Strengths, Limitations, and Future Research
To our knowledge, to date, 2 articles12,13 had systematically examined the effect of the regime of exercises combined with stabilized TNF inhibitors therapy interventions in AS patients, but they were only systematic reviews. Therefore, we firstly used a meta-analysis to systematically investigate the effect of regime of exercises plus stabilized TNF inhibitors therapy compared to stabilized TNF inhibitors therapy alone, although exercise is frequently advised as part of their managements. However, there are a number of limitations to this meta-analysis that should be acknowledged. Firstly, perhaps the most notably, only a small number of studies met the inclusion criteria, thus reducing the power of the analyses. Meanwhile, the publication bias test was not conducted due to insufficient number of eligible studies for each outcome, thus the pooled results will be negatively affected if small sample size effect existed. Secondly, the inclusion of only English-language literature may also have restricted the number of available relevant articles, at the same time, there are only 3 RCT studies, existence of publication bias for studies was included, and had a negative effect on the pooled results of current meta-analyses. Thirdly, the exercise intervention forms are different. Finally, randomized controlled trials investigating the short- and long-term benefits of combining stabilized TNF inhibitors therapy with rehabilitation in early, but in our included studies we selected the intervention time did not exceeding four months, only 1 study followed up 12 months, hence, the follow-up time would increase. Therefore, we hope future studies with a multicentered and large-scale sample, adequate follow-up, similar exercise duration, frequency and methods, and randomized methodology to draw conclusions about the effect of the regime of exercises combined with stabilized TNF inhibitors therapy in AS patients.
CONCLUSION
The findings do suggest that the regime of exercises combined with stabilized TNF inhibitor therapy may be more effective on reducing BASMI and BASDAI of AS than stabilized TNF inhibitors therapy alone. Insufficient high-quality evidence is available in the current literature regarding the effects of the regime of exercise plus stabilized TNF inhibitors therapy for the QoL with AS patients. Hence, the findings from the current meta-analyses are by no means definitive. So, the RCTs with high quality and large-scale and adequate follow-up are needed to further clarify the effectiveness of the regime of exercises plus stabilized TNF inhibitors therapy for AS patients.
Acknowledgment
We would like to the editor and anonymous referee due to all helps proposed by them to improve the quality of our manuscript.
Footnotes
Abbreviations: AS = ankylosing spondylitis, ASAS = Assessments of SpondyloArthritis international Society, BASDAI = Bath Ankylosing Spondylitis Disease Activity Index, BASFI = Bath Ankylosing Spondylitis Functional Index, BASMI = Bath Ankylosing Spondylitis Metrology Index, DMARDs = disease-modified antirheumatic drugs, EULAR = European League Against Rheumatism, HEP = home-based exercise program, HRQoL = health-related quality of life, ISE = Incentive Spirometer Exercise, NSAIDs = nonsteroidal antiinflammatory drugs, QoL = quality of life, TNF = tumor necrosis factor.
The authors have no conflicts of interest to disclose.
Author contributions: Wei Wei and Chunmei Wang conceived and completed the protocol. Hui Liang, Wenrong Li, and Hua Zhang searched the potential citations. Hui Liang and Wenrong Li screened and selected the eligible studies. Hui Liang, Xu Tian, and Wenrong Li abstracted the essential information. Hua Zhang and Xu Tian performed the synthesis. Hui Liang, Hua Zhang, and Xu Tian had written the manuscript. All authors reviewed the whole manuscript and agreed to submit it for publication in Medicine®.
REFERENCES
- 1.Kjeken I, Bo I, Ronningen A, et al. A three-week multidisciplinary in-patient rehabilitation programme had positive long-term effects in patients with ankylosing spondylitis: randomized controlled trial. J Rehabil Med 2013; 45:260–267. [DOI] [PubMed] [Google Scholar]
- 2.Edavalath M. Ankylosing spondylitis. J Ayurveda Integr Med 2010; 1:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sieper J, Braun J, Rudwaleit M, et al. Ankylosing spondylitis: an overview. Ann Rheum Dis 2002; 61 Suppl. 3:iii8–iii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karapolat H, Akkoc Y, Sari I, et al. Comparison of group-based exercise versus home-based exercise in patients with ankylosing spondylitis: effects on Bath Ankylosing Spondylitis Indices, quality of life and depression. Clin Rheumatol 2008; 27:695–700. [DOI] [PubMed] [Google Scholar]
- 5.Cakar E, Taskaynatan MA, Dincer U, et al. Work disability in ankylosing spondylitis: differences among working and work-disabled patients. Clin Rheumatol 2009; 28:1309–1314. [DOI] [PubMed] [Google Scholar]
- 6.Biasi D, Carletto A, Caramaschi P, et al. Efficacy of methotrexate in the treatment of ankylosing spondylitis: a three-year open study. Clin Rheumatol 2000; 19:114–117. [DOI] [PubMed] [Google Scholar]
- 7.Brandt J, Khariouzov A, Listing J, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003; 48:1667–1675. [DOI] [PubMed] [Google Scholar]
- 8.van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006; 54:2136–2146. [DOI] [PubMed] [Google Scholar]
- 9.Braun J, Baraliakos X, Listing J, et al. Persistent clinical efficacy and safety of anti-tumour necrosis factor alpha therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of response. Ann Rheum Dis 2008; 67:340–345. [DOI] [PubMed] [Google Scholar]
- 10.Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011; 70:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol 2015; 34:1737–1744. [DOI] [PubMed] [Google Scholar]
- 12.Giannotti E, Trainito S, Arioli G, et al. Effects of physical therapy for the management of patients with ankylosing spondylitis in the biological era. Clin Rheumatol 2014; 33:1217–1230. [DOI] [PubMed] [Google Scholar]
- 13.Lubrano E, Spadaro A, Amato G, et al. Tumour necrosis factor alpha inhibitor therapy and rehabilitation for the treatment of ankylosing spondylitis: a systematic review. Semin Arthritis Rheum 2015; 44:542–550. [DOI] [PubMed] [Google Scholar]
- 14.Zochling J, van der Heijde D, Burgos-Vargas R, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2006; 65:442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev 2004; CD002822. [DOI] [PubMed] [Google Scholar]
- 16.Analay Y, Ozcan E, Karan A, et al. The effectiveness of intensive group exercise on patients with ankylosing spondylitis. Clin Rehabil 2003; 17:631–636. [DOI] [PubMed] [Google Scholar]
- 17.Rosu MO, Topa I, Chirieac R, et al. Effects of Pilates, McKenzie and Heckscher training on disease activity, spinal motility and pulmonary function in patients with ankylosing spondylitis: a randomized controlled trial. Rheumatol Int 2014; 34:367–372. [DOI] [PubMed] [Google Scholar]
- 18.Silva EM, Andrade SC, Vilar MJ. Evaluation of the effects of Global Postural Reeducation in patients with ankylosing spondylitis. Rheumatol Int 2012; 32:2155–2163. [DOI] [PubMed] [Google Scholar]
- 19.Gyurcsik ZN, Andras A, Bodnar N, et al. Improvement in pain intensity, spine stiffness, and mobility during a controlled individualized physiotherapy program in ankylosing spondylitis. Rheumatol Int 2012; 32:3931–3936. [DOI] [PubMed] [Google Scholar]
- 20.So MW, Heo HM, Koo BS, et al. Efficacy of incentive spirometer exercise on pulmonary functions of patients with ankylosing spondylitis stabilized by tumor necrosis factor inhibitor therapy. J Rheumatol 2012; 39:1854–1858. [DOI] [PubMed] [Google Scholar]
- 21.Dubey SG, Leeder J, Gaffney K. Physical therapy in anti-TNF treated patients with ankylosing spondylitis. Rheumatology (Oxford) 2008; 47:1100–1101. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG. Higgins JP, Green S. Assessing risk of bias in included studies. Wiley-Blackwell, Cochrane Handbook for Systematic Reviews of Interventions: Version 5.0.0 [Internet]. Chichester:2008. [Google Scholar]
- 23.Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S). Arthritis Care Res (Hoboken) 2011; 63 Suppl. 11:S47–S58. [DOI] [PubMed] [Google Scholar]
- 24.Masiero S, Bonaldo L, Pigatto M, et al. Rehabilitation treatment in patients with ankylosing spondylitis stabilized with tumor necrosis factor inhibitor therapy: a randomized controlled trial. J Rheumatol 2011; 1335–1342. [DOI] [PubMed] [Google Scholar]
- 25.Spadaro A, De Luca T, Massimiani MP, et al. Occupational therapy in ankylosing spondylitis: short-term prospective study in patients treated with anti-TNF-alpha drugs. Joint Bone Spine 2008; 75:29–33. [DOI] [PubMed] [Google Scholar]
- 26.Ciprian L, Lo Nigro A, Rizzo M, et al. The effects of combined spa therapy and rehabilitation on patients with ankylosing spondylitis being treated with TNF inhibitors. Rheumatol Int 2013; 241–245. [DOI] [PubMed] [Google Scholar]
- 27.Masiero S, Poli P, Bonaldo L, et al. Supervised training and home-based rehabilitation in patients with stabilized ankylosing spondylitis on TNF inhibitor treatment: a controlled clinical trial with a 12-month follow-up. Clin Rehabil 2014; 28:562–572. [DOI] [PubMed] [Google Scholar]
- 28.Colina M, Ciancio G, Garavini R, et al. Combination treatment with etanercept and an intensive spa rehabilitation program in active ankylosing spondylitis. Int J Immunopathol Pharmacol 2009; 22:1125–1129. [DOI] [PubMed] [Google Scholar]
- 29.Yigit S, Sahin Z, Demir SE, et al. Home-based exercise therapy in ankylosing spondylitis: short-term prospective study in patients receiving tumor necrosis factor alpha inhibitors. Rheumatol Int 2013; 33:71–77. [DOI] [PubMed] [Google Scholar]
- 30.Xu T, Hui L, Juan YL, et al. Effects of moxibustion or acupoint therapy for the treatment of primary dysmenorrhea: a meta-analysis. Altern Ther Health Med 2014; 20:33–42. [PubMed] [Google Scholar]
- 31.Uhrin Z, Kuzis S, Ward MM. Exercise and changes in health status in patients with ankylosing spondylitis. Arch Intern Med 2000; 160:2969–2975. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-de-Las-Penas C, Alonso-Blanco C, Morales-Cabezas M, et al. Two exercise interventions for the management of patients with ankylosing spondylitis: a randomized controlled trial. Am J Phys Med Rehabil 2005; 84:407–419. [DOI] [PubMed] [Google Scholar]
- 33.Durmus D, Alayli G, Cil E, et al. Effects of a home-based exercise program on quality of life, fatigue, and depression in patients with ankylosing spondylitis. Rheumatol Int 2009; 29:673–677. [DOI] [PubMed] [Google Scholar]
- 34.van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005; 52:582–591. [DOI] [PubMed] [Google Scholar]
- 35.Lubrano E, D’Angelo S, Parsons WJ, et al. Effects of a combination treatment of an intensive rehabilitation program and etanercept in patients with ankylosing spondylitis: a pilot study. J Rheumatol 2006; 33:2029–2034. [PubMed] [Google Scholar]


