Abstract
The effect of hepatitis B virus (HBV) infection on bone mineral density in patients without advanced liver disease remains unclear. Hence, we assessed the association between HBV infection and the risk of osteoporosis.
From 2000 to 2011, patients older than 20 years with HBV infection were identified from the Longitudinal Health Insurance Database 2000. Of the 180,730 sampled patients, 36,146 and 144,584 patients were categorized into HBV infection and comparison cohorts, respectively.
Compared with the comparison cohort, the HBV infection patients had a higher risk of osteoporosis (adjusted hazard ratio [aHR]: 1.14, 95% confidence interval [CI]: 1.03–1.25) after adjusting for age, sex, frequency of medical visits, and comorbidities of diabetes, hypertension, hyperlipidemia, heart failure, cirrhosis, chronic kidney disease, thyroid diseases, medication of steroid, PPI, warfarin, aspirin, and estrogen replacement therapy. The patients with HBV infection exhibited a 1.13-fold (95% CI = 1.03–1.25) higher risk of developing osteoporosis, but the risk of osteoporotic fracture was comparable between patients with HBV infection and the comparison cohort (aHR = 1.20, 95% CI = 0.77–1.86). The incidence of osteoporosis increased with the increment of age (age ≤ 49: aHR = 1; age 50–64: aHR = 5.67, 95% CI = 5.09–6.32; age ≧ 65: aHR = 13.3, 95% CI = 11.8–14.9) and coexisting cirrhosis (aHR = 1.62, 95% CI = 1.24–2.12). However, the osteoporosis risk contributed by HBV infection decreased with age and the age-specific risk analyses showed that patients with HBV infection exhibited the highest risk of osteoporosis than patients without HBV infection for the patients aged ≤49 (aHR = 1.42, 95% CI = 1.19–1.70). Furthermore, the osteoporosis risk contributed by HBV infection has decreased with the presence of comorbidity (aHR = 1.27, 95% CI = 1.09–1.48 vs aHR = 1.04, 95% CI = 0.91–1.15).
HBV increases the risk of osteoporosis, but HBV infection may be less influential than other risk factors. Moreover, HBV has no detrimental effect on osteoporotic fracture in this study.
INTRODUCTION
Osteoporosis is characterized by reduced bone mineral density and deterioration in skeletal microarchitecture with consequent bone fragility and susceptibility to fractures.1 Current demographic trends of an increasing average life expectancy of over 65 years among the general population will lead to a major increase in osteoporosis and osteoporotic fracture.2,3 Moreover, osteoporosis has been ranked as having the 5th highest health care expenditure for age-related diseases, below diabetes, hyperlipidemia, hypertension, and heart diseases.4 Aging, immobility, hypertension, use of antihypertensive agents, hyperparathyroidism, menopause, diabetes mellitus, corticosteroid usage, low calcium intake, vitamin D deficiency, and genetic vulnerability are traditionally considered the risk factors for osteoporosis. Moreover, osteoporotic fracture is the most severe impact of osteoporosis on socioeconomics and the national general health.5 The risk of osteoporotic fracture mainly depends on the mechanical strength of bone and the forces acting on it; therefore, bone mineral density remains the most reliable predictor of osteoporotic fracture.6 It is important to identify and treat additional potential risk factors of osteoporosis for alleviating the burden of osteoporotic fracture.
Chronic hepatitis B virus (HBV) infection has emerged as a global health problem, and it was estimated that as many as 365 million people (6%) have been infected worldwide.7 Despite HBV vaccination having been introduced to Taiwan over 30 years ago, the seroprevalence of the HBV surface antigen decreased only slightly from 13.7% in 2002 to 12.65% in 2007.8 In contrast to hepatitis C virus, the effect of HBV on bone mineral density is rarely mentioned in the literature.9,10 Although prior studies have suggested that chronic HBV infection can induce tumor necrosis factors to inhibit bone formation and increase bone resorption, no longitudinal study has been conducted to evaluate the relationship between chronic HBV infection and osteoporosis or osteoporotic fracture.11,12
To assess the association between HBV infection and subsequent development of osteoporosis or osteoporotic fracture, we conducted a nationwide population-based cohort study by analyzing data from a nationwide medical database, the National Health Insurance Research Database.
METHODS
Data Source
Our study cohort was derived from the Longitudinal Health Insurance Database 2000 (LHID2000), a nationally representative dataset of 1,000,000 insurants.13 The LHID2000 contains all reimbursement claims data for each insurant, including registry of beneficiary, medical records, and medical services, and the database is updated annually. The LHID2000 consists of deidentified secondary data that are made available to researchers in Taiwan by the National Health Research Institutes (http://nhird.nhri.org.tw/date_01.html). Each insurant's diagnosis codes are classified using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). To ensure the accuracy of disease diagnoses, the Bureau of National Health Insurance (BNHI) randomly reviews the medical charts of one in every 100 ambulatory claims and one in every 20 inpatient claims. This study was approved by the Ethics Review Board of China Medical University Hospital (CMU-REC-101–012).
Sampled Patients
Figure 1 shows the selection process of the participants in the HBV infection cohort and the comparison cohort to assess the association between HBV infection and subsequent development of osteoporosis or osteoporotic fracture. The HBV infection cohort comprised patients with an initial HBV infection diagnosed (ICD-9-CM codes 070.20, 070.22, 070.30, 070.32, and V02.61) from 2000 to 2011. The HBV infection diagnosis date was defined as the index date. We selected comparison patients without a HBV infection diagnosis. The index date of comparison patients was the same year as the matched HBV infection patients and a month and day were randomly assigned. In our study, osteoporosis consists of osteoporosis alone (ICD-9-CM 733.0) and osteoporotic fracture (ICD-9-CM 733.1). Patients with a history of osteoporosis and hepatitis C virus infection (ICD-9-CM codes070.41, 070.44, 070.51, and 070.54) diagnosed before the index date and those with incomplete age or sex information were excluded from both the HBV infection and comparison cohorts. The HBV infection patients and comparison patients were matched at a 1:4 ratio based on propensity scores. The confounding factors in this study were determined according to Japanese 2011 guidelines for prevention and treatment of osteoporosis.14 We used logistic regression to calculate the propensity score for each patient by estimating the assignment probability based on baseline variables, including age, sex, frequency of medical visits (per year), comorbidities of diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), heart failure (ICD-9-CM code 428), stroke (ICD-9-CM codes 430-438), obesity (ICD-9-CM code 278), cirrhosis (ICD-9-CM codes 571.2, 571.5, and 571.6), chronic kidney disease (ICD-9-CM code 585) and thyroid diseases (ICD-9-CM codes 240-242, 244-246), medications of steroid, proton pump inhibitor (PPI), warfarin, aspirin, and estrogen replacement therapy. This would provide an equal probability to each HBV infection patient of being assigned to the comparison cohort.
FIGURE 1.
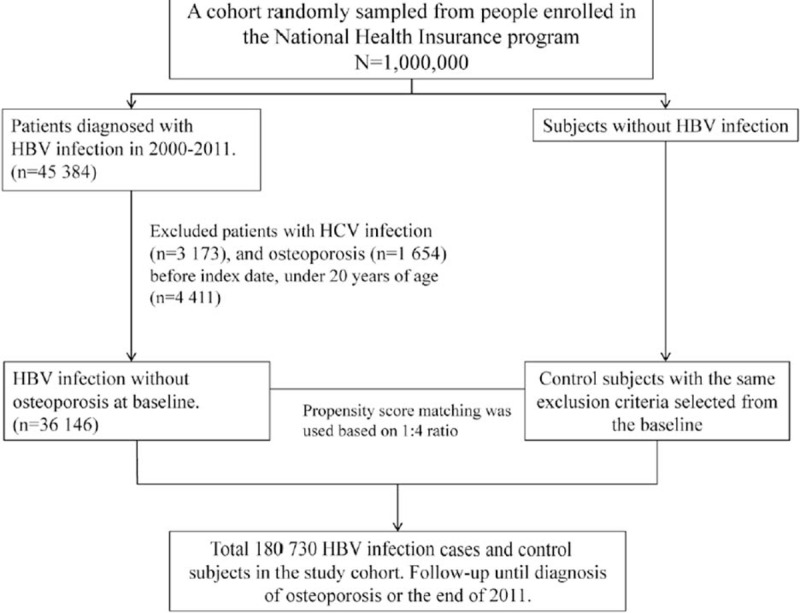
The selection process of the participants in the hepatitis B virus (HBV) infection cohort and the comparison cohort.
Outcome
The mean follow-up period was 5.74 ± 3.39 and 5.84 ± 3.41 years in the HBV infection and comparison cohorts, respectively (data not shown). The duration of follow-up in person-years was measured for each patient from the index date to osteoporosis diagnosis, or until the patient was censored because of death or withdrawal from the insurance system.
Statistical Analysis
The distributions of demographic factors, including age, sex, frequency of medical visits (per year), comorbidities, and medications in the HBV infection cohort and comparison cohort were matched on the propensity scores. The standardized difference was used to quantify differences in means or prevalence between the HBV infection cohort and comparison cohort for all matching variables. The incidence density of developing osteoporosis for each risk factor was measured as the number of osteoporosis events divided by the sum of follow-up time (per 1000 person-years). Univariate and multivariate Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for assessing the effects of HBV infection on the risk of osteoporosis. The multivariate models were simultaneously adjusted for age, sex, frequency of medical visits, and comorbidities of diabetes, hypertension, hyperlipidemia, heart failure, cirrhosis, chronic kidney disease, thyroid diseases, and medication of steroid, PPI, warfarin, aspirin, and estrogen replacement therapy. We estimated the disease-specific cumulative incidences by Kaplan–Meier survival curves for adjusted functions by considering age, sex, frequency of medical visits, and the aforementioned comorbidities and medications in the Cox model. The difference in cumulative incidence curves between the HBV infection and comparison cohorts was tested using the likelihood-ratio test. Data management and statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC); a 2-sided P < 0.05 indicated statistical significance.
RESULTS
The cumulative incidence curve for osteoporosis in the HBV infection cohort was significantly higher than that for the comparison cohort after adjustment for age, sex, frequency of medical visits, and comorbidities of diabetes, hypertension, hyperlipidemia, heart failure, cirrhosis, chronic kidney disease, thyroid diseases, and medication of steroid, PPI, warfarin, aspirin, and estrogen replacement therapy (Fig. 2, P for likelihood-ratio test = 0.009). In the patients with HBV infection, risk of osteoporosis increased progressively with increasing duration of follow-up, rather than with being limited to the immediate days, after a diagnosis of HBV infection.
FIGURE 2.
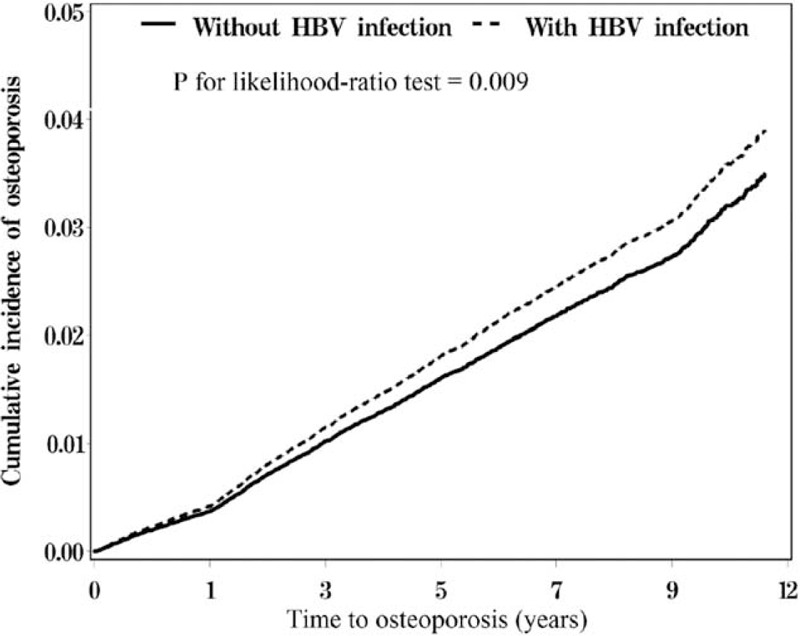
Cummulative incidence comparison of osteoporosis for patients with (dashed line) and without (solid line) hepatitis B virus (HBV) infection.
Table 1 shows the demographic characteristics and comorbidities in cohorts with and without HBV infection based on propensity score matching. The patients in both cohorts were predominantly men and aged ≤49 years. The mean ages of the patients in the HBV infection and comparison cohorts were 42.6 (standard deviation [SD] = 13.7) and 42.2 (SD = 15.7) years, respectively. The mean frequency of medical visits for the HBV infection cohort and comparison cohort were 21.4 years (SD = 16.3) and 20.7 years (SD = 18.9), respectively. Both cohorts were similar in distributions of comorbidities and medications.
TABLE 1.
Demographic Characteristics and Comorbidities in Cohorts With and Without HBV Infection
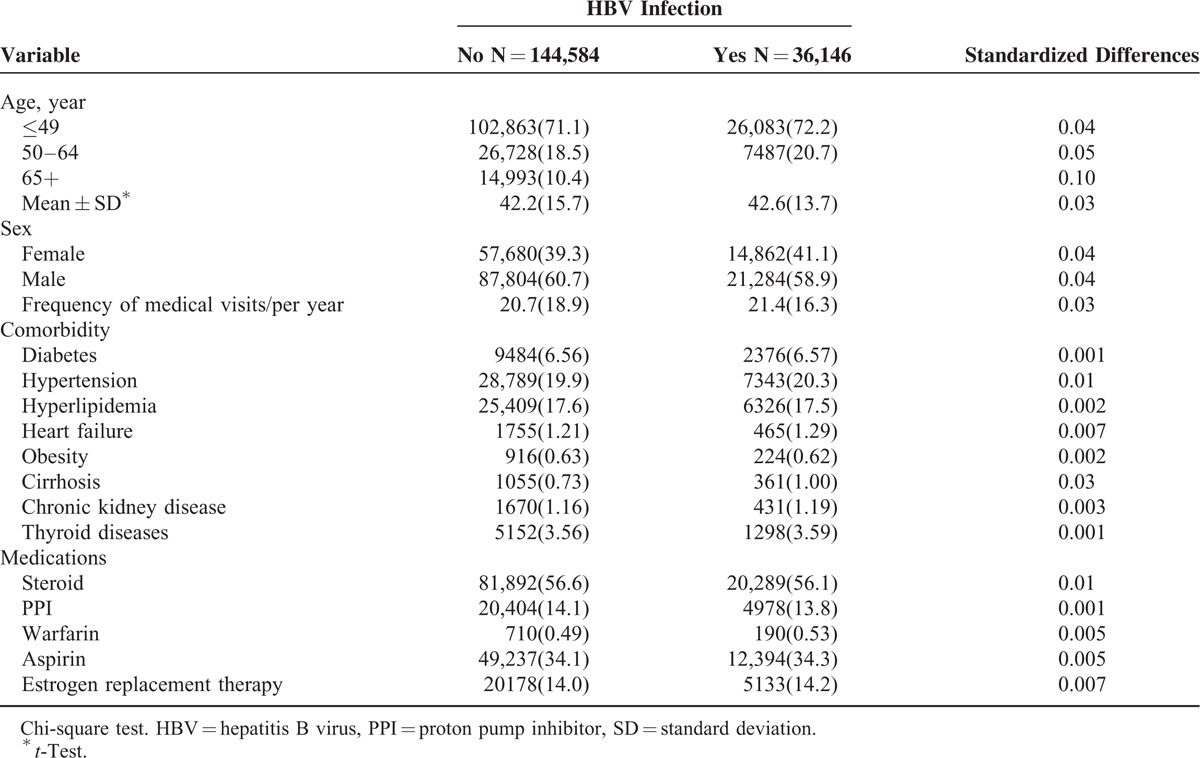
Table 2 shows the incidence and hazard ratios for osteoporosis and osteoporosis-associated risk factors. The incidence rates of osteoporosis were 2.59 and 2.81 per 1000 person-years for the HBV infection and comparison cohorts, respectively. Compared with the comparison cohort, the HBV infection patients had a higher risk of osteoporosis (adjusted hazard ratio [aHR]: 1.14, 95% CI: 1.03–1.25) after adjusting for age, sex, frequency of medical visits, and comorbidities of diabetes, hypertension, hyperlipidemia, heart failure, cirrhosis, chronic kidney disease, thyroid diseases, medication of steroid, PPI, warfarin, aspirin, and estrogen replacement therapy. HBV infection patients over the age of 65 years had a higher incidence of osteoporosis (15.7 per 1000 person-years) and this risk of osteoporosis increased with age. The risk of osteoporosis was higher in women than in men (aHR = 2.93, 95% CI = 2.70–3.19). Furthermore, cirrhosis and aspirin usage were also associated with a high risk of osteoporosis. The relative risk of osteoporosis increased significantly in the women aged greater than 50 years, and the relative risk of osteoporosis diminished after estrogen replacement therapy. In addition, HBV infection increased the relative risk of osteoporosis among the women with estrogen replacement therapy (supplementary Table 1).
TABLE 2.
Incidence and Hazard Ratios for Osteoporosis and Osteoporosis-Associated Risk Factors
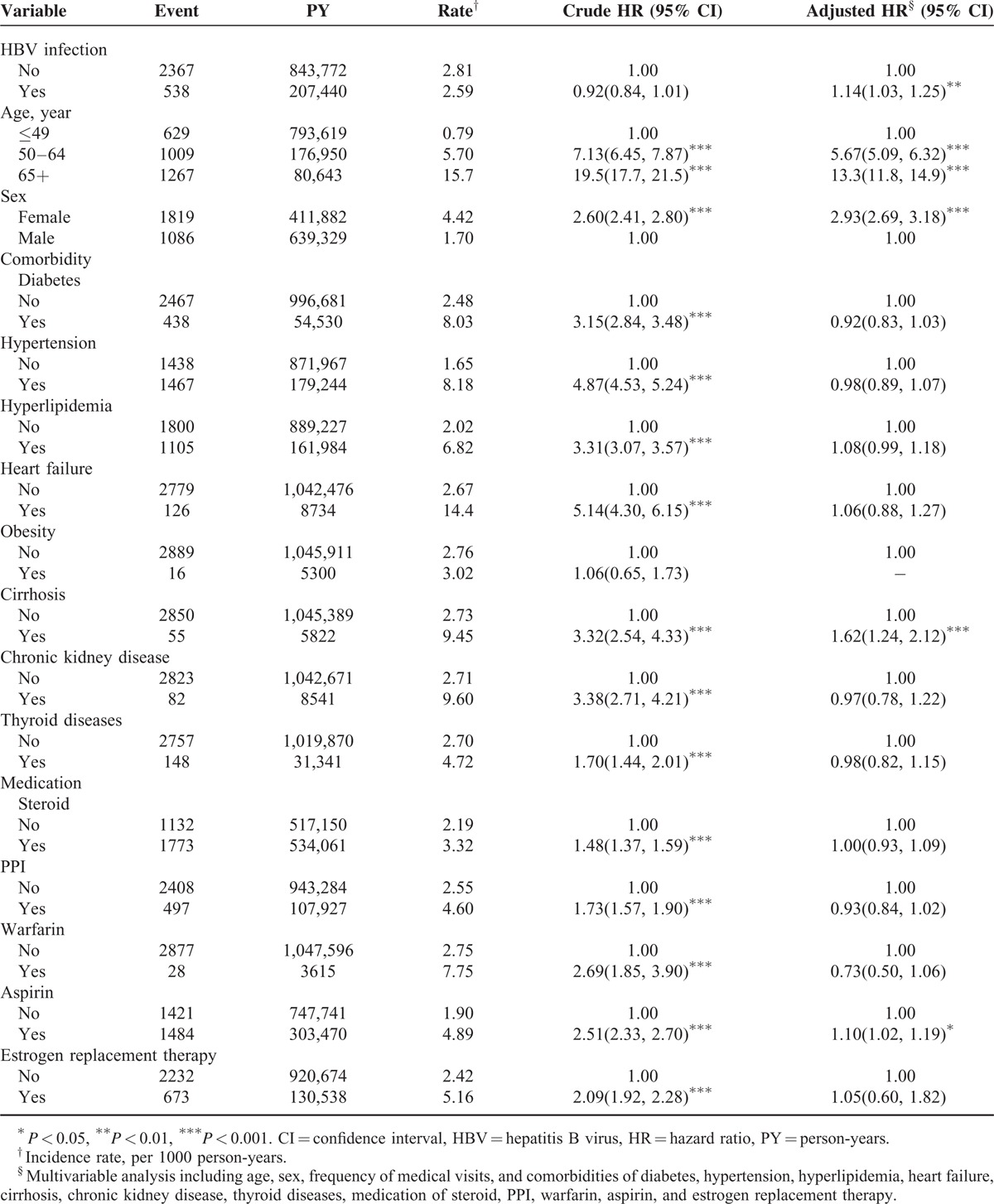
Table 3 shows the incidence of osteoporosis by age, sex, and comorbidity, and a Cox model of measured hazard ratios for patients with HBV infection compared with those without HBV infection. The osteoporosis risk contributed by HBV infection has decreased with age and the age-specific risk analyses showed that patients with HBV infection exhibited a significantly higher risk of osteoporosis than patients without HBV infection for the patients aged ≤49 (aHR = 1.42, 95% CI = 1.19–1.70). The risk of osteoporosis was higher in patients with HBV infection than in those without HBV infection for women (aHR = 1.19; 95% CI = 1.06–1.33). The osteoporosis risk contributed by HBV infection has decreased with the presence of comorbidity (aHR = 1.27, 95% CI = 1.09–1.48 vs aHR = 1.04, 95% CI = 0.91–1.15). The risk of osteoporosis was greater in the patients with HBV infection than that in the comparison cohort for medications of steroid and estrogen replacement therapy. However, the osteoporosis risk contributed by HBV infection was generally greater among the patients without the usage of osteoporosis-related medications.
TABLE 3.
Incidence of Osteoporosis by Age, Sex, and Comorbidity, and a Cox Model of Measured Hazard Ratios for Patients With HBV Infection Compared With Those Without HBV Infection
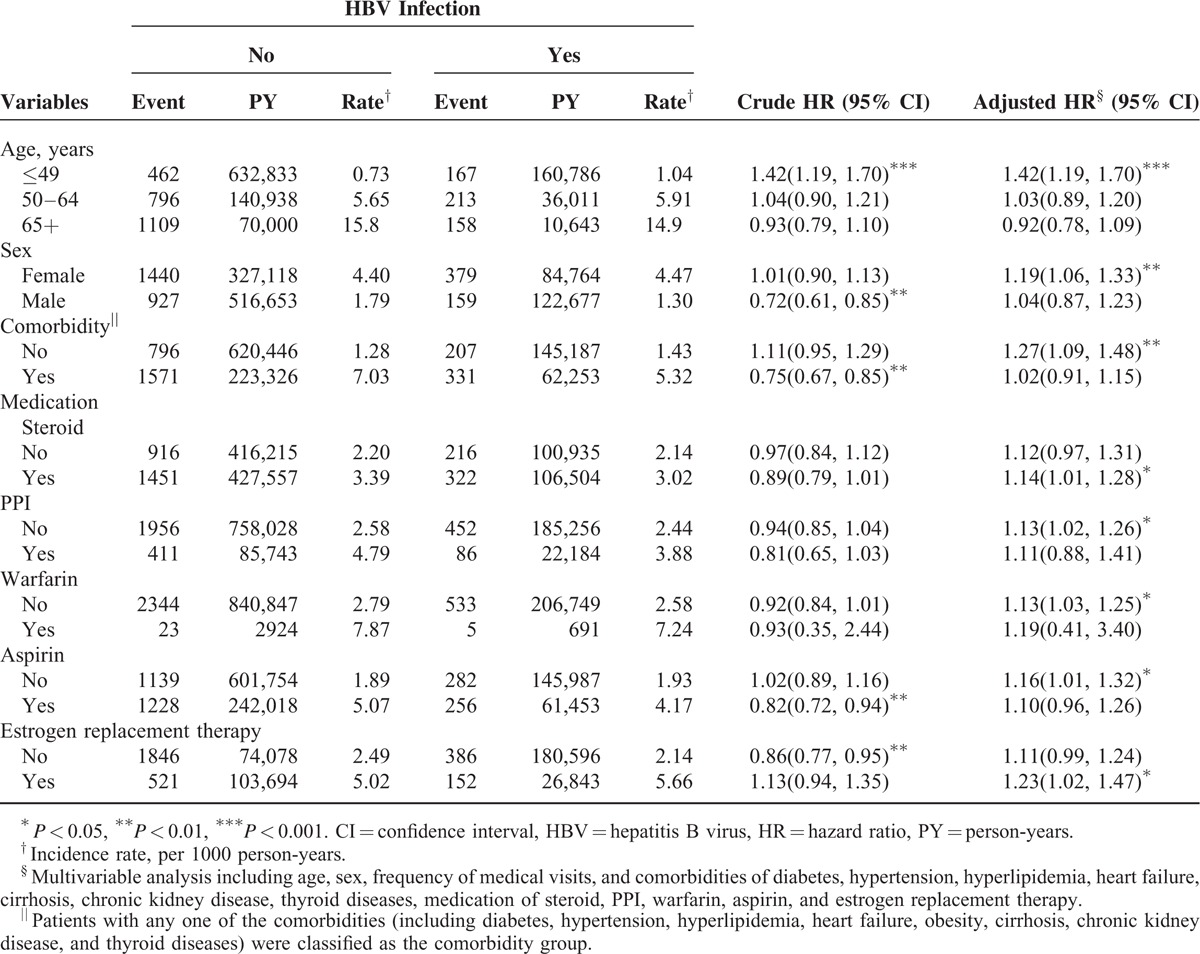
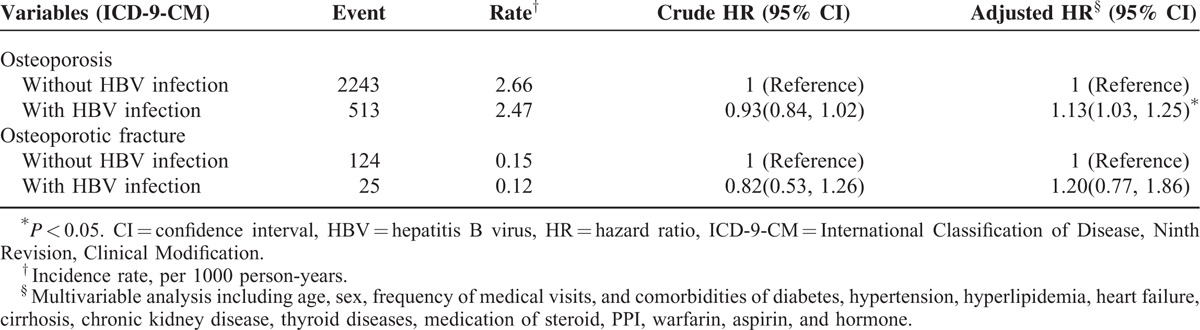
Table 4 shows the comparisons of hazard ratios between patients with and without HBV infection for osteoporosis and osteoporotic fracture. Analyses of the osteoporosis type showed that the patients with HBV infection had a higher risk of osteoporosis than that of the patients without HBV infection (aHR = 1.13; 95% CI = 1.03–1.25). However, the association between HBV infection and osteoporotic fracture was not statistically significant. Furthermore, antiviral therapy did not significantly alleviate the subsequent risk (adjusted HR = 0.46, 95% CI = 0.12–1.86) of developing osteoporosis in the HBV infection patients (supplementary Table 2). Among the HBV infection cohort, the risk of osteoporosis for the patients with combined HBV infection and cirrhosis was not significantly greater than those with HBV infection alone (supplementary Table 3).
TABLE 4.
Comparisons of Hazard Ratios Between Patients With and Without HBV Infection for Osteoporosis (or Osteoporotic Fracture) Outcomes
DISCUSSION
Byrne et al15 have conducted a large-scale population-based study to explore the association between HBV infection and hip fracture in the United States, but did not discuss osteoporosis development. They concluded that chronic HBV infection, even without advanced liver disease, increased the risk of hip fracture for patients of all races except for Asian patients. Black, White, and Hispanic patients with HBV infection and receiving no treatment had a higher rate of hip fracture compared with patients of these racial groups without HBV. Black and White patients with HBV infection and receiving treatment had a higher risk of hip fracture compared with patients of these races, but the association was not statistically significant. However, no association has been observed between hip fracture and Asian patients with HBV infection with or without treatment. Consistent with the literature, our epidemiological study demonstrated that HBV infection without advanced liver cirrhosis increases the risk of osteoporosis and no detrimental effect of HBV on osteoporotic fracture was observed in our Taiwan population.
The reported annual incidence of HBV among adults was about 1.5% to 2.7% in Taiwan.16,17 Many patients without symptoms may not be diagnosed as HBV infection if they have never received such examination, and our reported incidence may be underestimated. However, the annual incidence was about 1.99% in our study, which was within the reported incidence rage based on the literature (supplementary Table 4). Furthermore, consistent with the literature proposing that most Asian patients with HBV infection acquired the infection at birth or during childhood, and that men are predisposed to HBV infection; we conclude the peak age distribution of HBV infection was before age 49 years (71.4%), and then the age distribution of HBV infection decreased progressively with increasing age based on Table 1.18,19 In our study, the mean age in the HBV infection cohort was 42.9 ± 13.8 years and 60.2% of the HBV infection patients were men. Based on LHID2000, the age distribution of HBV infection patients in 2011 was consistent to peak before aged ≤49 years and then decreased with age (supplementary Table 4). It is noted that our study showed that the age-specific relative risk of osteoporosis contributed by HBV infection was greatest for the patients aged ≤49 years. This finding may be explained by that the greater prevalence of other osteoporosis-associated risk factors in the older patients decreased the contribution of HBV infection to the osteoporosis risk.
Compared with comparison patients, the HBV infection patients tended to have more comorbidities, including diabetes, hypertension, hyperlipidemia, heart failure, obesity, cirrhosis, chronic kidney disease, and thyroid diseases. The possible pathogenesis for the HBV infection patients having more comorbidities may include HBV-associated metabolic syndrome and atherosclerosis.20,21 Reports on the relationship between HBV and metabolic syndrome are inconsistent in the literature.22 However, HBV is generally considered to be capable of activating sterol regulatory element-binding protein 1 (SREBP1) and peroxisome proliferator-activated receptor (PPARγ) transcripts, inducing liver steatosis. Thus, HBV is involved in the transcriptional regulation of lipid and glucose, causing metabolic syndrome and insulin resistance.23 Furthermore, HBV was suggested to be strongly associated with atherosclerosis independent of insulin resistance and the components of metabolic syndrome.21
Despite the association between HBV infection and several traditional risk factors for osteoporosis, the risk of osteoporosis remained higher in the HBV infection cohort after we adjusted for age, sex, frequency of medical visits, and comorbidities of diabetes, hypertension, hyperlipidemia, heart failure, cirrhosis, chronic kidney disease, thyroid diseases, medication of steroid, PPI, warfarin, aspirin, and estrogen replacement therapy. The influence of HBV-related mortality on the risk of osteoporosis was insignificant as the reported annual incidences of HBV-related death for HBV infection patents and the controls were similar to be as low as 0.02%.24 Furthermore, our study shows the annual all-cause mortality for the HBV infection cohort and the comparison cohort similarly was 0.45% and 0.40%, respectively (data not shown). BNHI regulated that antiviral therapy could only be administered for HBV infection with active inflammation or cirrhosis, which implied more serious liver injury, and this might explain why antiviral therapy could not alleviate the subsequent risk of developing osteoporosis in our study (supplementary Table 2). We could not assess the performance or immobilization status of HBV infection patients with cirrhosis. However, our study concludes that HBV infection alone significantly increased the risk of osteoporosis, and the risk was not inferior to those with combined HBV infection and cirrhosis (supplementary Table 3). It is noted that the relative risk of osteoporosis was higher in cirrhotic patients without HBV infection than those with HBV infection, which might be explained by the presence of virus-unrelated cirrhosis, such as alcohol-related cirrhosis.
The osteoporosis incidence in our study increased with age and was higher in women than in men. Our study shows that the risk of osteoporosis increased significantly when the women were aged greater than 50 years, and estrogen replacement therapy could alleviate the risk of osteoporosis. In addition, HBV infection increased the risk of osteoporosis among the women with estrogen replacement therapy (supplementary Table 1). These findings were consistent with that postmenopausal osteoporosis and senile osteoporosis are the main etiologies of primary osteoporosis. Estrogen deficiency is the main cause of postmenopausal osteoporosis, which mainly affects trabecular bone and can be arrested by estrogen replacement. Estrogen can inhibit the secretion of cytokines, such as interleukin-1, interleukin-6, and tumor necrosis factors, which stimulate the development of osteoclasts.25 It has been reported that bone loss accelerates from a rate of 0.5% to 1.0% per year before menopause to 2.5% to 5% per year after menopause and the highest rate is in the first 3 to 6 years postmenopause. By contrast, the possible pathogenesis of senile osteoporosis, decreased bone mineral density in both cortical and trabecular bones, is assumed to increase serum parathyroid hormone and bone resorption caused by impaired calcium absorption and increased renal loss.26
Moreover, osteoporosis was associated with aspirin usage and cirrhosis in the present study. The effect of aspirin on the bone mineral density remains undetermined. Some studies suggested that aspirin decreased bone loss via the selective inhibition of cyclo-oxygenase 2 activity; nevertheless, some studies suggested that aspirin could inhibit the chondrocytes differentiation and increase apoptosis in the osteoblasts.27–29
The association between HBV infection and osteoporosis may be due to sharing the same risk factors. However, we can reasonably conclude that the increased risk of osteoporosis in these patients was likely due to the effect of HBV infection, because the possible confounding effects about osteoporosis was already substantially minimized in this study. The osteoporosis risk contributed by HBV infection was decreased with age because the prevalence of osteoporosis-associated risk factors in the older patients was greater. In addition, we already excluded patients with comorbidity at baseline in the subgroup analyses (Table 3) to confirm the validity of our findings. The study results coupled with the subgroup analyses to confirm the possible causal association between HBV infection and osteoporosis, which suggests that HBV infection is a possible risk factor for osteoporosis. However, HBV infection may be lower important than the traditional osteoporosis-associated risk factors (such as aging and female sex). The subsequently decreased effect of HBV infection on osteoporosis with age may reflect that senile osteoporosis is the more important risk factor of osteoporosis than HBV infection. In addition, the decreased effect of HBV infection with age on osteoporotic fracture may be because most Taiwanese with HBV infection were acquired at birth or childhood.15–19 Therefore, most Taiwanese with HBV infection were in the inactive phase of chronic HBV infection and not severe due to a low viral load. In addition, osteoporosis fracture could not be simply determined by bone mineral density but also by other factors (such as movement instability and the risk of falling), which might decrease the effects of HBV infection to osteoporotic fracture.30
The suggested mechanisms for the association between HBV and osteoporosis include HBV-related chronic inflammation and HBV-associated decompensated liver or cirrhosis.15 First, chronic HBV infection can induce the production of inflammatory cytokines, such as tumor necrosis factor-alpha, interleukin-1, and interleukin-6, which increase receptor activator of nuclear factor kappa-B ligand (RANKL) to stimulate osteoclastogenesis and bone resorption.11,12 Moreover, tumor necrosis factor-alpha can inhibit the osteoblast differentiation and promote osteoblast apoptosis. The combined effects of aforementioned inflammatory cytokines are the main reasons of osteoporosis caused by chronic HBV infection, which can result in coupling of decreased bone formation and increased bone resorption to diminish the bone mineral density. Malnutrition, muscle wasting, and low body weight mass will also lead to decreased bone density and sometimes can be observed in patients with chronic HBV infection. Second, the physiological changes associated with decompensated liver or cirrhosis can decrease bone mineral density. The liver production of insulin-like growth factor 1, which promotes the differentiation and proliferation of osteoblasts, will be impaired in the presence of decompensated liver.31 Accelerated bone loss mainly due to increased osteoclast activity caused by hypogonadism with diminished blood levels of estrogen and testosterone is also frequently observed in the presence of decompensated liver.32 In addition, advanced liver disease will impair the hydroxylation of vitamin D3 to D25 in the liver and fat absorption with a resultant diminished uptake of vitamin D to accelerate bone loss and decrease bone formation.33,34 Furthermore, decompensated liver will impair the collagen binding of the bone matrix by reducing the fibronectin production and inhibit the osteoblast function by increasing the production of oncofetal fibronectin, an isoform of fibronectin.35 In addition, metabolic acidosis in end-stage liver disease can also induce calcium efflux from the bone to reduce bone mineral density.36 Finally, increased bilirubin in decompensated liver has been proven to be capable of inhibiting osteoblast proliferation with decreased blood levels of osteocalcin, a marker of bone formation.37
Our study had several strengths. This is the first population-based study to assess the relationship between HBV infection and the risks of osteoporosis and osteoporotic fracture in an Asian population. The use of a nationwide database and the 12-year observation of participants selected from a representative cohort comprising 1,000000 patients covered by the National Health Insurance program benefitted the statistical analyses. The recruited subjects were sampled from approximately 99% of the stable population in Taiwan. Our study also provided a longitudinal study, rather than a cross-sectional approach, to evaluate the association between HBV infection and osteoporosis.
Our study had several limitations. First, we could not clarify the association between bone health, biochemistry, and viremic status. Nevertheless, all patients in our study coded as HBV infection exhibited positive HBV surface antigen, and our results supported the association between HBV infection and osteoporosis. Second, the osteoporosis-related lifestyle factors could not be fully ascertained in this study. Nevertheless, similar to the analyses in our published papers, we have controlled for the potential osteoporosis-associated comorbidities in our study.38,39 The confounding factors in this study were determined according to Japanese 2011 guidelines for prevention and treatment of osteoporosis.14 In addition to primary osteoporosis relating to aging and sex, the guidelines subclassify the causes of secondary osteoporosis into secondary to other diseases, such as hyperparathyroidism and rheumatoid arthritis; secondary to lifestyle-related diseases, such as diabetes, hypertension, hyperlipidemia, and chronic kidney disease; and secondary to treatment-associated osteoporosis, such as steroid and estrogen usage. Accordingly, we have adjusted comorbidities of diabetes, hypertension, hyperlipidemia, heart failure, stroke, obesity, cirrhosis, chronic kidney disease and thyroid diseases, and medications of steroid, PPI, warfarin, aspirin, and estrogen replacement therapy. Third, we could not validate the diagnosis of osteoporosis or osteoporotic fracture based on radiography or densitometry. However, to enhance the accuracy of diagnosis, we included only patients who had received medical care for osteoporosis or osteoporotic fracture more than 3 times. Furthermore, medical experts at the BNHI conduct regular audits to ensure the accuracy of diagnosis codes in insurance claims in Taiwan. The National Health Insurance program in Taiwan began since 1995, operated by the government which is the only buyer. Medical specialists have to survey all insurance claims by the peer review. Therefore, the related diagnoses of osteoporosis and osteoporotic fracture were based on the ICD-9 codes and checked by related physicians according to the standard clinical criteria (such as X-ray or bone densitometry). These wrong diagnoses or codes should result in being punished to pay a lot of penalty for these doctors or hospitals. Furthermore, the diagnoses and codes for osteoporosis and osteoporotic fracture used in our study should be reliable. Finally, many patients without symptoms may not be diagnosed as HBV infection if they have never received such examination. However, this misclassification would overestimate the risk of osteoporosis in the comparison cohort rather than in the HBV infection cohort and, therefore, the relative risk of osteoporosis contributed by HBV infection actually should be greater than that in our study. Moreover, the temporal association between HBV infection and osteoporosis or osteoporotic fracture development could not be ascertained in our study. However, it is generally believed that most Asian patients with HBV infection acquire the infection at birth or during childhood.15–19 In addition, our study shows the risk of osteoporosis increased progressively with increasing duration of follow-up, rather than with being limited to the immediate days, after a diagnosis of HBV infection (Fig. 2).
In conclusion, this nationwide population-based cohort study reveals that chronic HBV infection increases the risk of developing subsequent osteoporosis. However, HBV infection may be less influential than other risk factors and the risk of osteoporotic fracture was not associated with HBV infection.
Acknowledgments
The authors thank Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan for the support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, BNHI = Bureau of National Health Insurance, CI = confidence interval, HBV = hepatitis B virus, ICD-9-CM = International Classification of Disease Ninth Revision Clinical Modification, LHID2000 = Longitudinal Health Insurance Database 2000, PPI = proton pump inhibitor, SD = standard deviation.
Conception and design: CHC, C-HK; Administrative support: C-HK; Collection and assembly of data, Data analysis and interpretation, Manuscript writing, and Final approval of manuscript: All authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Consensus Development Conference: Prophylaxis and treatment of osteoporosis. Osteoporosis Int 1991; 1:114–117. [PubMed] [Google Scholar]
- 2.Kinsella K, Wan H. International Population Reports. P95/09-1. An Aging World: 2008. Washington, DC: US Census Bureau, US Government Printing Office; 2009. [Google Scholar]
- 3.Cooper C, Cole ZA, Holroyd CR, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 2011; 22:1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suman VB, Khalid P, Pratik KC. Risk factors associated with osteoporosis. Int J Health Sci Res 2013; 3:85–91. [Google Scholar]
- 5.Kanis JA, Melton LJ, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res 1994; 9:117–1141. [DOI] [PubMed] [Google Scholar]
- 6.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005; 16:581–589. [DOI] [PubMed] [Google Scholar]
- 7.Custer B, Sullivan SD, Hazlet TK, et al. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004; 38:S158–S168. [DOI] [PubMed] [Google Scholar]
- 8.Chen YL, Yang JY, Lin SF, et al. Slow decline of hepatitis B burden in general population: results from a population-based survey and longitudinal follow-up study in Taiwan. J Hepatol 2005; 63:354–363. [DOI] [PubMed] [Google Scholar]
- 9.Schiefke I, Fach A, Wiedmann M, et al. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol 2005; 11:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill US, Zissimopoulos A, Al-Shamma S, et al. Assessment of bone mineral density in Tenofovir-treated patients with chronic hepatitis B: can the fracture assessment tool identify those at greatest risk? JID 2015; 211:347–382. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem 2002; 277:2695–2701. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Calvin JL, Gallego-Rojo F, Fernandez-Perez R, et al. Osteoporosis, mineral metabolism, and serum soluble tumor necrosis factor receptor p55 in viral cirrhosis. J Clin Endocinol Metab 2004; 89:4325–4330. [DOI] [PubMed] [Google Scholar]
- 13.Database NHIR. Taiwan, http://nhird.nhri.org.tw/en/index.html.(cited in 2015/11/26). [Google Scholar]
- 14.Orimo H, Nakamura T, Hosoi T, et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis-executive summary. Arch Osteoporos 2012; 7:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne DD, Newcomb CW, Carbonari DM, et al. Risk of hip fracture associated with untreated and treated chronic hepatitis B virus infection. J Hepatol 2014; 61:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chien YC, Jan CF, Kuo HS, et al. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev 2006; 28:126–135. [DOI] [PubMed] [Google Scholar]
- 17.Beasley RP, Hwang LY, Lin CC, et al. Incidence of hepatitis among students at a university in Taiwan. Am J Epidemiol 1983; 117:213–222. [DOI] [PubMed] [Google Scholar]
- 18.Su WJ, Liu CC, Liu DP, et al. Effect of age on the incidence of acute hepatitis B after 25 years of a universal newborn hepatitis B immunization program in Taiwan. JID 2012; 205:757–762. [DOI] [PubMed] [Google Scholar]
- 19.Locarnini S, Hatzakis A, Chen DS, et al. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J Hepatol 2015; 62:S76–S86. [DOI] [PubMed] [Google Scholar]
- 20.Jinjuvadia R, Liangpunsakul S. Association between metabolic syndrome and its individual components with viral hepatitis B. Am J Med Sci 2014; 347:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Targher G, Bertolini L, Padovani R, et al. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol 2007; 46:1126–1132. [DOI] [PubMed] [Google Scholar]
- 22.Jan CF, Chen CJ, Chiu YH, et al. A population-based study investigating the association between metabolic syndrome and hepatitis B/C infection (Keelung community-based integrated screening study no. 10). Int J Obes Relat Metab Disord 2006; 30:794–799. [DOI] [PubMed] [Google Scholar]
- 23.Kim KH, Shin HJ, Kim K, et al. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SERBP1 and PPARgamma. Gastroenterology 2007; 132:1955–1967. [DOI] [PubMed] [Google Scholar]
- 24.Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol 2011; 26:628–638. [DOI] [PubMed] [Google Scholar]
- 25.Deal CL. Osteoporosis: prevention, diagnosis, and management. Am J Med 1997; 102:35S–39S. [DOI] [PubMed] [Google Scholar]
- 26.Riggs BL, Melton LT, III, Evans TG, et al. Involutional osteoporosis. In. Oxford Textbook of Geriatric Medicine. London: Oxford University Press,; 1992. [Google Scholar]
- 27.Carbone LD, Tylavsky FA, Cauley JA, et al. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res 2003; 18:1795–1802. [DOI] [PubMed] [Google Scholar]
- 28.Derakhshanfar A, Kheirandish R, Alidadi S, et al. Study of long effects of administration of aspirin (acetylsalicyclic acid) on bone in broiler chickens. Comp Clin Pathol 2013; 22:1201–1204. [Google Scholar]
- 29.De Luna-Bertos E, Ramos-Torrecillas J, Manzano-Moreno FJ, et al. Effects on growth of human osteoblast-like cells of three nonsteroidal anti-inflammatory drugs metamizole, dexketoprofen, and ketorolac. Biol Res Nurs 2015; 17:62–67. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Larramona G, Lucendo AJ, Gonzalez-Castillo S, et al. Hepatic osteodystrophy: an important matter for consideration in chronic liver disease. World J Gastroenterol 2011; 3:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouillard S, Lane NE. Hepatic osteodystropthy. Hepatology 2001; 33:301–307. [DOI] [PubMed] [Google Scholar]
- 32.Pignata S, Daniele B, Galati MG, et al. Oestradiol and testosterone blood levels in patients with viral cirrhosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol 1997; 9:283–286. [DOI] [PubMed] [Google Scholar]
- 33.Van Leeuwen JP, van Driel M, van den Bemd GJ, et al. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit Rev Eukaryot Gene expr 2001; 11:199–226. [PubMed] [Google Scholar]
- 34.Lindor KD, Janes CH, Crippen JS, et al. Bone disease in primary biliary cirrhosis: does ursodeoxycholic acid acid make a difference? Hepatology 1995; 21:389–392. [PubMed] [Google Scholar]
- 35.Nakchbandi IA. Osteoporosis and fractures in liver disease: relevance, pathogenesis and therapeutic implications. World J Gastroenterol 2014; 20:9427–9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krieger NS, Frick KK, Bushinsky DA. Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens 2013; 14:311–317. [DOI] [PubMed] [Google Scholar]
- 37.Janes CH, Dickson ER, Okazaki R, et al. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest 1995; 95:2581–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen SJ, Lin CS, Lin CL, et al. Osteoporosis is associated with high risk for coronary artery disease: a population-based cohort study. Medicine 2015; 94:e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang KH, Chang MY, Muo CH, et al. Exposure to air pollution increases the risk of osteoporosis: a nationwide longitudinal study. Medicine 2015; 94:e733. [DOI] [PMC free article] [PubMed] [Google Scholar]


