Abstract
Epidemiologic studies reporting the effect of soy intake on endometrial cancer risk conveyed conflicting results. We systematically reviewed the literature to investigate whether there was an inverse relation between dietary soy intake and endometrial cancer risk.
PubMed, EMBASE, the Cochrane Library, and 4 main Chinese literature databases were searched from their inception to August 25, 2015 for both case–control studies and cohort studies that assessed the effect of soy intake on endometrial cancer risk. Study-specific most-adjusted odds ratios (ORs) or relative risks (RRs) were combined by using fixed-effects or random-effects model to calculate pooled risk estimates (REs).
A total of 10 epidemiologic studies were included in this meta-analysis, including 8 case–control studies and 2 prospective cohort studies. Dietary soy intake was inversely associated with endometrial cancer risk with an overall RE of 0.81 (95% CI: 0.72, 0.91). In subgroup analyses, a statistically significant protective effect of soy intake was found for unfermented soy food (RE: 0.81, 95% CI: 0.67, 0.97), postmenopausal women (RE: 0.76, 95% CI: 0.61, 0.95), and Asian (RE: 0.79, 95% CI: 0.66, 0.95) and non-Asian population (RE: 0.83, 95% CI: 0.71, 0.96).
Current evidence indicates that soy food intake is associated with lower endometrial cancer risk. Further larger cohort studies are warranted to fully clarify such an association.
INTRODUCTION
Worldwide, endometrial cancer (EC) is the fifth most common cancer in women, occurring more frequently in developed countries.1 It has been highly suggested that increased EC risk is associated with prolonged estrogen exposure if not countervailed by progesterone.2–4 Estrogen exposure includes exogenous estrogen (oral contraceptive medicine or hormone replacement therapy), nulliparity, early menarche and late menopause, etc.2,5,6 Obesity and low plasma concentration of sex hormone binding globulin are also associated with elevation of EC risk.7,8
Phytoestrogens are nonsteroidal plant-derived compounds, structurally similar to endogenous estrogens, and suggested to protect against hormone-dependent cancers.9,10 The anticancer mechanisms might include inhibition of enzymes that synthesize estrogen, promotion of the production of sex hormone binding globulin, modulation of cell proliferation, apoptosis, and inhibition of angiogenesis and tumor cells.11–14 Isoflavones, one major class of phytoestrogens, principally including genistein and daidzein, have been vigorously investigated. An almost exclusive dietary source of isoflavones, by far, is soybean which exists in most commonly consumed diet and especially as a staple in Asian-type diet. Evidence from several recent meta-analyses has suggested that soy may have a protective effect against hormone-dependent cancers, such as lung cancer, prostate cancer, ovarian cancer, and breast cancer.15–20
In addition to these hormone-related cancers, an inhibitory effect of genistein against endometrial carcinogenesis was also found in experimental studies.21,22 However, epidemiologic studies reporting the effect of soy intake on EC risk conveyed conflicting results. Furthermore, due to small sample sizes, most studies were not adequately powered to detect the effect of soy intake on EC risk. Thus, in order to provide the latest and more convincing evidence, we systematically reviewed the current available epidemiologic studies to investigate whether soy intake was associated with lower EC risk.
METHODS
This systematic review and meta-analysis was conducted and reported in adherence to MOOSE (Meta-Analysis of Observational Studies in Epidemiology).23 Since our study was a review of previous published studies, ethical approval or patient consent was not required. Two investigators (GQ-Z and JL-C) independently carried out literature search, eligibility evaluation, data extraction, and quality assessment. Discrepancies between authors were resolved by consensus.
Literature Search and Selection Criteria
PubMed, EMBASE, the Cochrane Library, and 4 main Chinese literature databases, that is, Wan Fang, VIP, SinoMed, and China National Knowledge Infrastructure (CNKI), were searched from their inception to August 25, 2015 by using a combination of Medical Subject Headings or Emtree and related common keywords in all fields. No language restriction was applied. The search strategy is shown in Table 1. The cited references of retrieved articles and previous reviews were also manually checked to identify any additional eligible studies.
TABLE 1.
Search Strategy

Studies meeting the following inclusion criteria were included: observational studies (prospective or retrospective cohort studies and case–control studies); investigated the association between soy or soy isoflavones or soy product intake and EC in adult women; reported adjusted odds ratios (ORs) or relative risks (RRs) and its 95% confidence intervals (CIs). If articles were from the same study population, the largest study was included to avoid duplication of information. Studies were excluded if evaluating: plasma or urinary isoflavones; dietary isoflavones from other sources instead of soy in association with EC.
Data Extraction and Quality Assessment
Relevant data from each included study were documented by using a unified data form. The following information were extracted from each study: study (the first author's name); country, study design, and study period; no. of cases/controls, mean or median age, and ethnicity; soy food assessed; most-adjusted ORs or RRs with 95% CIs for the highest compared with lowest quantile; and matched or adjusted factors in the design or data analysis. Since study-specific data can be obtained from original articles, no authors were contacted.
A 9-star system on the basis of the Newcastle-Ottawa Scale was used to assess the study quality of observational studies.24 However, because there is a correlation between caloric intake and nutrient consumption, and the possibility that a relation might exist between caloric intake and EC risk cannot be excluded, we modified the Newcastle-Ottawa Scale by adding one item “energy adjusted residual or nutrient-density model in data analyses.”16,25 If studies used the energy adjusted residual or nutrient-density model in their data analyses, an extra star would be assigned. Finally, the total score was 10, and studies with ≥7 stars were defined as high-quality studies.
Statistical Analysis
The study-specific most-adjusted ORs or RRs with its 95% CIs (highest compared with lowest amounts of soy intake) were used to compute a summary risk estimate (RE) with its 95% CI. From the REs, the SE of the estimate (SEE) was directly derived as SEE = [log (95% CI, upper limit) − log (95% CI, lower limit)]/3.92 and was used in the calculation of the pooled estimates. Subgroup analyses were performed according to study design (cohort studies vs. case–control studies), soy-derived isoflavones (genistein vs. daidzein vs. glycitein vs. formononetin vs. biochanin A), soy food (fermented vs. unfermented), menopausal status (premenopausal vs. postmenopausal), and race (Asians vs. non-Asians). Heterogeneity across studies was tested by using the I2 statistic, which was a quantitative measure of inconsistency across studies.26 Studies with an I2 statistic of ≥50% were considered to have significant heterogeneity. The random effects model was used to calculate pooled REs and its 95% CIs if significant heterogeneity existed. Otherwise, the fixed effects model was applied to calculate the pooled RE.
Funnel plot was carried out to investigate publication bias of all the included studies. A sensitivity analysis was conducted to assess the influence of individual studies on the pooled result, by excluding each study one by one and recalculating the combined RE on the remaining studies. All statistical analyses were performed with RevMan 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
RESULTS
Study Identification and Selection
Our systematic literature search yielded a total of 10 studies on soy intake and EC risk in the final analysis.27,28,30–37 One thousand fifty-eight records were identified by searching 7 databases and hand-searching relevant bibliographies. Three hundred five records were excluded for duplicates and an additional 711 records were excluded based on the titles and abstracts. The remaining 42 full-text articles were assessed for eligibility, and 32 were further excluded due to the following reasons: reviews (N = 8); dietary patterns or lifestyle and EC risk (N = 7); specific food (not relevant to phytoestrogen) and EC risk (N = 9); studies which reported OR by different gene polymorphisms (N = 1)38; total flavonoid intake and EC risk (N = 1)39; studies did not report OR or its 95% CI (N = 1)40; and studies based on the same study population (N = 5).41–45 The selection process is shown in Figure 1.
FIGURE 1.
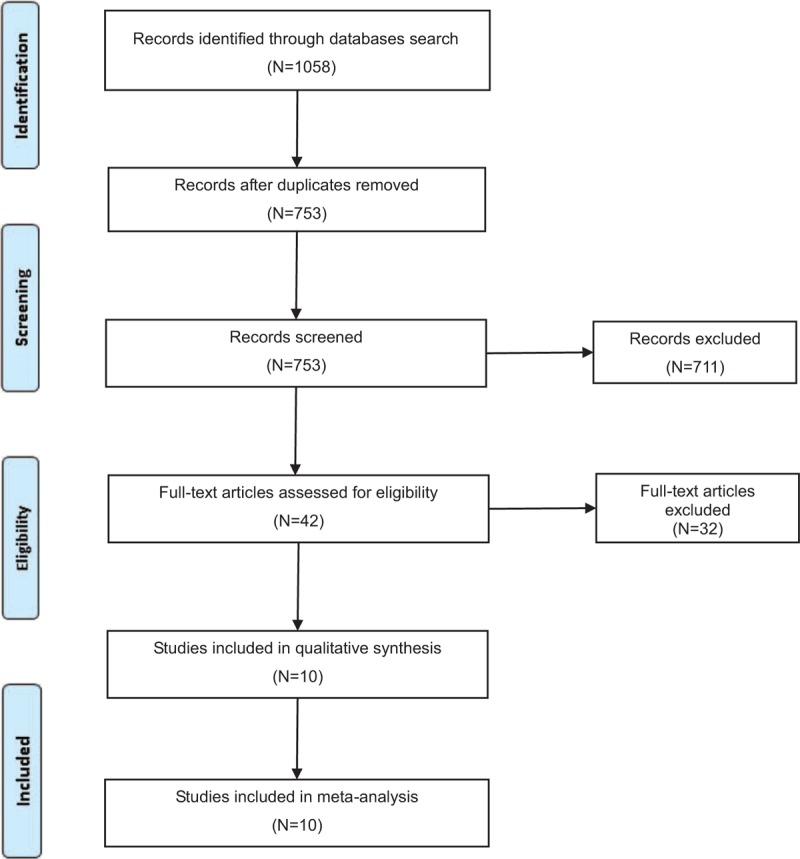
Selection process for the studies included in the meta-analysis.
Study Characteristics
The main characteristics of included studies are described in Table 2. All the included studies were published in English and between 1996 and 2014. Of the included studies, 2 were prospective cohort studies,36,37 6 were population-based case–control studies,27,28,30,31,33,35 and 2 were hospital-based case–control studies.32,34 Among the 10 studies, 2 were conducted in Japan,32,37 1 in China,35 1 in Italy,34 1 in Australia,28 and 5 in the United States.27,30,31,33,36 Several studies presented results by menopausal status, 2 for premenopausal,27,34 and 3 for postmenopausal.27,34,36 Most studies were matched or adjusted for a wide range of potential confounders, such as age, age at menarche, parity, energy intake, body mass index, oral contraceptive use, and hormone replacement therapy.
TABLE 2.
Characteristics of Included Observational Studies
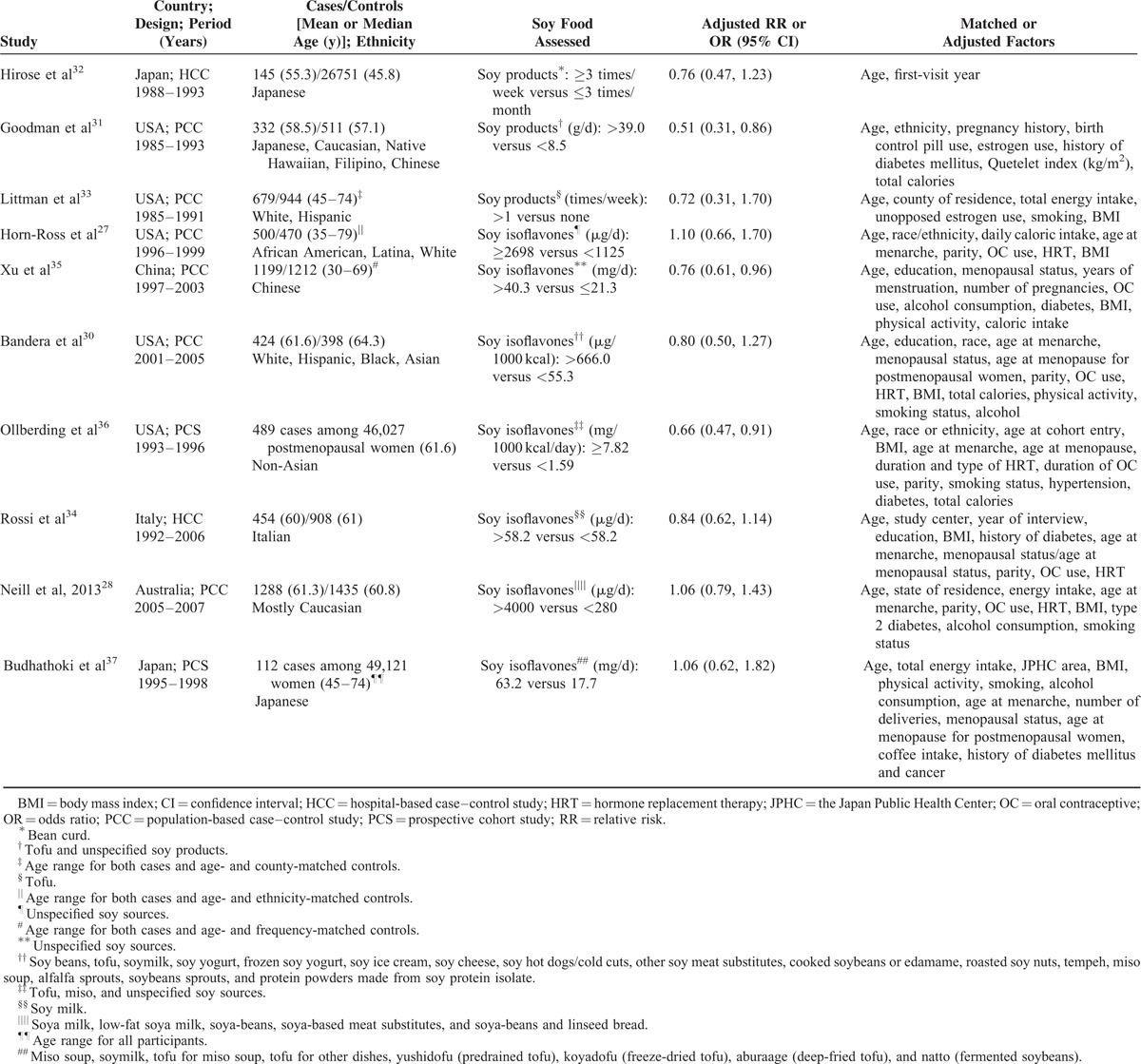
Quality Assessment
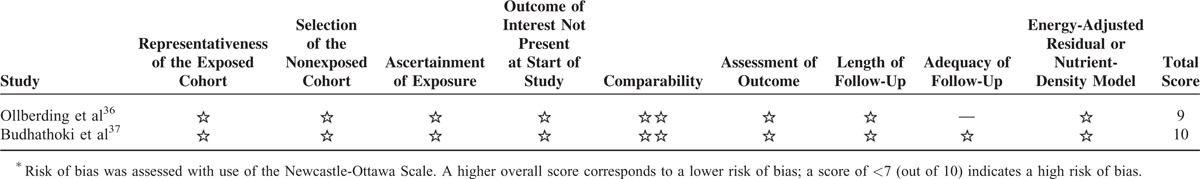
Risk-of-bias assessment of the included studies is presented in Tables 3 and 4. Eight studies were rated as a total score of more than 727,28,30,31,33,35–37 and the other 2 studies as a score of 5 indicating a high risk of bias.32,34
TABLE 3.
Risk-of-Bias Assessment of Case–Control Studies∗
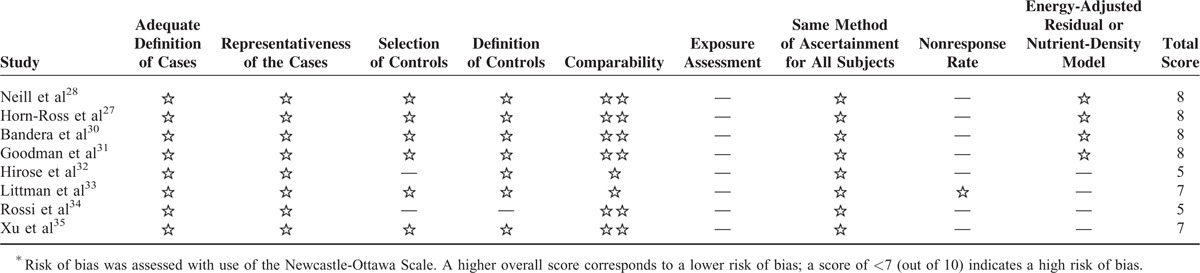
TABLE 4.
Risk-of-Bias Assessment of Prospective Cohort Studies∗
Highest Versus Lowest Category
Our overall analysis of 10 studies showed a 19% reduction in risk of EC with high intake of soy foods (RE: 0.81, 95% CI: 0.72, 0.91), without significant heterogeneity (I2 = 20%), shown in Figure 2. There was no evidence of significant publication bias by inspection of the funnel plot, shown in Figure 3.
FIGURE 2.
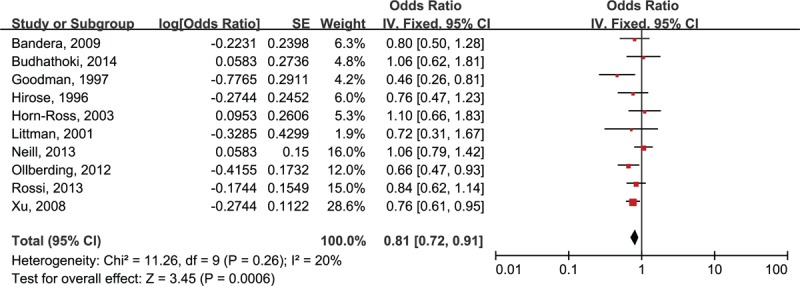
Risk estimates (95% CIs) of soy intake and risk of endometrial cancer.
FIGURE 3.
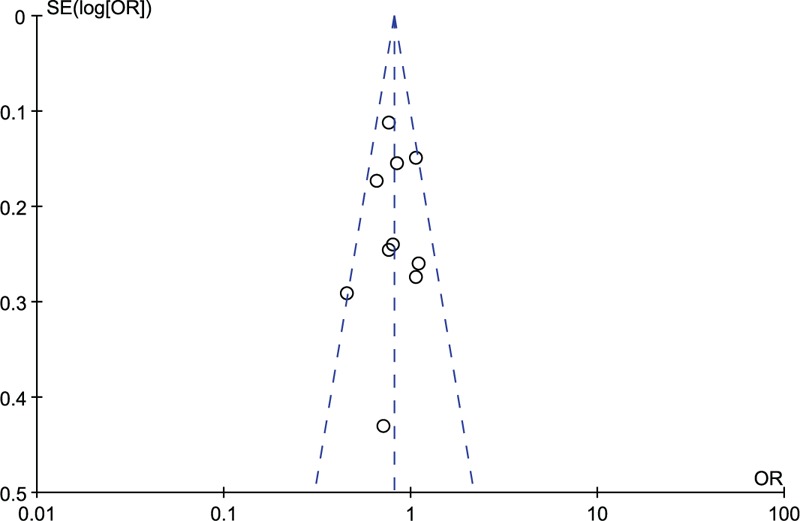
Funnel plot of risk estimates of all studies included in the meta-analysis.
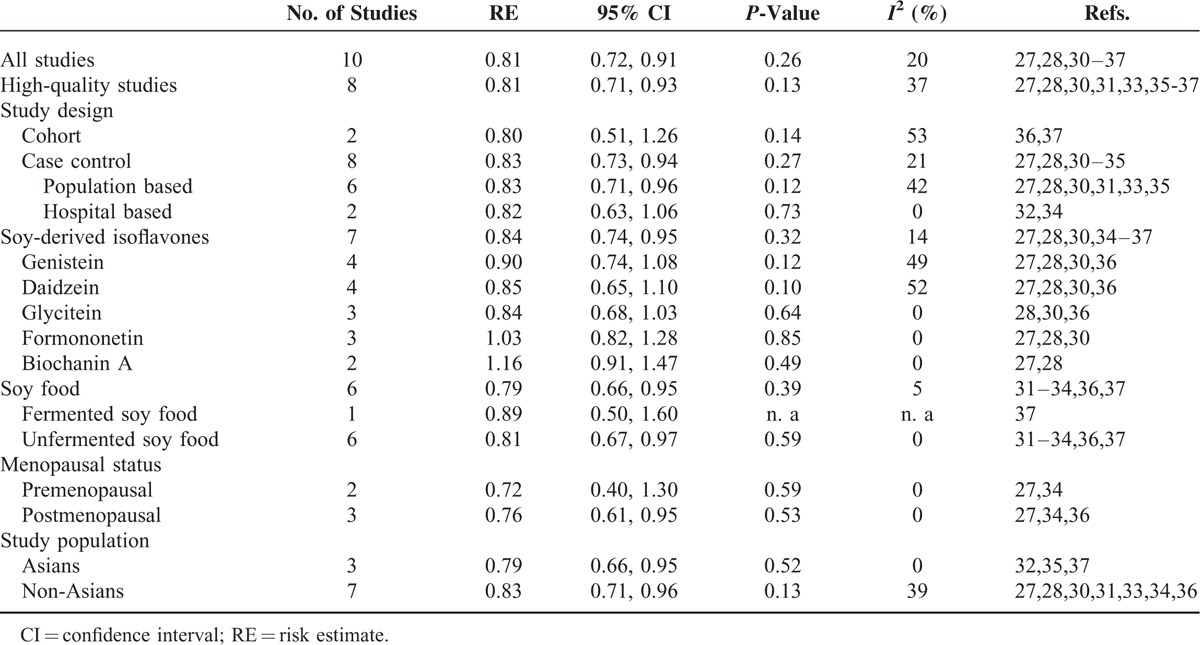
Table 5 reports the pooled REs for soy intake on EC risk in selected subgroups. In the subgroup analyses, by study design, case–control studies showed a preventive effect of soy intake on EC with a RE of 0.83 (95% CI: 0.73, 0.94), similar to the overall analysis. Regarding the type of soy intake, both soy food (RE: 0.79, 95% CI: 0.66, 0.95) and soy-derived isoflavones (RE: 0.84, 95% CI: 0.74, 0.95) had a preventive effect on EC. This beneficial effect remained in unfermented soy food (RE: 0.81, 95% CI: 0.67, 0.97). However, no association was found in any subtypes of isoflavones. When stratified by menopausal status, there was a statistically significant inverse association in postmenopausal women (RE: 0.76, 95% CI: 0.61, 0.95), but not in premenopausal women (RE: 0.72, 95% CI: 0.40, 1.30). This inverse association remained in both Asian population (RE: 0.79, 95% CI: 0.66, 0.95) and non-Asian population (RE: 0.83, 95% CI: 0.71, 0.96).
TABLE 5.
Subgroup Analyses for Dietary Soy Intake on Endometrial Cancer Risk
In sensitivity analyses, further exclusion of any single study did not materially alter the overall combined RE, with a range from a low of 0.77 (95% CI: 0.68, 0.88) to a high of 0.84 (95% CI: 0.74, 0.95) via omission of the study by Neill et al28 and the study by Ollberding et al,36 respectively.
DISCUSSION
The present systematic review and meta-analysis identified 10 observational studies investigating soy intake on EC risk. Our analysis showed that soy intake was associated with a 19% reduction in risk of EC when highest reported soy intake was compared with lowest reported soy intake. Moreover, this finding was consistent across sensitivity analyses and most subgroup analyses, and no publication bias was observed.
Comparison With Previous Studies
A meta-analysis by Myung et al18 was conducted in 2009 on association between soy intake and endocrine-related gynecological cancer. An inverse relation was found between soy intake and EC risk on the basis of only 3 population-based case–control studies. However, the erratum result27 instead of the corrected data29 by Horn-Ross et al was analyzed in this meta-analysis, which could probably arouse spurious association between soy intake and risk of EC. Furthermore, we conducted sensitivity analyses according to the data presented in this meta-analysis, and the protective effect of soy was unstable from an overall RE of 0.78 (95% CI: 0.62, 0.97), to 0.86 (95% CI: 0.67, 1.11) when omitting Goodman et al, or to 0.74 (95% CI: 0.52, 1.06) when omitting Xu et al. In contrast with the previous meta-analysis, the present one strongly suggested that soy intake was inversely associated with lower EC risk. Moreover, subgroup analyses and sensitivity analyses did not materially alter the pooled result and no publication bias was observed, which added robustness to our main findings.
The plausible biological mechanisms through which soy products may protect against EC include estrogen-dependent mechanisms via the estrogen receptor (ER) signaling pathway and estrogen-independent mechanisms. Prolonged estrogen exposure has been indicated to be associated with higher risk of EC.2–4 Estrogen acts via the ER, which consists of 2 subtypes in endometrium, ER alpha and ER beta. It is thought that ER alpha mediates the proliferative effect of estrogens and functions as a tumor promoter,46 while ER beta is proved to act as an ER alpha antagonist and function as a tumor suppressor.47–50 Isoflavones, which are structurally similar to estrogen, show weak estrogenic activity and could compete with estrogen at the ER complex.51 It has been shown that genistein, one major type of isoflavones, has a higher affinity for ER beta than for ER alpha.18,52 Estrogen-independent mechanisms include inhibition of 17beta-hydroxysteroid dehydrogenase, antioxidative effects, induction of apoptosis, inhibition of angiogenesis and cancer cell growth, and promotion of the production of sex hormone binding globulin.14,53–60
The association between soy intake and EC in cohort studies becomes null based on only 2 studies. However, the study by Ollberding et al,36 1 of the 2 included studies, was conducted in postmenopausal women. When stratified by menopausal status, we observed a protective role in postmenopausal women, but not in premenopausal women, which suggested that menopausal status maybe an important modifier of the effect of isoflavones on EC. One probable reason is that soy isoflavones may be effective only at low sex hormone concentrations as shown in postmenopausal women. Another explanation is that the mechanism by which isoflavones act might involve the ovarian synthesis of sex hormone or the alteration of other menstrual cycle characteristics.61,62 Of note, the lack of effect in premenopausal women could also be due to the small sample sizes included in this subgroup analysis. Nevertheless, further studies that assess the effects of soy intake on EC risk separately in pre- and postmenopausal women are still warranted.
Our findings indicated that unfermented soy foods was inversely associated with EC risk, whereas a null association was found between fermented soy foods and EC risk based on only one cohort study by Budhathoki et al.37 The result was consistent with the meta-analysis by Yang et al,16 which suggested that unfermented soy foods was inversely associated with lung cancer risk, not fermented soy foods. Another meta-analysis63 studied the effects of fermented and unfermented soy foods separately on gastric cancer risk, which also showed that a high intake of unfermented soy foods was associated with lower gastric cancer risk, whereas fermented soy foods was associated with an increased risk of gastric cancer. Only few experimental studies found that fermented soy foods intake could exert an inhibitory effect on breast, stomach, and colon tumorgenesis.64–67 To our knowledge, no experimental or observational studies explored the probable effects of fermented and unfermented soy foods separately on EC. Since soy foods exist in most commonly consumed diet worldwide, and especially as a staple in Asian-type diet, our study lays stress on the need for further studies to clarify the difference between fermented and unfermented soy foods in the etiology and prevention of EC.
Notably, the exact mechanisms by which soy products may protect against endocrine-related cancers have not been fully elucidated. Isoflavones were shown to inhibit growth of breast cancer cells at high concentration, but stimulate the growth at low concentration.18,68,69 Soy isoflavones at particularly high consumption resulting in relatively high incidence of endometrial hyperplasia has also been found in a randomized, double-blinded, placebo-controlled trial.70 Hence, the protective effects of soy isoflavones on EC should be viewed with caution, since the possibility of biphasic effects of soy isoflavones still cannot be excluded.
Several potential limitations should be taken into consideration when interpreting the results. First, among the 10 included studies, only 2 were cohort studies, whereas the other 8 were case–control studies. Case–control studies were highly subject to recall bias and selection bias. Hence, additional well-designed cohort studies are needed to confirm our findings. Second, the adjusted confounding variables were highly variable across the included studies. However, the study-specific most-adjusted ORs or RRs were used to pool the REs, so we could minimize the effects of confounding factors as much as possible, such as age, age at menarche, body mass index, oral contraceptive use, hormone replacement therapy, energy intake, and parity. Third, the sufficient amount of soy intake for the prevention of EC cannot be defined in our study, because the included studies used different quantile ranges to categorize their data which resulted in different increments of quantitative exposure were used for pooling. In addition, some food composition databases were incomplete because of not containing the whole range of soy foods consumed by the study population, which could probably lead to underestimate soy foods consumed. Also, the analytical methods in these databases used to determine the soy food values varied across included studies. Hence, the optimal amount of soy food remains to be explored in future studies.
In conclusion, the present systematic review and meta-analysis suggested that soy intake was associated with lower EC risk. Because of methodological shortcomings of included studies, various soy sources, and different methods used to assess soy consumption across studies, these findings from our study should be interpreted cautiously and confirmed in future research.
Acknowledgments
Author contributions were as follows: Guo-Qiang Zhang and Jin-Liang Chen: study design, literature search, systematic review and data collection, statistical analysis, interpretation of results, and preparation of the manuscript; Qin Liu, Yong Zhang, Huan Zeng: contribution to critical review of the manuscript; Yong Zhao: principal investigator, study design, statistical analysis, and interpretation of results.
Footnotes
Abbreviations: CI = confidence interval, CNKI = China National Knowledge Infrastructure, EC = endometrial cancer, ER = estrogen receptor, MOOSE = Meta-Analysis of Observational Studies in Epidemiology, OR = odds ratio, RE = risk estimate, RR = relative risk.
This work was supported by Chinese Nutrition Society (CNS) Nutrition Research Foundation-DSM Research Fund.
Guo-Qiang Zhang and Jin-Liang Chen contributed equally to this work.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2014; 136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet 2005; 366:491–505. [DOI] [PubMed] [Google Scholar]
- 3.Key TJ, Pike MC. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 1988; 57:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siiteri PK. Steroid hormones and endometrial cancer. Cancer Res 1978; 38:4360–4366. [PubMed] [Google Scholar]
- 5.Beral V, Bull D, Reeves G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005; 365:1543–1551. [DOI] [PubMed] [Google Scholar]
- 6.Parazzini F, La Vecchia C, Bocciolone L, et al. The epidemiology of endometrial cancer. Gynecol Oncol 1991; 41:1–16. [DOI] [PubMed] [Google Scholar]
- 7.Ratajczak T, Twaddle E, Hahnel R. Sex hormone-binding globulin and estrogen receptor in endometrial and cervical cancer. Gynecol Oncol 1980; 10:262–266. [DOI] [PubMed] [Google Scholar]
- 8.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 2002; 11:1531–1543. [PubMed] [Google Scholar]
- 9.Zhang JC, Xie ZB, Zai SF, et al. Matched case-control study for detecting risk factors of breast cancer in women living in Xinxiang, Henan Province. He Nan Zhong Liu Xue Za Zhi 2003; 16:201–203.(In Chinese). [Google Scholar]
- 10.Tao P, Yu XH, Liu L, et al. Gene polymorphism of estrogen receptor, dietary soy intake and breast cancer risk. Zhong Liu Yu Fang Yu Zhi Liao 2015; 28:8–12.(In Chinese). [Google Scholar]
- 11.Wuttke W, Jarry H, Seidlova-Wuttke D. Isoflavones—safe food additives or dangerous drugs? Ageing Res Rev 2007; 6:150–188. [DOI] [PubMed] [Google Scholar]
- 12.Molteni A, Brizio-Molteni L, Persky V. In vitro hormonal effects of soybean isoflavones. J Nutr 1995; 125:751s–756s. [DOI] [PubMed] [Google Scholar]
- 13.Baker ME. Endocrine activity of plant-derived compounds: an evolutionary perspective. Proc Soc Exp Biol Med 1995; 208:131–138. [DOI] [PubMed] [Google Scholar]
- 14.Gao M, Cao YK, Xue XO. Effect of daidzein on proliferation of endometrial carcinoma. J Beijing Univ Traditional Chinese Med 2010; 33:162–165.(In Chinese). [Google Scholar]
- 15.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst 2006; 98:459–471. [DOI] [PubMed] [Google Scholar]
- 16.Yang WS, Va P, Wong MY, et al. Soy intake is associated with lower lung cancer risk: results from a meta-analysis of epidemiologic studies. Am J Clin Nutr 2011; 94:1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Die MD, Bone KM, Williams SG, et al. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int 2014; 113:E119–130. [DOI] [PubMed] [Google Scholar]
- 18.Myung SK, Ju W, Choi HJ, et al. Soy intake and risk of endocrine-related gynaecological cancer: a meta-analysis. BJOG 2009; 116:1697–1705. [DOI] [PubMed] [Google Scholar]
- 19.Shen DP, Wang XP, Qin LQ. Isoflavones intake and risk of ovarian cancer: a meta-analysis of epidemiological study. Soybean Sci 2013; 32:814–817.(In Chinese). [Google Scholar]
- 20.Li XT, Hua L. Isoflavones intake and risk of breast cancer: a meta-analysis. J Math Med 2015; 28:53–56.(In Chinese). [Google Scholar]
- 21.Sampey BP, Lewis TD, Barbier CS, et al. Genistein effects on stromal cells determines epithelial proliferation in endometrial co-cultures. Exp Mol Pathol 2011; 90:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mylonas I, Jeschke U, Makovitzky J, et al. Immunohistochemical expression of steroid receptors and glycodelin A in isolated proliferative human endometrial glandular cells after stimulation with tamoxifen and phytoestrogens (genistein and daidzein). Anticancer Res 2003; 23:1119–1125. [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (cited 12 October 2015). [Google Scholar]
- 25.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997; 65:1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn-Ross PL, John EM, Canchola AJ, et al. Phytoestrogen intake and endometrial cancer risk. J Natl Cancer Inst 2003; 95:1158–1164. [DOI] [PubMed] [Google Scholar]
- 28.Neill AS, Ibiebele TI, Lahmann PH, et al. Dietary phyto-oestrogens and the risk of ovarian and endometrial cancers: findings from two Australian case-control studies. Br J Nutr 2014; 111:1430–1440. [DOI] [PubMed] [Google Scholar]
- 29.Horn R. Erratum: “Phytoestrogen intake and endometrial cancer risk” (Journal of the National Cancer Institute (2003) vol. 95 (1158–1164)). J Natl Cancer Inst 2006; 98:1501. [DOI] [PubMed] [Google Scholar]
- 30.Bandera EV, Williams MG, Sima C, et al. Phytoestrogen consumption and endometrial cancer risk: a population-based case-control study in New Jersey. Cancer Causes Control 2009; 20:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman MT, Wilkens LR, Hankin JH, et al. Association of soy and fiber consumption with the risk of endometrial cancer. Am J Epidemiol 1997; 146:294–306. [DOI] [PubMed] [Google Scholar]
- 32.Hirose K, Tajima K, Hamajima N, et al. Subsite (cervix/endometrium)-specific risk and protective factors in uterus cancer. Jpn J Cancer Res 1996; 87:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Littman AJ, Beresford SA, White E. The association of dietary fat and plant foods with endometrial cancer (United States). Cancer Causes Control 2001; 12:691–702. [DOI] [PubMed] [Google Scholar]
- 34.Rossi M, Edefonti V, Parpinel M, et al. Proanthocyanidins and other flavonoids in relation to endometrial cancer risk: a case-control study in Italy. Br J Cancer 2013; 109:1914–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu WH, Zheng W, Cai Q, et al. The Asp327Asn polymorphism in the sex hormone-binding globulin gene modifies the association of soy food and tea intake with endometrial cancer risk. Nutr Cancer 2008; 60:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ollberding NJ, Lim U, Wilkens LR, et al. Legume, soy, tofu, and isoflavone intake and endometrial cancer risk in postmenopausal women in the multiethnic cohort study. J Natl Cancer Inst 2012; 104:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Budhathoki S, Iwasaki M, Sawada N, et al. Soy food and isoflavone intake and endometrial cancer risk: the Japan Public Health Center-based prospective study. BJOG 2014; 122:304–311. [DOI] [PubMed] [Google Scholar]
- 38.Deming SL, Zheng W, Xu WH, et al. UGT1A1 genetic polymorphisms, endogenous estrogen exposure, soy food intake, and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 2008; 17:563–570. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Lee IM, Zhang SM, et al. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr 2009; 89:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman MT, Hankin JH, Wilkens LR, et al. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res 1997; 57:5077–5085. [PubMed] [Google Scholar]
- 41.Xu WH, Zheng W, Xiang YB, et al. Soya food intake and risk of endometrial cancer among Chinese women in Shanghai: population based case-control study. BMJ 2004; 328:1285–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu WH, Dai Q, Xiang YB, et al. Interaction of soy food and tea consumption with CYP19A1 genetic polymorphisms in the development of endometrial cancer. Am J Epidemiol 2007; 166:1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu WH, Dai Q, Xiang YB, et al. Nutritional factors in relation to endometrial cancer: a report from a population-based case-control study in Shanghai, China. Int J Cancer 2007; 120:1776–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao MH, Xu WH, Zheng W, et al. A case-control study in Shanghai of fruit and vegetable intake and endometrial cancer. Br J Cancer 2005; 92:2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai Q, Xu WH, Long JR, et al. Interaction of soy and 17(beta)-HSD1 gene polymorphisms in the risk of endometrial cancer. Pharmacogenet Genomics 2007; 17:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Utsunomiya H, Suzuki T, Harada N, et al. Analysis of estrogen receptor alpha and beta in endometrial carcinomas: correlation with ER beta and clinicopathologic findings in 45 cases. Int J Gynecol Pathol 2000; 19:335–341. [DOI] [PubMed] [Google Scholar]
- 47.Pujol P, Rey JM, Nirde P, et al. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res 1998; 58:5367–5373. [PubMed] [Google Scholar]
- 48.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv 2003; 3:281–292. [DOI] [PubMed] [Google Scholar]
- 49.Li AJ, Baldwin RL, Karlan BY. Estrogen and progesterone receptor subtype expression in normal and malignant ovarian epithelial cell cultures. Am J Obstet Gynecol 2003; 189:22–27. [DOI] [PubMed] [Google Scholar]
- 50.Chen GG, Zeng Q, Tse GM. Estrogen and its receptors in cancer. Med Res Rev 2008; 28:954–974. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar FH, Li Y. The role of isoflavones in cancer chemoprevention. Front Biosci 2004; 9:2714–2724. [DOI] [PubMed] [Google Scholar]
- 52.Jarry H, Harnischfeger G, Duker E. [The endocrine effects of constituents of Cimicifuga racemosa. 2. In vitro binding of constituents to estrogen receptors]. Planta Med 1985; 4:316–319. [DOI] [PubMed] [Google Scholar]
- 53.Hale GE, Hughes CL, Cline JM. Endometrial cancer: hormonal factors, the perimenopausal “window of risk,” and isoflavones. J Clin Endocrinol Metab 2002; 87:3–15. [DOI] [PubMed] [Google Scholar]
- 54.Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest Suppl 1990; 201:3–23. [PubMed] [Google Scholar]
- 55.Rose DP. Dietary fiber, phytoestrogens, and breast cancer. Nutrition 1992; 8:47–51. [PubMed] [Google Scholar]
- 56.Adlercreutz H, Fotsis T, Heikkinen R, et al. Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian postmenopausal women and in women with breast cancer. Lancet 1982; 2:1295–1299. [DOI] [PubMed] [Google Scholar]
- 57.Setchell KD, Lawson AM, Borriello SP, et al. Lignan formation in man—microbial involvement and possible roles in relation to cancer. Lancet 1981; 2:4–7. [DOI] [PubMed] [Google Scholar]
- 58.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 1998; 68:1333s–1346s. [DOI] [PubMed] [Google Scholar]
- 59.Adlercreutz H, Mousavi Y, Clark J, et al. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J Steroid Biochem Mol Biol 1992; 41:331–337. [DOI] [PubMed] [Google Scholar]
- 60.Adlercreutz H, Hockerstedt K, Bannwart C, et al. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG). J Steroid Biochem 1987; 27:1135–1144. [DOI] [PubMed] [Google Scholar]
- 61.Lu LJ, Anderson KE, Grady JJ, et al. Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev 1996; 5:63–70. [PubMed] [Google Scholar]
- 62.Cassidy A, Bingham S, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr 1994; 60:333–340. [DOI] [PubMed] [Google Scholar]
- 63.Kim J, Kang M, Lee JS, et al. Fermented and non-fermented soy food consumption and gastric cancer in Japanese and Korean populations: a meta- analysis of observational studies. Cancer Sci 2011; 102:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kinouchi FL, Maia DC, de Abreu Ribeiro LC, et al. A soy-based product fermented by Enterococcus faecium and Lactobacillus helveticus inhibits the development of murine breast adenocarcinoma. Food Chem Toxicol 2012; 50:4144–4148. [DOI] [PubMed] [Google Scholar]
- 65.Chang WH, Liu JJ, Chen CH, et al. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by fermented soy milk. Nutr Cancer 2002; 43:214–226. [DOI] [PubMed] [Google Scholar]
- 66.Ohara M, Lu H, Shiraki K, et al. Inhibition by long-term fermented miso of induction of gastric tumors by N-methyl-N′-nitro-N-nitrosoguanidine in CD (SD) rats. Oncol Rep 2002; 9:613–616. [PubMed] [Google Scholar]
- 67.Ohuchi Y, Myojin Y, Shimamoto F, et al. Decrease in size of azoxymethane induced colon carcinoma in F344 rats by 180-day fermented miso. Oncol Rep 2005; 14:1559–1564. [PubMed] [Google Scholar]
- 68.de Lemos ML. Effects of soy phytoestrogens genistein and daidzein on breast cancer growth. Ann Pharmacother 2001; 35:1118–1121. [DOI] [PubMed] [Google Scholar]
- 69.Dampier K, Hudson EA, Howells LM, et al. Differences between human breast cell lines in susceptibility towards growth inhibition by genistein. Br J Cancer 2001; 85:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unfer V, Casini ML, Costabile L, et al. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fertil Steril 2004; 82:145–148. [DOI] [PubMed] [Google Scholar]


