Abstract
To investigate the association between iodinated contrast medium (ICM) exposure during computed tomography (CT) and the subsequent development of thyroid disorders in patients without known thyroid disease in Taiwan, an iodine-sufficient area.
We conducted a population-based cohort study by using data from 1996 to 2012 in the Taiwan National Health Insurance Research Database. A total of 33,426 patients who underwent ICM-enhanced CT were included as the study cohort. To avoid selection bias, we used propensity score and matched for the index year (defined as the year of first ICM exposure) to retrieve 33,426 patients as the comparison cohort. No patients in the 2 cohorts had any known thyroid disease before the index year. Patients with a history of amiodarone treatment or coronary angiography and those with <1 year follow-up were excluded. Participants were followed until a new diagnosis of thyroid disorder or December 31, 2011. Hazard ratios (HRs) with 95% confidence interval (95% CI) were calculated using the Cox proportional hazards regression.
An association was identified between ICM exposure and the subsequent development of thyroid disorders after adjustment for potential confounders (adjusted HR = 1.17; 95% CI: 1.07–1.29; P = 0.001). Male patients and patients’ ages ≥40 years in the ICM-exposure cohort had a higher adjusted HR for developing thyroid disorders than did those in the non-ICM-exposure cohort. Hypothyroidism had the highest adjusted HR (HR = 1.37; 95% CI: 1.06–1.78; P < 0.05) among all thyroid disorders and had a higher risk of development or detection during >0.5-year post-ICM exposure compared with that during ≤0.5-year post-ICM exposure (HR = 1.26; 95% CI: 1.01–1.58; P < 0.05). Repeated ICM exposure increased the risk of thyroid disorders in patients who accepted >1 time of ICM per year on average compared with those who accepted ≤1 time per year on average (adjusted HR = 3.04; 95% CI: 2.47–3.73; P < 0.001).
This study identified ICM exposure during CT as a risk factor for the subsequent development of thyroid disorders in patients without known thyroid disease, particularly in patients with repeated exposure.
INTRODUCTION
Iodine is an essential substrate for thyroid hormone production. The recommended daily intake of iodine is 150 μg.1 Excessive ingestion of or exposure to iodine is well-tolerated by the human body because of its intrinsic autoregulatory mechanisms. However, susceptible individuals, such as patients with known thyroid diseases, may fail to adapt to the iodine overload, resulting in hypo- or hyperthyroidism.2,3 The major sources of excess iodine in clinical practice are iodinated contrast medium (ICM), amiodarone, and povidone-iodine. ICM is widely used in imaging procedures, including cardiac catheterization and computed tomography (CT). A usual ICM dose for CT contains approximately 13,500 μg of free iodide and 15 to 60 g of bound iodine, several thousand times higher than the recommended daily intake dose.4,5 Bolus loading of these iodine-rich substances may induce transient or prolonged thyroid dysfunction even in patients without known thyroid disease.2 Although the use of modern diagnostic modalities with contrast has increased substantially, large nationwide studies with long-term follow-up to investigate the risk of thyroid disorder development after ICM exposure are lacking.
Hyper- and hypothyroidism increase the risk of cardiovascular diseases and associated mortality.6–13 Although the incidence rate (IR) of ICM exposure-related thyroid disorder is low, ICM is frequently used in modern diagnostic modalities and thus its occurrence becomes an important issue.
Taiwan is an iodine-sufficient area.14 For the present study, we aimed to determine the association between ICM exposure during CT and the subsequent development of thyroid disorders in patients without known thyroid disease by using the Taiwan National Health Insurance Research Database (NHIRD).
METHODS
Data Sources
We conducted a nationwide population-based cohort study by using data from the NHIRD. The National Health Insurance program in Taiwan was launched on March 1, 1995, by the National Health Insurance Administration (NHIA), and provided coverage to more than 23.03 million residents in Taiwan (approximately 99.2% of the population) at the time. The NHIA released identification-encrypted data to the National Health Research Institute (NHRI) to establish the NHIRD. The Longitudinal Health Insurance Database 2000 (LHID2000), used in this study, contains medical information of 1 million beneficiaries randomly sampled from the registry of all beneficiaries in 2000. Claims data in the LHID2000 were extended from January 1, 1996 to December 31, 2011. Age- and sex-related distributions in the original claims data and the sampled data do not differ significantly. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used for diagnoses.
The NHRI scrambles patient identification and replaces it with surrogate numbers to ensure privacy. Furthermore, data confidentiality is maintained in accordance with NHIA and NHRI data regulations. Because the NHIRD contains deidentified secondary data for research, our study was exempted from the informed consent of participants. This study was approved by the Institutional Review Board of China Medical University (CMUH104-REC2-115).
Study Population and Outcomes
Figure 1 shows participants selection in the study and comparison cohorts. Patients with known thyroid diseases (ICD-9-CM 240–246) before the first CT (with or without ICM-enhanced) were excluded. In addition, patients who underwent amiodarone treatment or coronary angiography during the study period (1996–2012) were excluded. All the study participants accepted a follow-up of at least 1 year. A total of 33,426 patients were selected from the NHIRD as the study cohort (ICM-exposure). The first instance of ICM exposure during CT was defined as the index time, and the index year was defined as the calendar year of the index time. In addition, 33,426 patients (comparison cohort, non-ICM-exposure) were selected from the database after matching with the study cohort for age, sex, the index year, and propensity score. Propensity score matching reduced the selection bias because it could bundle many confounding covariates that might be present in an observation study. During the study period, more than 90% of the contrast medium was ionic contrast medium. The incidence of thyroid disorders (ICD-9-CM 240–242 and 244–246, excluded 243 [congenital hypothyroidism]) was evaluated until December 31, 2011. Patients were defined as having thyroid disorders if they had at least 2 outpatient service claims with a diagnosis of thyroid disorder or if they had a single hospitalization in which thyroid disorder was found in 1 of the 5 spaces used to report their diagnosis when hospitalized. To improve the accuracy of the diagnosis of hyper- and hypothyroidism, patient prescriptions, such as methimazole, carbimazole, and propylthiouracil for hyperthyroidism and eltroxin for hypothyroidism were matched with the ICD-CM codes in the claims data.
FIGURE 1.
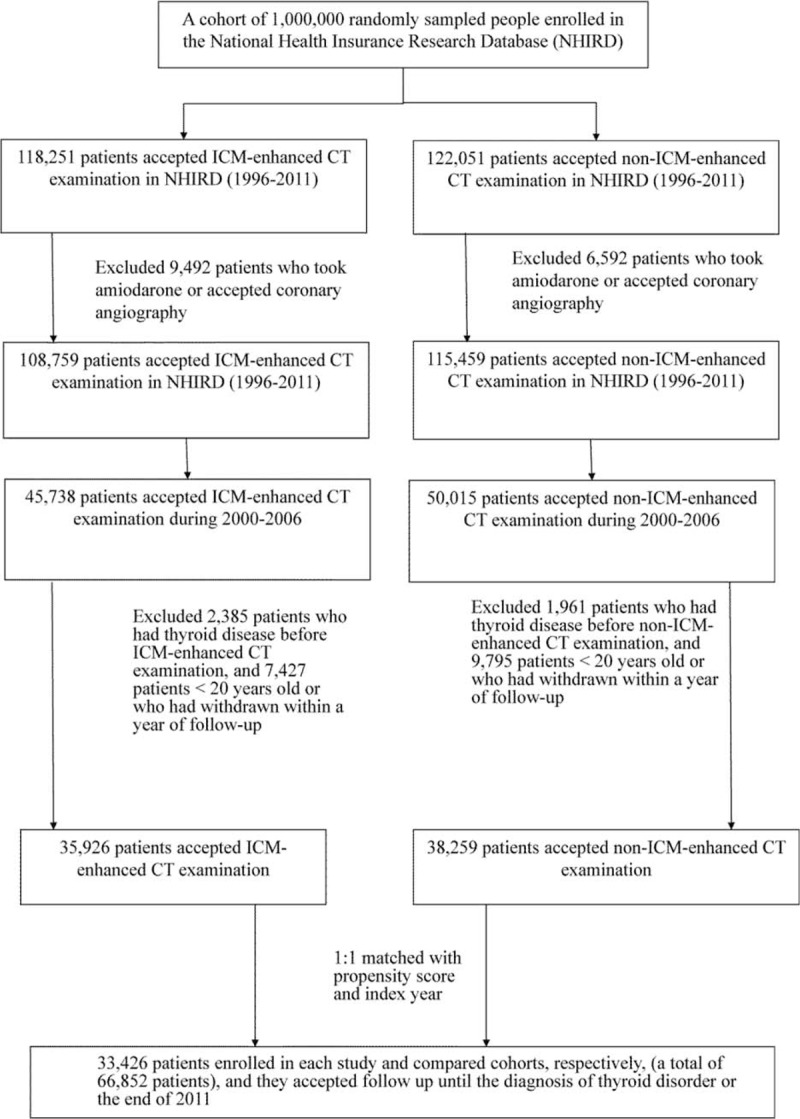
Participant selection for the study and comparison cohorts.
Potential Confounders
We systematically identified the potential confounders for thyroid disorders by referring to the ICD-9-CM codes in the claims data: hypertension (ICD-9-CM 401–405), hyperlipidemia (ICD-9-CM 272), diabetes mellitus (DM) (ICD-9-CM 250, 357.2, 362.01, 362.02, 366.41), ischemic heart disease (IHD) (ICD-9-CM 411–414), peripheral arterial occlusive disease (PAOD) (ICD-9-CM 440–444), arrhythmia (ICD-9-CM 427), stroke (ICD-9-CM 430–438), chronic kidney disease (CKD) (ICD-9-CM 581–588, 403–404, 285.21, 250.4), and asthma (ICD-9-CM 493). Diagnoses given ahead of or in concurrence with the first CT (with or without ICM-enhanced) were considered underlying comorbidities.
Statistical Analyses
Differences in demographic characteristics and comorbidities between the study and comparison cohorts were examined using the Chi-squared and 2-sample t tests. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated for each variable using Cox proportional hazards regression. Difference in the incidence of thyroid disorders between the study and comparison cohorts was estimated using Kaplan–Meier curves by performing the log-rank test. Statistical analyses were performed using SAS 9.4 statistical package (SAS Institute Inc., Cary, NC), and the significance level was set at 0.05.
RESULTS
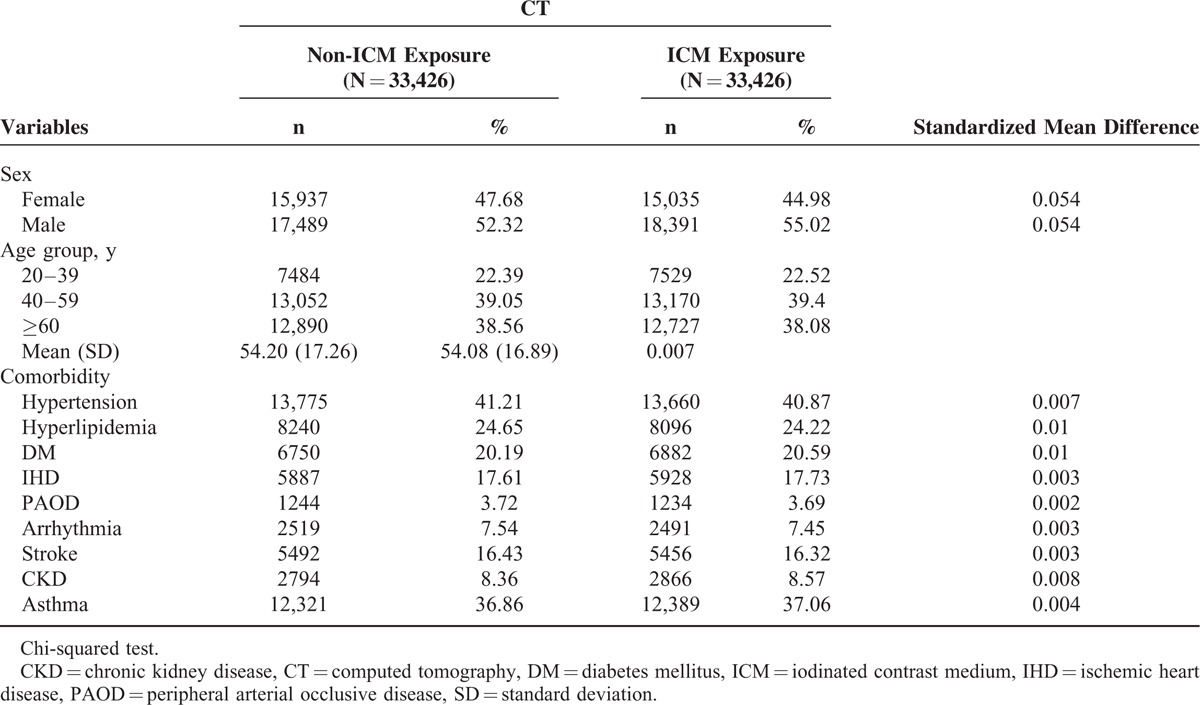
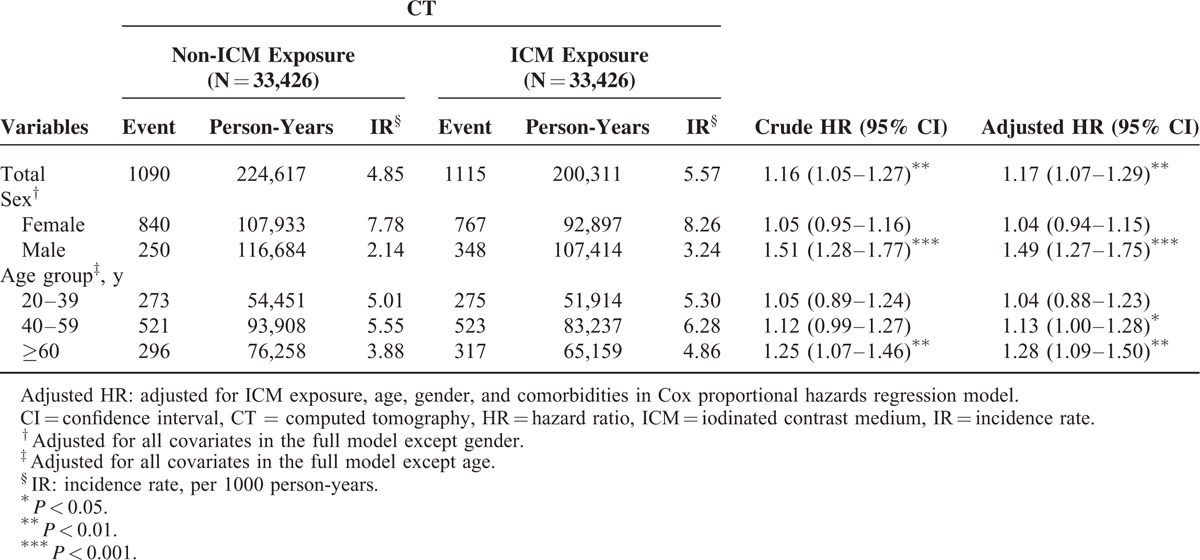
A total of 33,426 patients and an equal number of comparable patients were included in the ICM-exposure and non-ICM-exposure cohorts, respectively. The mean age of the ICM-exposure and non-ICM-exposure cohorts was 54.20 ± 17.26 and 54.08 ± 16.89 years (standardized mean difference 0.007), respectively. The male to female ratio in the ICM-exposure and non-ICM-exposure cohorts was 1.09 and 1.22, respectively. Table 1 summarized the demographic characteristics and comorbidities between the study and comparison cohorts.
TABLE 1.
Demographic Characteristics and Comorbidities
The median (range) follow-up time in the ICM-exposure and non-ICM-exposure cohorts were 5.99 (3.01–6.18) and 6.72 (2.71–6.80) years, respectively. The median (range) duration between the index time and diagnosis of thyroid disorders were 3.45 (2.53–3.10) and 3.58 (2.57–3.09) years in the ICM-exposure and non-ICM-exposure cohorts, respectively. During the follow-up period, the IRs of thyroid disorders in the ICM-exposure and non-ICM-exposure cohorts were 5.57 and 4.85 per 1000 person-years, respectively.
The covariates including hypertension, hyperlipidemia, DM, IHD, PAOD, arrhythmia, stroke, CKD, and asthma did not increase the HR of developing thyroid disorders in Cox regression analyses, except ICM exposure and female gender (HR = 1.16; 95% CI: 1.05–1.27 and HR = 1.66; 95% CI: 1.07–2.57, respectively). The adjusted HRs of ICM exposure and female gender were highly significant after adjustment for hypertension, hyperlipidemia, DM, IHD, PAOD, arrhythmia, stroke, CKD, and asthma (adjusted HR = 1.17; 95% CI: 1.07–1.29 and adjusted HR = 1.96; 95% CI; 1.19–3.22, respectively; Table 2). The Kaplan–Meier analysis showed a higher IR for thyroid disorders in the ICM-exposure cohort compared with the non-ICM-exposure cohort (log-rank test, P < 0.001; Figure 2).
TABLE 2.
Hazard Ratio of Thyroid Disorders Associated With ICM Exposure
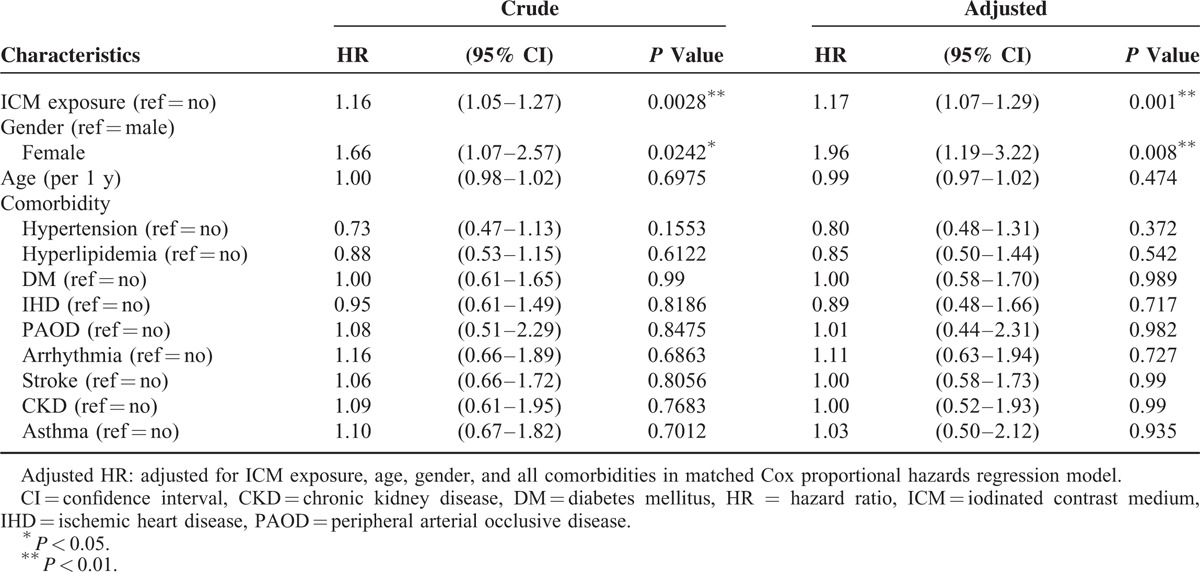
FIGURE 2.
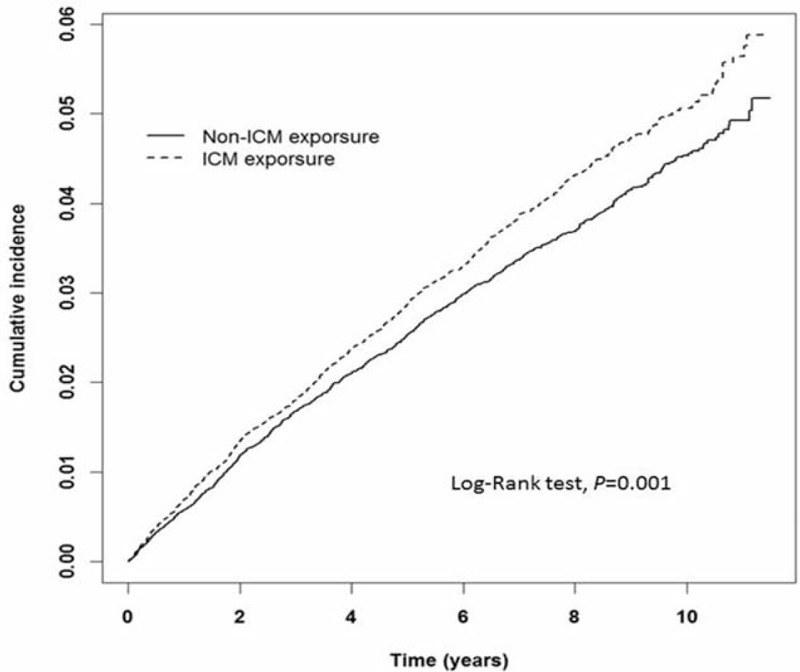
Kaplan–Meier analysis of the cumulative incidence of thyroid disorders for the ICM-exposure and non-ICM-exposure cohorts. (X-axis: follow-up time in years; Y-axis: cumulative incidence per 1000 person-years.) ICM = iodinated contrast medium.
In the subgroup analysis for gender, we found that ICM exposure in male patients had a higher adjusted HR of thyroid disorders than non-ICM exposure (adjusted HR = 1.49; 95% CI: 1.27–1.75). With respect to age, patients’ ages 40 to 59 years and ≥60 years had higher adjusted HR of thyroid disorders after ICM exposure (adjusted HR = 1.13; 95% CI: 1.00–1.28 and adjusted HR = 1.28; 95% CI: 1.09–1.50, respectively; Table 3).
TABLE 3.
Subgroup Analysis of Incidence Rate and Hazard Ratio of Thyroid Disorders After ICM Exposure
Furthermore, when stratified by thyroid disorders, thyrotoxicosis showed the highest IR (IR = 1.81 and 1.65 per 1000 person-years in the ICM-exposure and non-ICM-exposure cohorts, respectively). However, only hypothyroidism had increased adjusted HR that was significant (adjusted HR = 1.37; 95% CI: 1.06–1.78; P < 0.05; Figure 3). To further analyze hypothyroidism development, the follow-up period was dichotomized into ≤0.5-year and >0.5-year. The IR for developing hypothyroidism in the ≤0.5-year and >0.5-year periods were 0.99 and 0.8 per 1000 person-years, respectively. Developing or detecting hypothyroidism had an increased HR in >0.5-year post-ICM exposure (HR = 1.26; 95% CI: 1.01–1.58; P < 0.05).
FIGURE 3.

Adjusted hazard ratios of different thyroid disorders after iodinated contrast medium exposure.
To analyze the cumulative effect of ICM exposure, ICM-exposure patients were dichotomized into ≤1 time per year and >1 time per year according to the average times of ICM exposure per year. The IRs of thyroid disorders in the ≤1 and >1 time per year groups were 5.2 and 15.1 per 1000 person-years, respectively. The adjusted HR of ICM exposure >1 time per year was 3.04 (95% CI: 2.47–3.73). The Kaplan–Meier analysis revealed a higher IR for developing thyroid disorders in the >1 time per year group compared with the ≤1 time per year group (log-rank test, P < 0.001; Figure 4).
FIGURE 4.
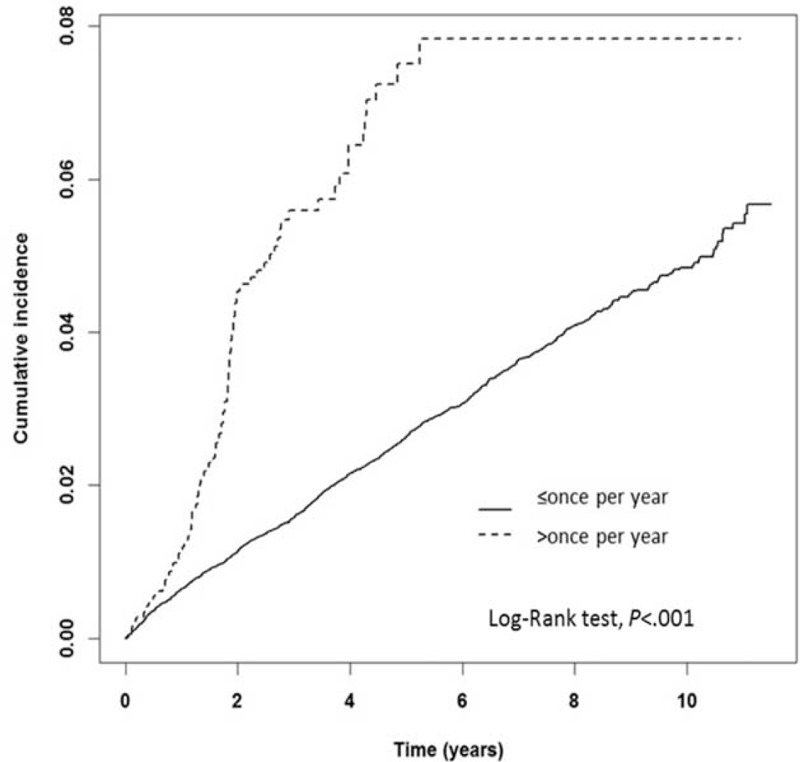
Kaplan–Meier analysis of the cumulative incidence of thyroid disorders for the 2 groups stratified by different times of iodinated contrast medium exposure per year on average. (X-axis: follow-up time in years; Y-axis: cumulative incidence per 1000 person-years).
DISCUSSION
To the best of our knowledge, this is the first nationwide cohort study on the association between ICM exposure and the subsequent development of thyroid disorders in patients without known thyroid disease in an iodine-sufficient area. A 1.17-fold increased risk (adjusted HR = 1.17; 95% CI: 1.07–1.29) of developing thyroid disorders was observed among the patients with ICM exposure during CT compared with those without ICM exposure. Among all thyroid disorders, hypothyroidism had the highest adjusted HR (adjusted HR = 1.37; 95% CI: 1.06–1.78), particularly after 0.5-year post-ICM exposure.
The Contrast Media Safety Committee of the European Society of Urogenital Radiology recommends that high-risk patients such as those with known hyperthyroidism should be monitored for thyroid function after ICM exposure during radiologic examinations.5 Indeed, it appears that this precaution should also include patients without known thyroid disease, particularly in patients with repeated ICM exposure and the male patients’ ages ≥40 years.
The acute Wolff–Chaikoff effect, a transient reduction in thyroid hormone synthesis after exposure to high amounts of iodine, was first described by Drs Jan Wolff and Israel Lyon Chaikoff in 1948.15 The decrease in thyroid hormone production is usually transient and resumes after adaption.16 This adaption is associated with a decreased expression of the sodium-iodine symporter of thyroid follicular cells.17 Several studies have proposed that the subsequent development of hypothyroidism after ICM exposure was induced by the failure of adaption or escape from the Wolff–Chaikoff effect. Susceptible patients include specific groups (eg, fetus, neonates, and elderly patients) and patients with underlying thyroid diseases (eg, thyroiditis, Graves disease post-131I, thyroidectomy, or anti-thyroid drug therapy).2,18–20 Although ICM-induced hypothyroidism is usually transient and recovers within 2 to 3 weeks after withdrawal of excess iodine, some patients develop permanent hypothyroidism and require treatment.20 Rhee et al2 demonstrated that ICM exposure was associated with the subsequent development of incident overt hypothyroidism in individuals without known thyroid disease. Although clinical laboratory data were lacking in the present study, the ICD code was matched with the drug prescriptions to ensure reliability of diagnosis. Moreover, patients with possible confounders, such as amiodarone use and acceptance of coronary angiography, were excluded, and follow-up was prolonged. A result similar to Rhee et al that hypothyroidism had the highest adjusted HR, was observed; however, a higher HR was observed during >0.5-year post-ICM exposure compared with ≤0.5-year post-ICM exposure. The delayed development or detection of hypothyroidism post-ICM exposure might be explained by the ambiguous presentation of hypothyroidism, the diagnosis of which could be easily missed. Because the thyroid function was routinely checked in the previous study, the patients in our study were examined only for the development of clinic symptoms or in concurrence with other major associated diseases. Hypothyroidism was associated with all types of mortality, particularly cardiovascular accident, if undetected or untreated.6,7,9,11–13 On the basis of our finding, we suggest follow-up for patients with a substantially increased risk of cardiovascular accident if ICM-related thyroid disorders were to develop.
The Jod–Basedow phenomenon (iodine-induced hyperthyroidism) was first described in the early 1800s. The mechanism of this phenomenon is used to explain ICM-associated thyrotoxicosis.2 The risk factors for iodine-induced hyperthyroidism include nontoxic or diffuse nodular goiter with thyroid autonomy, old age, and residency in an iodine-deficient area.2,18,21 Several studies have reported the rare condition of iodine-induced thyrotoxicosis in patients without underlying thyroid disease.2,22–26 The present study was conducted in Taiwan, an iodine-sufficient area. In addition, patients with known thyroid diseases were excluded. However, ICM increased the adjusted risk of hyperthyroidism, although nonsignificantly. In conclusion, ICM exposure should not only be considered as a possible cause of recurrent hyperthyroidism but also be a cause of concern in patients without known hyperthyroidism.5
Furthermore, the cumulative effect of ICM was observed in our study. Repeated ICM exposure increased the risk of thyroid disorders in patients exposed more than once per year on average compared with those exposed at lower rates (adjusted HR = 3.04; 95% CI: 2.47–3.73). When repeated ICM-enhanced image-based examination is necessary, an alternative image study without ICM, such as magnetic resonance imaging, should be considered.
The major strength of this study was the use of a large sample size from a nationwide database. The most challenging aspect of this study was the elimination of selection bias for ICM use in CT. To overcome this bias, propensity score matching was employed to select the comparison cohort: the 2 cohorts had undergone CTs with the only difference being the presence and absence of ICM. Third, we excluded patients who underwent amiodarone treatment or coronary angiography during the study period. Other potential confounders, such as age, gender, and underlying comorbidities, were adjusted for through Cox proportional regression analysis. Fourth, the cumulative effect of repeated ICM exposure was evaluated, and follow-up time was prolonged.
There were limitations in this study. First and also the most important limitation was the lacking in clinical information about the previous thyroid function, anti-thyroid autoantibody status, TSH levels, and urinary iodine levels in the studied participants before and after ICM exposure. And these were all known important risk factors for subsequent development of thyroid disorders, especially hypothyroidism. Second, this study did not eliminate the possible impact from the radiotherapy and it was also an important risk factor for the development of overt hypothyroidism. Third, our database did not include information on diet, lifestyle, body mass index, and smoking status, which could be risk factors for thyroid disorders.
CONCLUSION
An association was identified between ICM exposure and the subsequent development of thyroid disorders, particularly hypothyroidism, in individuals without known thyroid disease. We recommend that monitoring of patients after ICM exposure should include not only those with known thyroid disorder but also those with normal thyroid condition; particularly close monitoring is warranted for patients with a markedly increased risk of mortality if thyroid disorders were to develop. When repeated ICM-enhanced image examination is necessary, an alternative image study without ICM use should be considered.
Footnotes
Abbreviations: CI = confidence interval, CKD = chronic kidney disease, CT = computed tomography, DM = diabetes mellitus, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, ICM = iodinated contrast medium, IHD = ischemic heart disease, IR = incidence rate, LHID2000 = Longitudinal Health Insurance Database 2000, NHIA = National Health Insurance Administration, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institute, PAOD = peripheral arterial occlusive disease.
These authors’ individual contributions were as follows. Conception and design: M-SH, C-SC, S-YL, and W-CC; data analysis and interpretation: J-HC and S-LC; manuscript writing: M-SH; and final approval and critical revision: M-LS and S-YH.
We confirm that we have read the Journal's position on issues involved with unethical publication and affirm that this study is consistent with those guidelines.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital; Academia Sinica Taiwan Biobank; Stroke Biosignature Project (BM103010096); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kuriti M, Pearce EN, Braverman LE, He X, Leung AM. Iodine content of U.S. weight-loss food. Endocr Pract 2014; 20:232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee CM, Bhan I, Alexander EK, et al. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med 2012; 172:153–159. [DOI] [PubMed] [Google Scholar]
- 3.Martin FI, Tress BW, Colman PG, et al. Iodine-induced hyperthyroidism due to nonionic contrast radiography in the elderly. Am J Med 1993; 95:78–82. [DOI] [PubMed] [Google Scholar]
- 4.Katzberg RW, Haller C. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int Suppl 2006; 69:S3–S7. [DOI] [PubMed] [Google Scholar]
- 5.van der Molen AJ, Thomsen HS, Morcos SK. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol 2004; 14:902–907. [DOI] [PubMed] [Google Scholar]
- 6.Sgarbi JA, Matsumura LK, Kasamatsu TS, et al. Subclinical thyroid dysfunctions are independent risk factors for mortality in a 7.5-year follow-up: the Japanese-Brazilian thyroid study. Eur J Endocrinol 2010; 162:569–577. [DOI] [PubMed] [Google Scholar]
- 7.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304:1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation 2010; 122:385–393. [DOI] [PubMed] [Google Scholar]
- 9.Haentjens P, Van Meerhaeghe A, Poppe K, et al. Subclinical thyroid dysfunction and mortality: an estimate of relative and absolute excess all-cause mortality based on time-to-event data from cohort studies. Eur J Endocrinol 2008; 159:329–341. [DOI] [PubMed] [Google Scholar]
- 10.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev 2005; 26:704–728. [DOI] [PubMed] [Google Scholar]
- 11.Imaizumi M, Akahoshi M, Ichimaru S, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab 2004; 89:3365–3370. [DOI] [PubMed] [Google Scholar]
- 12.Parle JV, Maisonneuve P, Sheppard MC, et al. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358:861–865. [DOI] [PubMed] [Google Scholar]
- 13.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001; 344:501–509. [DOI] [PubMed] [Google Scholar]
- 14.Tang KT, Wang FF, Pan WH, et al. Iodine status of adults in Taiwan 2005–2008. 5 years after the cessation of mandatory salt iodization. J Formos Med Assoc 2015; pii: S0929-6646(15)00238-7. doi: 10.1016/j.jfma.2015.06.014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem 1948; 174:555–564. [PubMed] [Google Scholar]
- 16.Pramyothin P, Leung AM, Pearce EN, et al. Clinical problem-solving. A hidden solution. N Engl J Med 2011; 365:2123–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng PH, Cardona GR, Fang SL, et al. Escape from the acute Wolff–Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology 1999; 140:3404–3410. [DOI] [PubMed] [Google Scholar]
- 18.Leung AM, Braverman LE. Iodine-induced thyroid dysfunction. Curr Opin Endocrinol Diabetes Obes 2012; 19:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markou K, Georgopoulos N, Kyriazopoulou V, et al. Iodine-induced hypothyroidism. Thyroid 2001; 11:501–510. [DOI] [PubMed] [Google Scholar]
- 20.Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol 2014; 10:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann MB, Andersson M. Update on iodine status worldwide. Curr Opin Endocrinol Diabetes Obes 2012; 19:382–387. [DOI] [PubMed] [Google Scholar]
- 22.Burgi H. Iodine excess. Best Pract Res Clin Endocrinol Metab 2010; 24:107–115. [DOI] [PubMed] [Google Scholar]
- 23.Burman KD, Wartofsky L. Iodine effects on the thyroid gland: biochemical and clinical aspects. Rev Endocr Metab Disord 2000; 1:19–25. [DOI] [PubMed] [Google Scholar]
- 24.Fradkin JE, Wolff J. Iodide-induced thyrotoxicosis. Medicine (Baltimore) 1983; 62:1–20. [DOI] [PubMed] [Google Scholar]
- 25.Leger AF, Massin JP, Laurent MF, et al. Iodine-induced thyrotoxicosis: analysis of eighty-five consecutive cases. Eur J Clin Invest 1984; 14:449–455. [DOI] [PubMed] [Google Scholar]
- 26.Savoie JC, Massin JP, Thomopoulos P, et al. Iodine-induced thyrotoxicosis in apparently normal thyroid glands. J Clin Endocrinol Metab 1975; 41:685–691. [DOI] [PubMed] [Google Scholar]


