Abstract
Traumatic intracranial hemorrhage (ICH) is prevalent worldwide with long-term consequences, including disabilities. However, studies on the association of traumatic ICH with coronary artery disease (CAD) are scant. Therefore, this study explored the aforementioned association in a large-scale, population-based cohort.
A total of 128,997 patients with newly diagnosed traumatic ICH and 257,994 age- and sex-matched patients without traumatic ICH from 2000 to 2010 were identified from Taiwan's National Health Insurance Research Database. The Kaplan–Meier method was used for measuring the cumulative incidence of CAD in each cohort. Cox proportional regression models were used for evaluating the risk of CAD in patients with and without traumatic ICH and for comparing the risk between the 2 cohorts.
The Kaplan–Meier analysis revealed that the cumulative incidence curves of CAD were significantly higher in patients with traumatic ICH than in those without ICH (log-rank test, P < 0.001). After adjustment for age, sex, and comorbidities, patients with traumatic ICH were associated with a higher risk of CAD compared with those without traumatic ICH (adjusted hazard ratio = 1.16, 95% confidence interval = 1.13–1.20). Compared with the general population, patients with traumatic ICH and having underlying comorbidities, including diabetes, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, chronic kidney disease, and congestive heart failure, exhibited multiplicative risks of developing CAD.
This cohort study revealed an increased risk of CAD in patients with traumatic ICH. Therefore, comprehensive evaluation and aggressive risk reduction for CAD are recommended in these patients.
INTRODUCTION
Traumatic intracranial hemorrhage (ICH), a worldwide health problem, is a major cause of mortality in young adults, and it is associated with high rates of lifelong disabilities among survivors.1,2 In addition to disabilities, survivors of traumatic ICH often experience neurocognitive deficits and psychological problems, such as depression, poor decision-making ability, and impulsive aggressive behaviors, leading to poor community, social, and vocational integration.1 Previous studies have suggested that the detrimental effects of traumatic brain injury (TBI) often extend beyond the hemorrhage area. Metabolic changes have also been observed in systemic regions distant from the focal hemorrhagic lesions.3,4 Moreover, moderate-to-severe TBI induces several inflammatory signals, which increase proinflammatory cytokine and chemokine release, resulting in monocyte activation and infiltration, glial activation, neuronal and myelin loss, and persistent inflammation.5,6 This evidence indicates the systemic injuries and inflammatory reactions following TBI.
Coronary artery disease (CAD), with atherosclerosis being the main pathophysiological mechanism, is the leading cause of morbidity and mortality worldwide.7 Atherosclerosis, a chronic inflammatory disease of the arterial walls, is characterized by the formation of lipid-laden lesions. Furthermore, lipid deposition, both innate and adaptive inflammation, such as T-cell and macrophage activation, and oxidative stress contribute to atherosclerosis development.8–10 Among them, inflammation mechanically alters conventional CAD risk factors, such as diabetes mellitus (DM), hyperlipidemia, hypertension, and biological modifications of the arterial walls, which play a major role in atherosclerosis.10
Although inflammatory mechanisms potentially link traumatic ICH and CAD, evidence regarding this association is limited. By analyzing Taiwan's National Health Insurance Research Database (NHIRD), we explored the risk of CAD in patients with traumatic ICH and observed an association between them. Therefore, both patients and clinicians should consider the risk of CAD following traumatic ICH.
METHODS
Data Source
The National Health Insurance (NHI) program, launched by the Taiwan government in 1995, provides comprehensive health care coverage for 99% of the residents in Taiwan (registered in NHIRD, Taiwan; http://nhird.nhri.org.tw/en/Background.html). The National Health Research Institute audits and releases the NHIRD for use in health service studies. The NHIRD comprises reimbursement claims data, including registry of beneficiary, medical record for each insured patient, and medical services. Following regulations implemented by the Department of Health, the identity of each patient was encrypted for privacy and data security. For this study, we used a subset of the NHIRD, including files of inpatient claims and Registry of Beneficiaries. This study was exempted from a complete ethical review by the International Review Board, China Medical University, and Hospital Research Ethics Committee (IRB permit number: CMUH104-REC2-115).
Patients
From the inpatient claims data, we identified patients newly diagnosed with traumatic ICH (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 852 and 853) from 2000 to 2010. The initial traumatic ICH diagnosis admission date was set as the index date. The exclusion criteria are outlined as follows: diagnosis of CAD (ICD-9-CM 410-414) at the baseline, aged <20 years, or having incomplete demographic information. Patients with traumatic ICH were frequency-matched to those without traumatic ICH and CAD at the baseline and by the year of the index date and age (5-year intervals) by using the aforementioned exclusion criteria. All patients were monitored until CAD was diagnosed, loss to follow-up, death, withdrawal from the NHI program, or December 31, 2011, whichever occurred first.
Comorbidities
Each patient was screened at the baseline for preexisting comorbidities, including DM (ICD-9-CM 250), hypertension (ICD-9-CM 401-405), hyperlipidemia (ICD-9-CM 272), chronic obstructive pulmonary disease (COPD; ICD-9-CM 490-496), chronic kidney disease (CKD; ICD-9-CM 580-589), congestive heart failure (CHF; ICD-9-CM 428), depression (ICD-9-CM 296.2, 296.3, 300.4, and 311), and anxiety (ICD-9-CM 300).
Statistical Analyses
The demographic and clinical characteristics, including sex, age (≤49, 50–64, and ≥65 years), and baseline comorbidity, of all patients were compared using the χ2 test. We used the Student t test for examining the continuous variables; we plotted the Kaplan–Meier curve for measuring the cumulative incidence of CAD for each cohort and tested the difference by using the log-rank test. The overall sex-, age-, and comorbidity-specific incidences of CAD (per 1000 person-year) were calculated for each cohort. Univariate and multivariate Cox proportional hazard regression models (hazard ratios [HRs] and 95% confidence intervals [CIs]) were used for assessing the risk of CAD associated with traumatic ICH and then comparing the risk between the 2 cohorts. The multivariate model was simultaneously adjusted for age, sex, DM, hypertension, hyperlipidemia, COPD, CKD, CHF, depression, and anxiety. Data were analyzed for evaluating the CAD-associated risk factors and the coeffects of CAD and traumatic ICH. All analyses were conducted using SAS statistical software (Version 9.4 for Windows; SAS Institute, Inc, Cary, NC). A 2-tailed P value of 0.05 was considered significant.
RESULTS
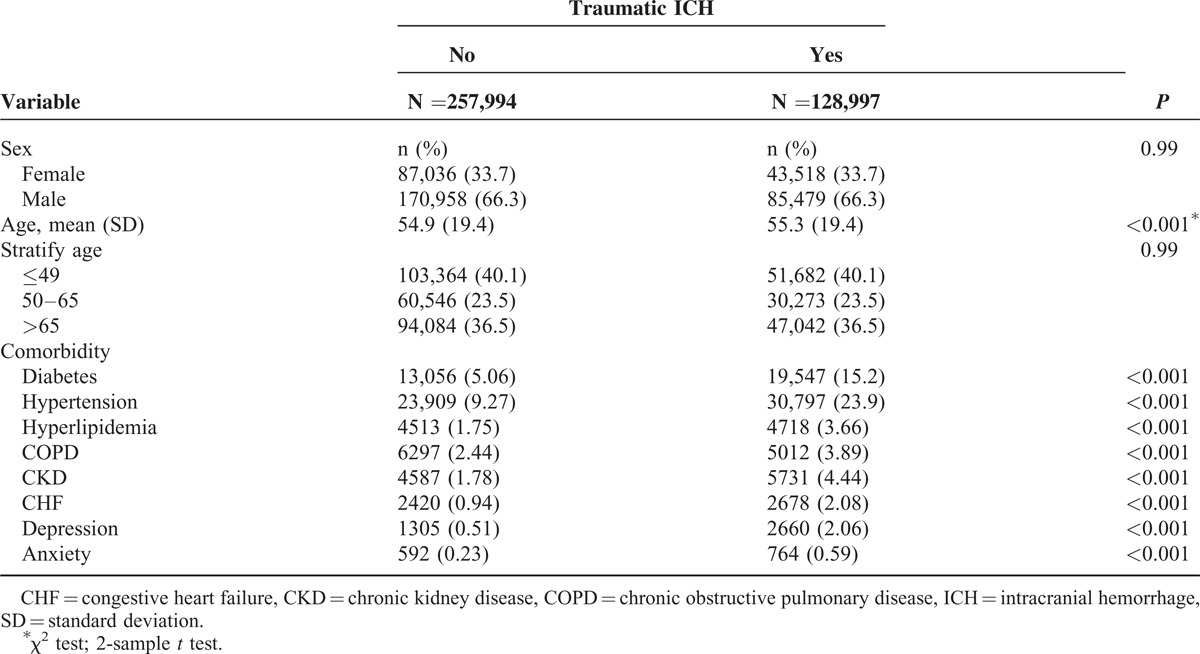
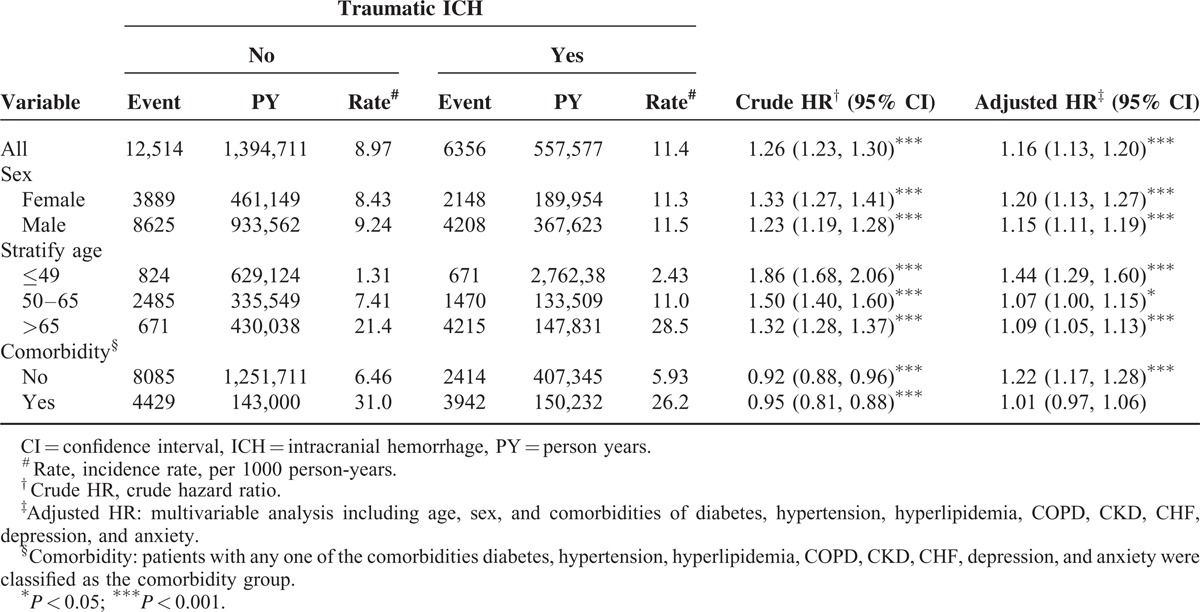
Our study included 128,997 patients with traumatic ICH and 257,994 patients without traumatic ICH. Among patients with traumatic ICH, 66.3% were males, and 40.1% were aged <49 years (Table 1). The mean age of patients with and without traumatic ICH was 55.3 ± 19.4 and 54.9 ± 13.8 years, respectively. Furthermore, patients with traumatic ICH were more predisposed to the comorbidities, such as DM, hypertension, hyperlipidemia, COPD, CKD, CHF, depression, and anxiety, compared with those without traumatic ICH. The mean follow-up duration was 4.32 ± 3.41 and 5.41 ± 3.16 years in patients with and without traumatic ICH, respectively. The Kaplan–Meier analysis results show that the cumulative incidence curves of CAD were significantly higher in the traumatic ICH cohort than in the comparison cohort (log-rank test P < 0.001). The overall incidence of CAD in the traumatic ICH cohort was 11.4 per 1000 person-years, which was 1.26-fold higher than that in the comparison cohort (8.97 per 1000 person-years). After adjustment for age, sex, and comorbidity, the risk of CAD was higher in patients with traumatic ICH than in those without traumatic ICH (adjusted HR [aHR] = 1.16, 95% CI = 1.13–1.20). Furthermore, the risk of CAD in patients with traumatic ICH, but without comorbidities, was significantly higher than that in patients without traumatic ICH stratified by sex (females: aHR = 1.20, 95% CI = 1.27–1.41; males: aHR = 1.15, 95% CI = 1.11–1.19), age (≤49 years: aHR = 1.44, 95% CI = 1.29–1.60; 50–64 years: aHR = 1.07, 95% CI = 1.00–1.15; >65 years: aHR = 1.09, 95% CI = 1.05–1.13), and comorbidity (patients without comorbidity: aHR = 1.22, 95% CI = 1.17–1.28).
TABLE 1.
Demographic Characteristics and Comorbidity in Patient With and Without Traumatic ICH
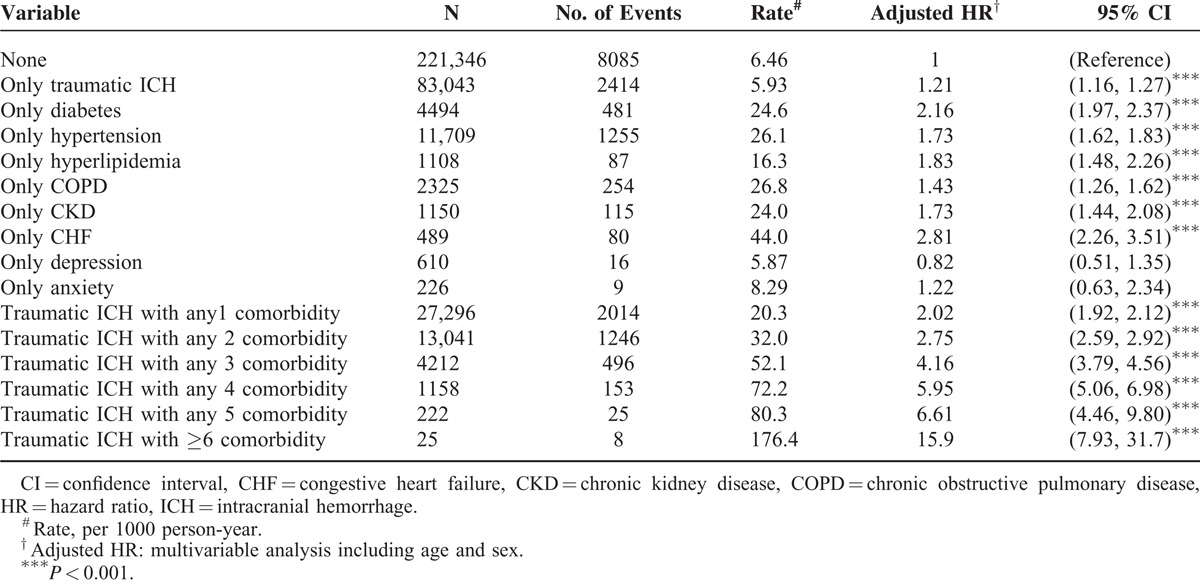
Compared with patients without traumatic ICH or underlying comorbidities, those with only traumatic ICH or comorbidities were associated with a higher risk of CAD (Table 3). Compared with patients with traumatic ICH without comorbidities, those with ≥6 comorbidities had a significantly increased risk of CAD (aHR = 15.9, 95% CI = 7.93–31.7), followed by those with 5 (aHR = 6.61, 95% CI = 4.46–9.80), 4 (aHR = 5.95, 95% CI = 5.06–6.98), 3 (aHR = 4.16, 95% CI = 3.79–4.56), and 2 (aHR = 2.75, 95% CI = 2.59–2.92) comorbidities and those with only 1 comorbidity (aHR = 2.02, 95% CI = 1.92–2.12).
TABLE 3.
Joint Effects for Coronary Artery Disease Between Traumatic ICH and Coronary Artery Disease-Associated Risk Factor
DISCUSSION
This large-scale, population-based study indicated that patients with traumatic ICH, both men and women, have a significantly higher risk of CAD than those without traumatic ICH, particularly those without comorbidities (Fig. 1, Table 2). After adjustment for age, sex, and underlying comorbidities, such as DM, hypertension, and hyperlipidemia, patients with ICH were associated with a 16% increased risk of CAD (Table 2). ICH is an independent risk factor for CAD; patients with ICH have additive effects on the risk of CAD when combined with other comorbidities (Table 3), suggesting an association between traumatic ICH and CAD.
FIGURE 1.
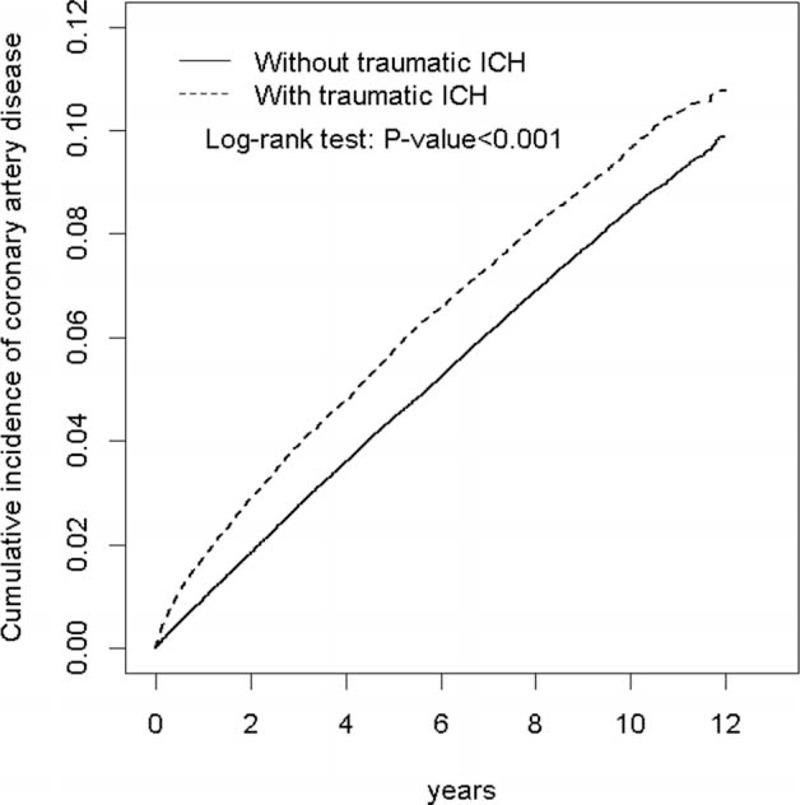
Comparison of the cumulative incidence of CAD between patients with and without traumatic ICH by using the Kaplan–Meier method. ICH = intracranial hemorrhage.
TABLE 2.
Comparison of Incidence and Hazard Ratio of Coronary Artery Disease Stratified by Sex, Age, and Comorbidity Between With and Without Traumatic ICH Patients
The NHIRD is a large-scale, nationwide, population-based database, covering approximately 23 million residents in Taiwan and thus permitting several study designs, such as cross-sectional, case-control, retrospective cohort analyses, and family studies.11,12 Because all patients in the NHI program receive optimal medical care at low costs, loss to follow-up is low, which may provide approximately 20 years of follow-up data of the enrolled population. Advantages of the NHIRD include its large sample size and lack of selection and participation bias.12 In this study, specialists diagnosed and coded (ICD-9-CM) CAD and traumatic ICH according to the standard diagnosis criteria, including typical symptoms and signs, laboratory data, and imaging findings. Because these data are monitored and strictly evaluated by the Bureau of NHI for reimbursement purposes, the diagnoses of CAD and traumatic ICH are highly reliable.
The underlying mechanisms in the association between traumatic ICH and CAD remain unexplored. Previous studies have suggested that TBI induces interleukin (IL)-1 and tumor necrosis factor-alpha (TNF-α), which triggers an inflammatory cascade and results in an impaired blood–brain barrier and neuronal and myelin loss.6,13–16 Moreover, TBI activates the innate immune system, resulting in the generation of reactive oxygen species (ROS), such as superoxide or peroxynitrite, which then cause oxidative damage and long-term neurological deficits.17–20 These inflammatory reactions occur in the central nervous and systemic circulatory system.21 In atherosclerosis, proinflammatory cytokines, such as IL-1 and TNF-α, play central roles in the inflammatory cascade.22,23 Both in vitro and in vivo investigations suggest that the deposition of cholesterol crystals activates inflammasome, leading to IL-1β production, further initiating fatty streaks and promoting local atherosclerotic progression.24,25 Tsimikas et al demonstrated that proinflammatory IL-1 genotypes were associated with the CAD risk and cardiovascular events, which was mediated by oxidized phospholipids and lipoprotein(a).22 On the basis of the inflammatory nature of atherosclerosis, trials involving anti-inflammatory therapies targeting IL-1 and TNF-α in cardiovascular disease are ongoing.26 Moreover, the retained low-density lipoproteins undergo oxidation and modification by ROS and then activate endothelial cells and facilitate foam cell formation, which is considered the initial step in the formation of atherosclerotic plaque.8,9,27,28 The inflammatory mechanisms linking TBI and atherosclerosis may partly explain the association between traumatic ICH and CAD in the present study.
Our study suggests that the incidence of CAD increased in elderly patients and in those with comorbidities, which is consistent with the observation in previous reports.29–31 However, after adjustment for the potential confounding effects by conducting a multivariate Cox proportional hazard regression analysis, young age (<49 years) and the absence of comorbidities remained the independent predictors of CAD in patients with traumatic ICH (Table 2). Younger patients are considered to have a lower incidence of conventional CAD risk factors, such as DM, hypertension, and hyperlipidemia. After stratification for age, aHRs of CAD were the highest in younger patients. This is possibly because these patients are more predisposed to traumatic ICH alone or the presence and accumulation of complex comorbidities and potential confounding factors reduce the effects of traumatic ICH.
We evaluated the coeffects for CAD in patients with traumatic ICH and CAD-related risk factors and observed that although traumatic ICH significantly contributes to the CAD risk, conventional CAD risk factors, such as DM, hypertension, and hyperlipidemia, predominate the development of CAD (Table 3). However, compared with the general population, patients with traumatic ICH and DM, hypertension, hyperlipidemia, COPD, CKD, and CHF exhibited multiplicative risks of developing CAD. These interaction analysis results revealed that the potential effects of traumatic ICH on CAD must be considered in clinical practice.
There were some limitations in the study: not available personal data of the lifestyles or habits as potential confounding factors for CAD in the NHIRD (such as body mass index, socioeconomic status, family history, smoking, and alcohol); lack of the individual data of the severity of ICH and CAD, functional status after traumatic ICH, and outcomes with ICH and CAD; the medication compliance could not be evaluated by the NHIRD, leading to the underestimation of the medication effects; we could not use the additional procedure codes to verify the CAD population, although the diagnosis of CAD was strictly monitored by certified medical reimbursement specialists in the NHIRD; because we used ICD-9 codes to evaluate the risk of CAD instead of the screening program, therefore these asymptomatic patients might not ask health care until they had the symptoms or sign of CAD, leading to the underestimation of the risk of asymptomatic CAD in these ICH patients; we could not specify the spectrums of CAD (such as stable and unstable angina and acute myocardial infarction) in this study; and the evidence of the methodological quality is usually lower by a retrospective cohort study (which lacks these necessary adjustments including these possibly unmeasured or unknown confounding factors) than that by prospective randomized trials.
As per our review of relevant literature, this is the first study to analyze data from the NHIRD and reveal the association between traumatic ICH and an increased risk of CAD in a large-scale, population-based cohort. Patients with traumatic ICH, particularly those with DM, hypertension, and hyperlipidemia, should be educated about the risk of CAD. Although additional prospective randomized studies are necessary for verifying the effects of traumatic ICH on CAD, meticulous evaluation and aggressive risk reduction for CAD are recommended in patients with traumatic ICH.
Footnotes
Abbreviations: CAD = coronary artery disease, CIs = confidence intervals, HRs = hazard ratios, ICH = intracranial hemorrhage, NHIRD = National Health Insurance Research Database, TBI = traumatic brain injury.
I-CL and C-HK equally contributed to this article.
W-SL was supported by grants from Taiwan's Ministry of National Defense (MAB-104-048). This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Author contributions: All authors have substantially contributed to the study and are in agreement with the content of the manuscript—conception/design: W-SL and C-HK; provision of study materials: C-HK; collection and assembly of data: W-SL C-LL, and C-HK; data analysis and interpretation: all authors; manuscript preparation: all authors; final approval of manuscript: all authors.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 2013; 9:231–236. [DOI] [PubMed] [Google Scholar]
- 2.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008; 7:728–741. [DOI] [PubMed] [Google Scholar]
- 3.Lok J, Leung W, Murphy S, et al. Intracranial hemorrhage: mechanisms of secondary brain injury. Acta Neurochir Suppl 2011; 111:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu HM, Huang SC, Hattori N, et al. Subcortical white matter metabolic changes remote from focal hemorrhagic lesions suggest diffuse injury after human traumatic brain injury. Neurosurgery 2004; 55:1306–1317. [DOI] [PubMed] [Google Scholar]
- 5.Fan L, Young PR, Barone FC, et al. Experimental brain injury induces differential expression of tumor necrosis factor-alpha mRNA in the CNS. Brain Res Mol Brain Res 1996; 36:287–291. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Polo JR, Rea HC, Johnson KM, et al. Inflammatory consequences in a rodent model of mild traumatic brain injury. J Neurotrauma 2013; 30:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tousoulis D, Psarros C, Demosthenous M, et al. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J Am Coll Cardiol 2014; 63:2491–2502. [DOI] [PubMed] [Google Scholar]
- 8.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011; 145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011; 12:204–212. [DOI] [PubMed] [Google Scholar]
- 10.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SH, Chiang TL. The effect of universal health insurance on health care utilization in Taiwan. Results from a natural experiment. JAMA 1997; 278:89–93. [DOI] [PubMed] [Google Scholar]
- 12.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med 2015. [DOI] [PubMed] [Google Scholar]
- 13.Knoblach SM, Faden AI. Cortical interleukin-1 beta elevation after traumatic brain injury in the rat: no effect of two selective antagonists on motor recovery. Neurosci Lett 2000; 289:5–8. [DOI] [PubMed] [Google Scholar]
- 14.Taupin V, Toulmond S, Serrano A, et al. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol 1993; 42:177–185. [DOI] [PubMed] [Google Scholar]
- 15.Fan L, Young PR, Barone FC, et al. Experimental brain injury induces expression of interleukin-1 beta mRNA in the rat brain. Brain Res Mol Brain Res 1995; 30:125–130. [DOI] [PubMed] [Google Scholar]
- 16.Shohami E, Gallily R, Mechoulam R, et al. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J Neuroimmunol 1997; 72:169–177. [DOI] [PubMed] [Google Scholar]
- 17.Das M, Mohapatra S, Mohapatra SS. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J Neuroinflammation 2012; 9:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu GX, Mueller M, Hawkins BE, et al. Traumatic brain injury in vivo and in vitro contributes to cerebral vascular dysfunction through impaired gap junction communication between vascular smooth muscle cells. J Neurotrauma 2014; 31:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontos HA, Wei EP, Povlishock JT, et al. Cerebral arteriolar damage by arachidonic acid and prostaglandin G2. Science 1980; 209:1242–1245. [DOI] [PubMed] [Google Scholar]
- 20.DeWitt DS, Mathew BP, Chaisson JM, et al. Peroxynitrite reduces vasodilatory responses to reduced intravascular pressure, calcitonin gene-related peptide, and cromakalim in isolated middle cerebral arteries. J Cereb Blood Flow Metab 2001; 21:253–261. [DOI] [PubMed] [Google Scholar]
- 21.Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 2015; 72:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsimikas S, Duff GW, Berger PB, et al. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a). J Am Coll Cardiol 2014; 63:1724–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duff GW. Peptide regulatory factors in non-malignant disease. Lancet 1989; 1:1432–1435. [DOI] [PubMed] [Google Scholar]
- 24.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010; 464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajamaki K, Lappalainen J, Oorni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 2010; 5:e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J 2014; 35:1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2010; 12:125–169. [DOI] [PubMed] [Google Scholar]
- 28.Lin HJ, Chen WL, Chen TH, et al. Vascular Endothelial Growth Factor-460 C/T BstUI Gene Polymorphism is associated with Primary Open Angle Glaucoma. Biomedicine (Taipei) 2014; 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee FY, Chen WK, Lin CL, et al. Carbon monoxide poisoning and subsequent cardiovascular disease risk: a nationwide population-based cohort study. Medicine (Baltimore) 2015; 94:e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh SR, Cheng WC, Su YM, et al. Molecular targets for anti-oxidative protection of green tea polyphenols against myocardial ischemic injury. Biomedicine (Taipei) 2014; 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]


