Abstract
The transanal eversion and prolapsing technique is a well-established procedure, and can ensure an adequate distal margin for patients with low rectal neoplasms. Potential leakage risks, however, are associated with bilateral dog ear formation, which results from traditional double-stapling anastomosis. The authors determined the feasibility of combining these techniques with a commercial stapling set to achieve a nondog ear (end-to-end) anastomosis for patients with mid- and distal rectal neoplasms.
Patients with early-stage (c/ycT1–2N0), mid- to distal rectal neoplasms and good anal sphincter function were included in this study. Laparoscopic low anterior resection was performed with a standard total mesorectal excision technique downward to the pelvic floor as low as possible. The bowel was resected proximal to the lesion with an endoscopic linear stapler. An anvil was inserted extracorporeally into the proximal colon via an extended working pore. The distal rectum coupled with the lesion was prolapsed and everted out of the anus. The neoplasm was resected with a sufficient margin above the dentate line under direct sight. A transrectal anastomosis without dog ears was performed intracorporeally to reconstitute the continuity of the bowel.
Eleven cases, 6 male and 5 female patients, were included in this study. The mean operative time was 191 (129–292) minutes. The mean blood loss was 110 (30–300) mL. The median distal margin distance from the lower edge of the lesion to the dentate line was 1.5 (0.5–2.5) cm. All the resection margins were negative. Most patients experienced uneventful postoperative recoveries. No patient had anastomotic leak. Most patients had an acceptable stool frequency after loop ileostomy closure.
Our preliminary data demonstrated the safety and feasibility of achieving a sound anastomosis without risking potential anastomotic leakage because of dog ear formation.
INTRODUCTION
At the time of this writing, local excisions, including traditional transanal resection, endoscopic submucosal dissection, and transanal endoscopic microsurgery, are established approaches for selected patients with early-stage distal rectal cancer. Local excisions, however, should only be offered to patients with T1N0 lesions with favorable clinical and pathologic features.1 Owing to potential lymph node metastases (incidence range: 0%–3% for T1 sm1, up to 15% for T1 sm2–3 and up to 25% for T2 tumors),2–5 rectal resection with total mesorectal excision (TME) is the current gold standard for the treatment of mid- and distal rectal neoplasms.1,6,7 This technique offers the oncologic benefit of reduced local recurrence and increased 5-year survival rates when combined with neoadjuvant radiation therapy.8–10
Laparoscopic colorectal surgery has progressed steadily since Jacobs’ first introduction in 1990.11 Laparoscopic low anterior resection (Lap LAR) is an ideal, minimally invasive surgical procedure for mid- and distal rectal cancer. Compared with open surgery, the laparoscopic approach has similar surgical safety, resection completeness, resection margins, and improved in-hospital recovery.12–15 Recent studies of patients with nonlocally advanced rectal cancer have confirmed that both approaches have similar oncologic results, including local recurrence, disease-free survival and overall survival, after at least 3 years and up to 10 years.16–18
The prolapsing procedure for rectal cancer is also known as the eversion technique. It was first performed by Maunsel in 1892 (initially published in Lancet 1892: 2: 473–476).19 There are several classic modifications of this procedure, that is, the Welch, Bacon, and Parks procedures.20 Most, however, are applicable to lesions within the middle-third of the rectum. In an era without staplers, considerable postoperative morbidity and mortality occurred. As a consequence of modern surgical and anesthetic advancements and the introduction of the circular stapler for colorectal anastomoses, the number of sphincter-saving operations for patients with rectal cancer has increased greatly.21,22 More advances were achieved with the introduction of the double-stapling technique (DST).23–25
Double-stapling technique facilitates the feasibility of performing a sphincter-saving operation intracorporeally and laparoscopically.26–29 Moreover, with the introduction of intersphincteric resection,30–32 the possibility of sphincter preservation for patients with ultralow rectal cancer33,34 has increased steadily. Double-stapling technique, however, is inevitably associated with bilateral intersecting margins at the distal rectal stump (the so-called dog ears). The dog ears have been reported to be the weak spots and are associated with potential anastomotic leaks.24,35
In this preliminary study, we investigated the feasibility of a perineal staple set to achieve a nondog ear (end-to-end) anastomosis with a combined Lap LAR and eversion technique to treat mid- and distal rectal neoplasms. Our primary objectives were to report our technical compliance, surgical safety, and early postoperative outcomes.
PATIENTS AND METHODS
Patients
A total of 11 consecutive patients with rectal cancer were documented. All patients were surgically treated by 1 surgical team (Xinxiang Li, MD) between June 2014 and May 2015. Patients with nonlocally advanced rectal cancer (cT1–2N0 or ycT1–2N0), good anal sphincter function (Wexner continence score <4), small tumor (maximum diameter <4 cm, and <1/2 circumference), and mid- and distal tumor locations (3–8 cm above the dentate line) were included in this study. Patients with recurrent disease, previous large open surgery with severe abdominopelvic sticks, inflammatory bowel disease, or familial adenomatous polyposis were excluded. Written informed consent was obtained from all patients, and the study protocol was approved by the Medical Ethics Committee of Fudan University Shanghai Cancer Center.
Operative Technique
Our technical procedure included the 4 following main parts: transabdominal Lap LAR with TME; preparation of the proximal end of the colon with purse string; transanal specimen extraction, transection, and stapler preparation of the distal end; and transanal colorectal anastomosis.
The patient was placed supine in the modified lithotomy position, and 5 ports were inserted (Figure 1). The Trendelenburg position was used following abdominopelvic exploration. At the iliac trigone, the medial mesenteric attachments were incised using an ultrasonic activated scalper (Harmonic®, Johnson & Johnson, NJ), and the superior hypogastric nerve plexus was identified and preserved. Proximal lymph node dissection was performed at the origin of the superior rectal artery. The superior rectal artery was dissected and transected following the application of Hem-o-Lock clips (Teleflex Inc., USA). The mesorectum was separated with the TME technique, which is similar to previously described reports.28,36 The mesentery of the sigmoid colon was manipulated as little as possible. Toldt fusion fascia was moved posteriorly, and the gonadal vessels and ureters were preserved. The retrorectal space was entered, and the separation was continued to the “holy plane” extending to the rectosacral ligament. The right and left lateral attachments of the sigmoid colon were incised, and the sigmoid colon and rectum were mobilized. Posteriorly, dissection of the presacral space was performed from right to left and proceeded distally into the pelvis. Dissection of the anterior rectum was initiated by exposing Denonvilliers fascia with identification of the vaginal wall or seminal vesicles. Dissection was extended distally to expose the levator ani muscle. Lateral, posterior and anterior levels of dissection were checked to complete the circumferential dissection to the pelvic floor as low as possible (Figure 2).
FIGURE 1.
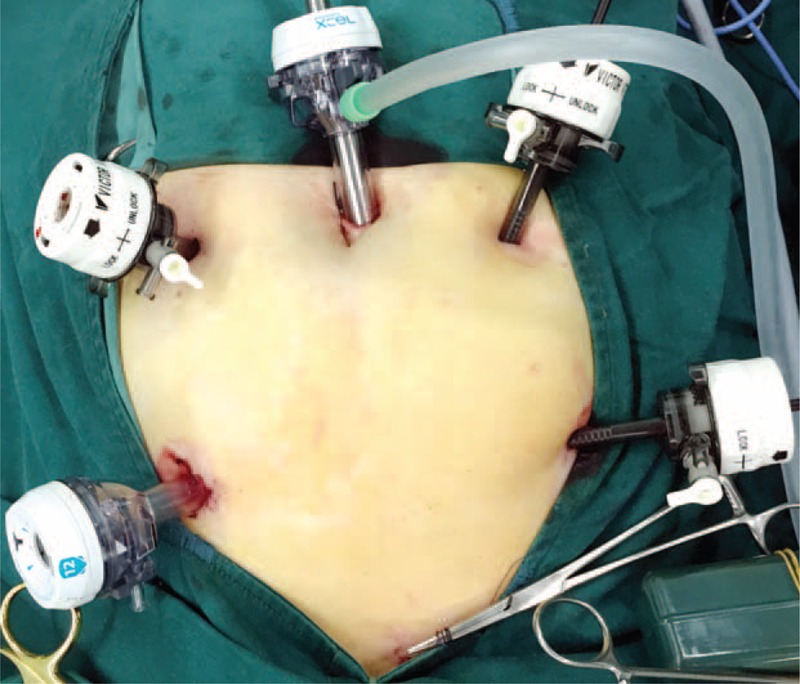
Five working ports were selected for the laparoscopic procedure. The bottom of the image presents the patient's caudal side. For a female patient, uterus hanging may be recommended. This can be fulfilled by placement of a 2–0 Prolene suture on the uterus. The stitch was then introduced outside the abdominal wall and fixed with adequate tension.
FIGURE 2.
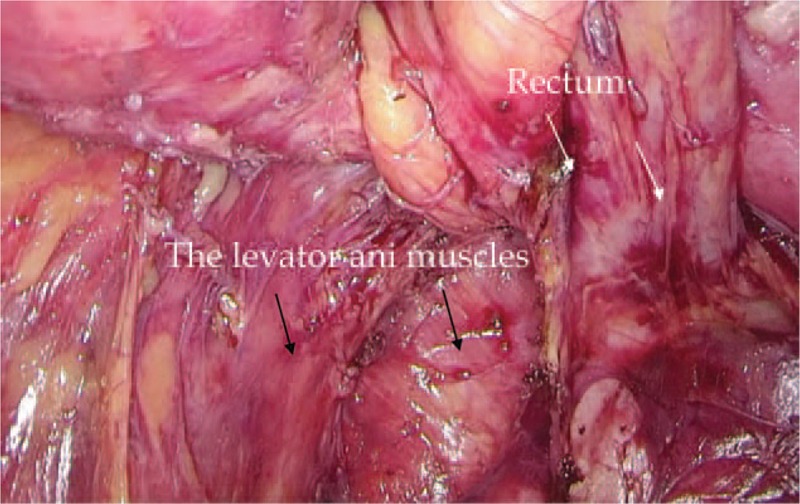
Dissection of the rectum. The rectum (white arrows) was dissected with the TME approach and proceeded circumferentially into the pelvic floor as distal as possible. The levator ani muscles were clearly displayed (black arrows).
The colon (10 cm proximal to the lesion) was transected laparoscopically using an endoscopic linear cutter (Ethicon® ET60a, Johnson & Johnson, NJ). The proximal colon was extracted through a small incision of 3 to 4 cm extended from the bottom-right pore, which was also used as the site of loop ileostomy (LI) formation. An extra 5 to 10 cm of proximal colon was resected under direct vision to ensure a safe proximal margin. An anvil from a 29- or 33-mm circular stapler (CSC-29 or CSC33, KOL®, Touchstone International, Suzhou, China) was secured to the colon with a purse string suture. Then, the proximal colon was returned, followed by closure of the abdominal wall and re-establishment of the pneumoperitoneum.
The rectum was irrigated with Betadine solution, and the anus was gently distended digitally by an assistant surgeon. A grasping forcep was slowly inserted through the anus, and the stump of the distal rectum was grasped securely with laparoscopic guidance. Although withdrawing the forcep, the distal rectal segment accompanied by the rectal neoplasm was everted gradually and pulled gently outside of the anus with the inner mucosa everted (Figure 3). The resection line (1–2 cm distal to the lower edge of the lesion) was plotted under direct vision. Transection of the rectum was performed with another firing of the same linear cutting stapler (mentioned above; Figure 4). The specimen was carefully inspected to ensure an adequate distal margin (Figure 5). Two 2–0 Prolene sutures (Ethicon®, Johnson & Johnson, NJ were secured on the both extremities of the resection line, which was created by the second linear cutter. The tails of the stitches were introduced through the windows (both side of the stapler), and the rectal stump including the suture line (dog ears) was completely loaded into the cartridge of the stapler (Figure 6).
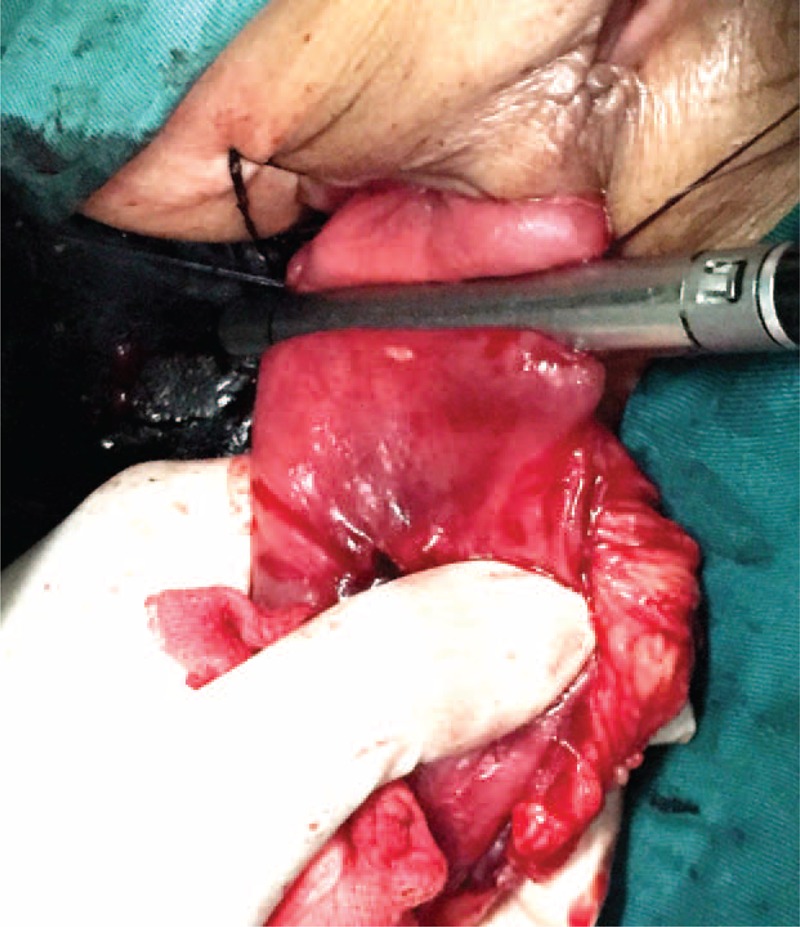
FIGURE 3.
The distal rectum was everted and pulled outside the anus. The inner mucosa and the lesion were everted. The distance from the lower edge of the lesion to the dentate line was determined under direct vision.
FIGURE 4.
Transection of the rectum. This was performed using another firing of a linear cutter under direct vision after an adequate distal margin was confirmed.
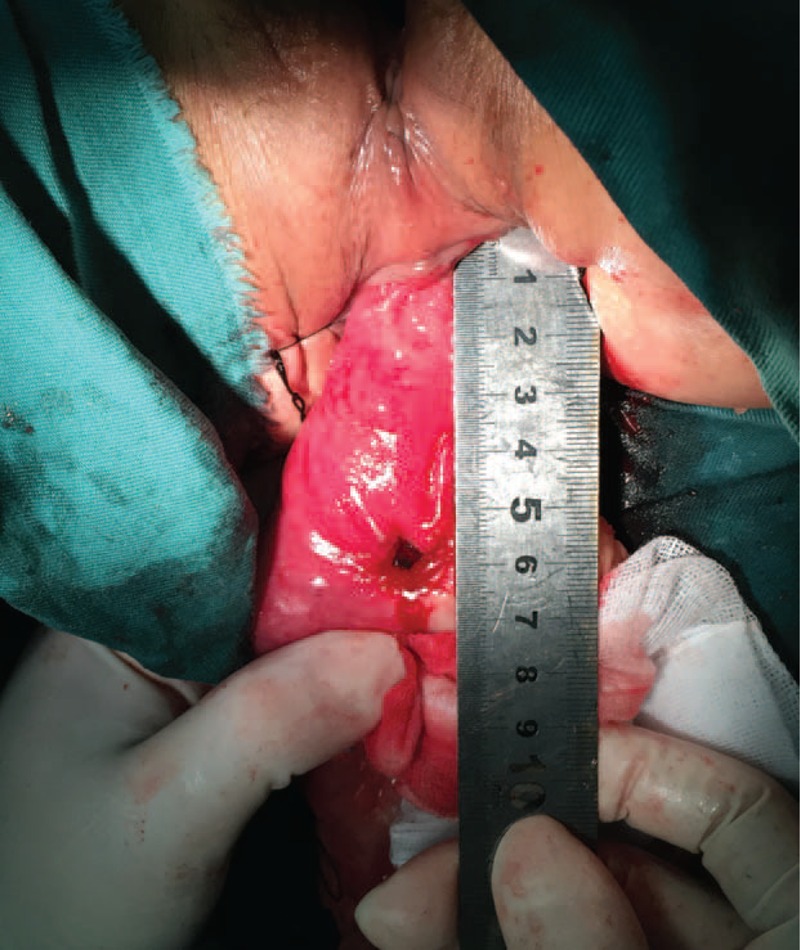
FIGURE 5.
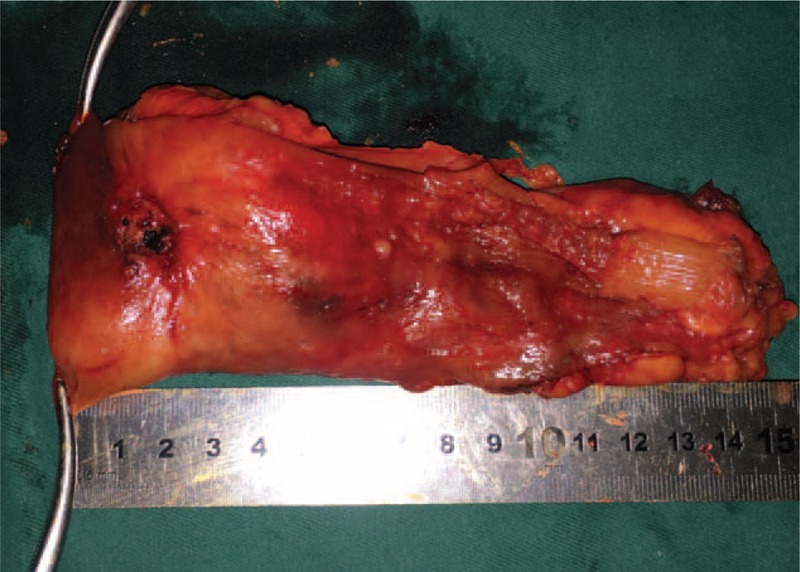
The specimen was removed and inspected. For this case, the distance from the lower edge of the lesion to the resection line was approximately 1 cm (ensuring an adequate distal margin). The margin was confirmed by pathologic examination.
FIGURE 6.
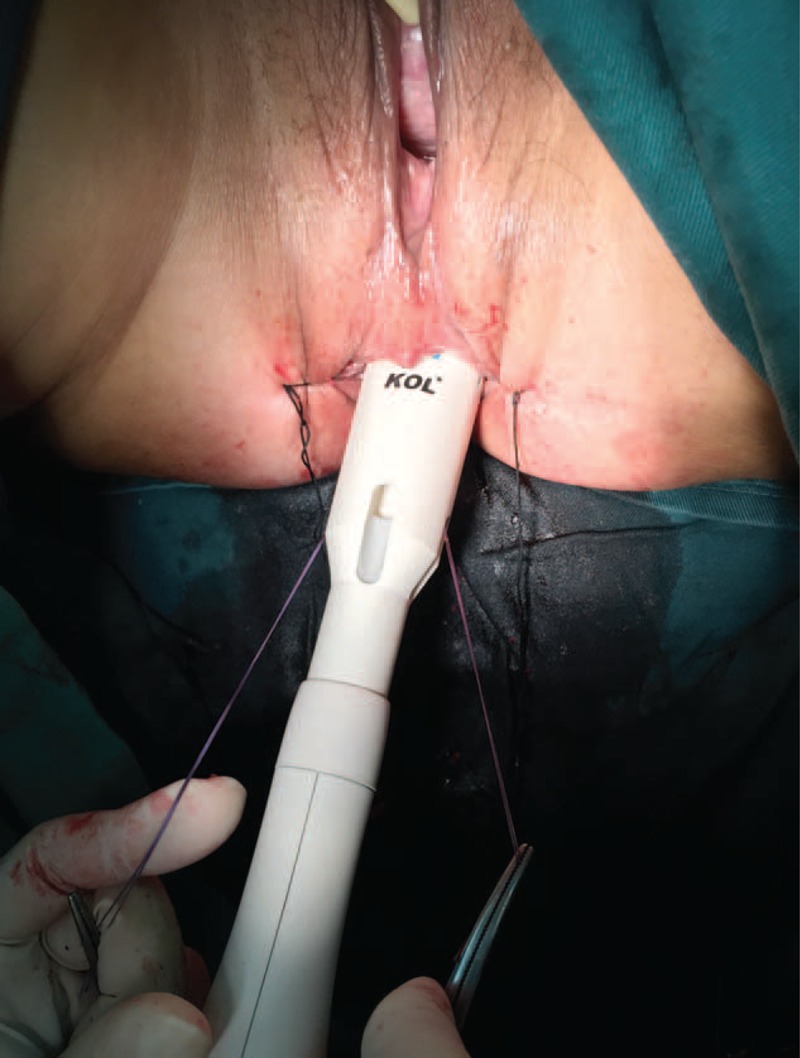
Preparation of the distal rectum. Two 2–0 Prolene sutures were placed on both extremities of the resection line, and the rectal stump including both “dog ears” of the resection line was completely loaded into the cartridge of the KOL® stapler by pulling the tails of the stitches through the windows on the instrument.
The distal rectum was returned gently to the pelvis through the anus. Intracorporeally, the anvil and center rod of the stapler were connected, the stapler was fired, and an end-to-end anastomosis was performed with laparoscopic guidance (Figure 7). The doughnuts were inspected carefully, and a routine transanal air leak test was performed. Following pelvic wash-out, a drainage tube was placed posteriorly to the anastomosis through the bottom-left working pore. For ultralow anastomoses, or for patients who received neoadjuvant therapy, a protecting LI was performed through the extended bottom-right working pore (Figure 8).
FIGURE 7.
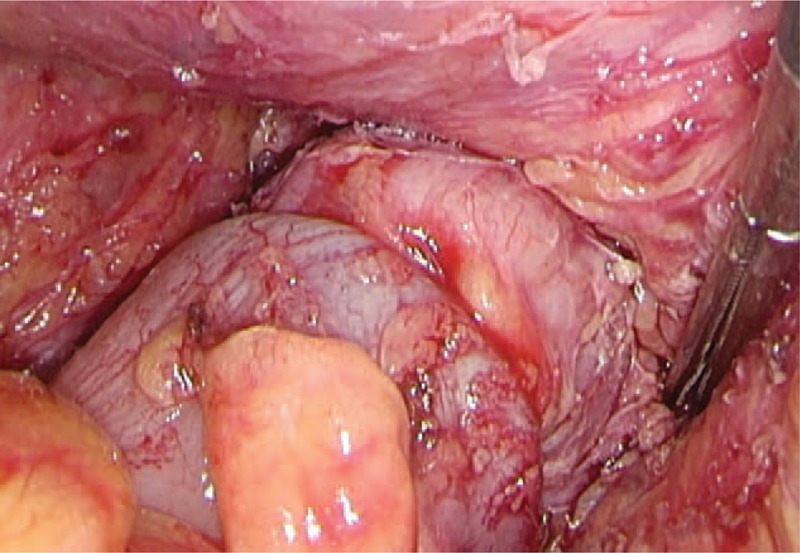
A nondog ear anastomosis was performed under laparoscopic guidance. Using the KOL® stapler set, an end-to-end colorectal anastomosis was completed for patients with ultralow rectal cancer using this combined lap laparoscopic low anterior resection and eversion technique.
FIGURE 8.
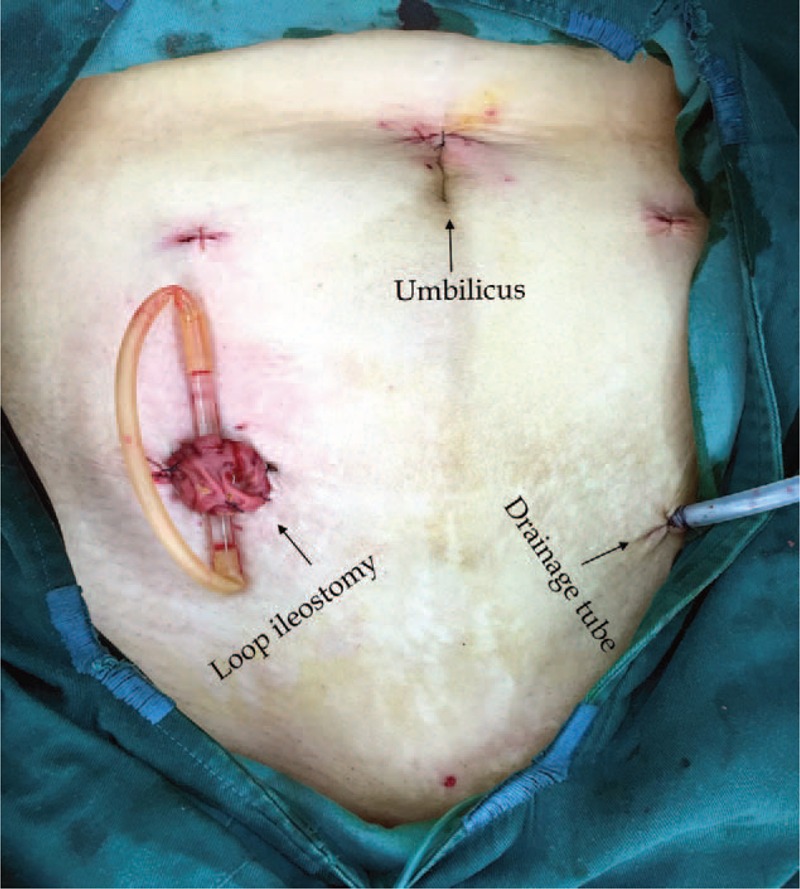
The abdominal appearance after surgery. The extended bottom-right working pore can be used to extract the proximal colon and perform a protecting loop ileostomy when required. The extended bottom-left working pore can be used to place a pelvic drainage tube.
Primary Evaluation Variables
We analyzed surgical safety, pathology outcomes, postoperative recovery, and early follow-up data for each patient (see below for details). Follow-up evaluations consisted of periodic physical and endoscopic examinations. The date of the last follow-up visit was May 15, 2015.
RESULTS
Patient Characteristics
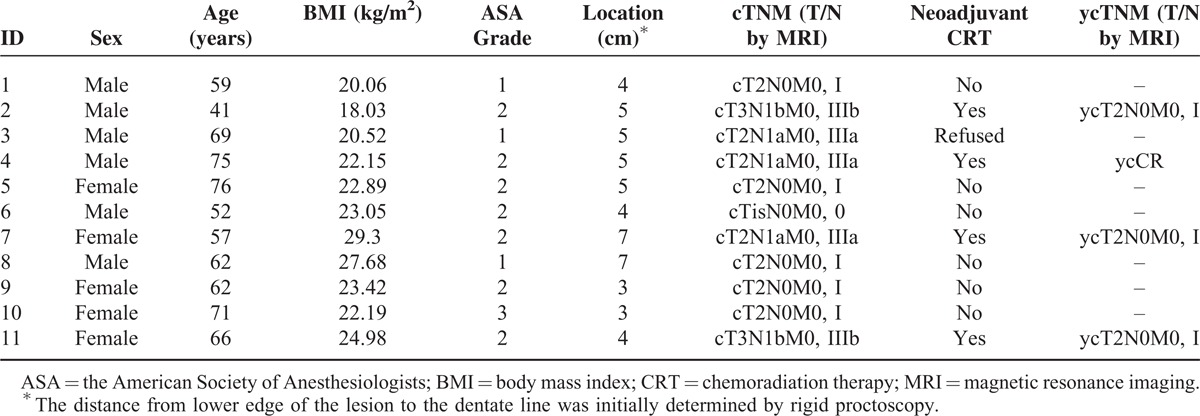
In approximately 1-year period, a total of 11 patients, 6 (54.5%) men and 5 women, underwent surgery. The median age of the patients was 62 (41–76) years. The median body mass index (BMI) was 22.89 (18.03–29.3) kg/m2. The patient characteristics are summarized in Table 1.
TABLE 1.
Patients’ Characteristics
Operative Outcomes
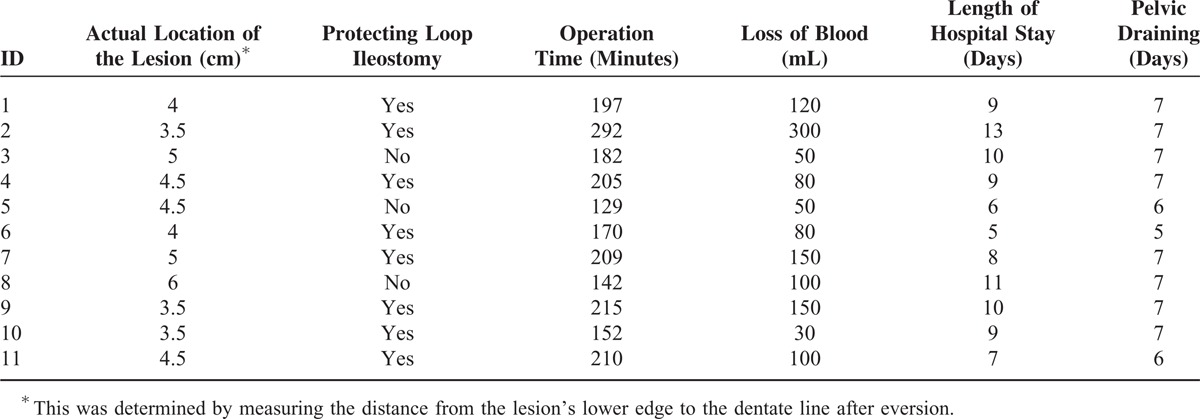
The actual location of the lesions was determined for each after eversion. The median distance from the lower edge of the lesion to the dentate line was 4.5 (3.5–6) cm. The mean operative time was 191 (129–292) minutes. The mean blood loss was 110 (30–300) mL. No patient required a blood transfusion. None had a positive result of air leak test after anastomosis. Eight of the 11 (77.2%) patients received a protecting LI. Other operative outcomes are presented in Table 2.
TABLE 2.
The Patient's Operative Outcomes
Pathologic Assessments
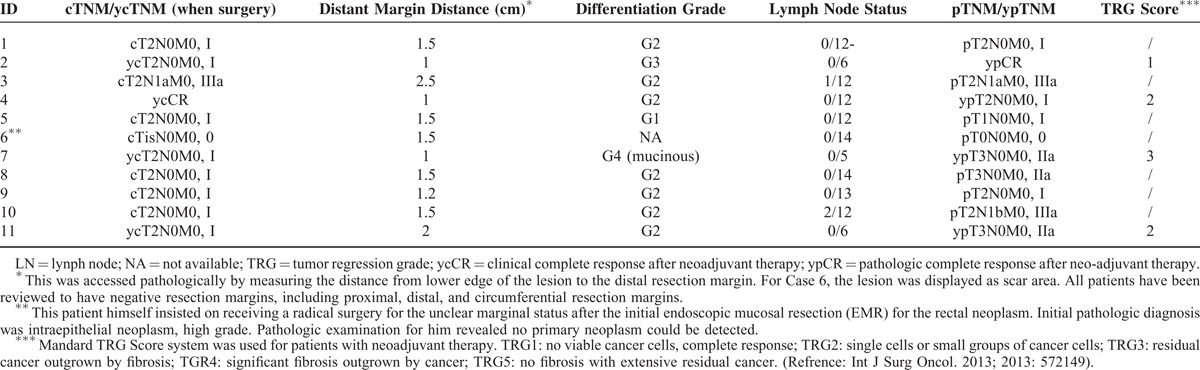
The median distance of distal margin was 1.5 (0.5–2.5) cm above from the dentate line. All of the resection margins, including the proximal, distal, and circumferential resection margins, were negative. All margins were negative for lymphovascular invasion, perineural invasion, and extranodal tumor deposits. The pathologic assessments, including pre- and postoperative TNM staging, are presented in Table 3.
TABLE 3.
Pathologic Assessments for Patients
Postoperative Complications and Early Follow-Up Data
The patients completed a median length of follow-up of 4.9 (0.2–11.2) months. Most patients had uneventful postoperative recoveries. No patient experienced anastomotic leaks or rupture after surgery. One patient (No. 2) experienced anastomotic bleeding on postoperative day 2, and he was treated with endoscopic hemostatic therapy. His LI had not been reversed till now owing to the moderate to severe radiation proctitis. Another patient (No. 7) developed mild anastomotic stricture 2 months following surgery and underwent endoscopic follow-up without any intervention. Most patients demonstrated an acceptable stool frequency following LI closure. Table 4 showed the postoperative recovery and early follow-up data. A representative endoscopic finding of the anastomosis 3 months after surgery (before LI closure) is presented for one patient (No. 6) (Figure 9).
TABLE 4.
Postoperative Complication and Other Follow-Up Data
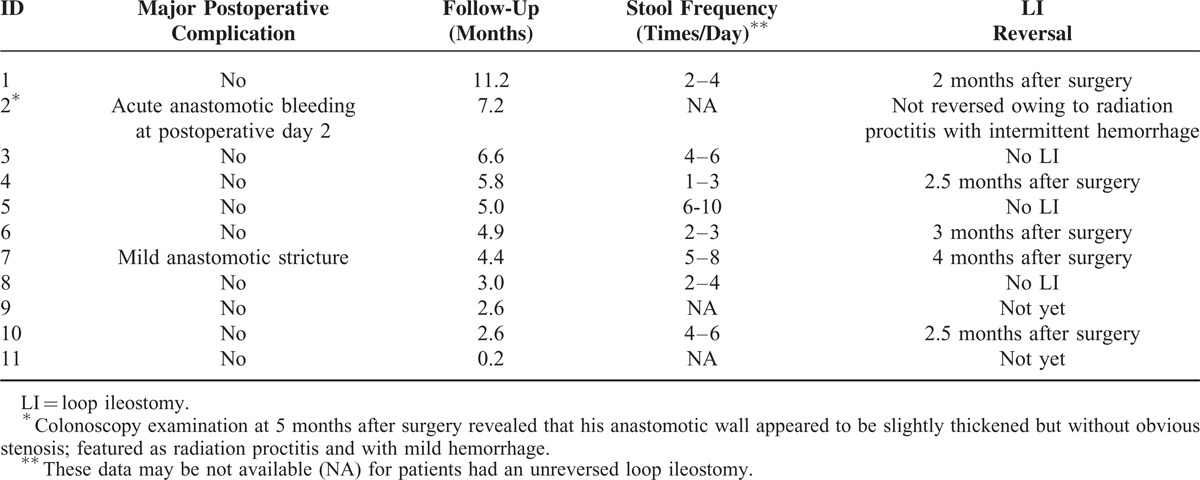
FIGURE 9.
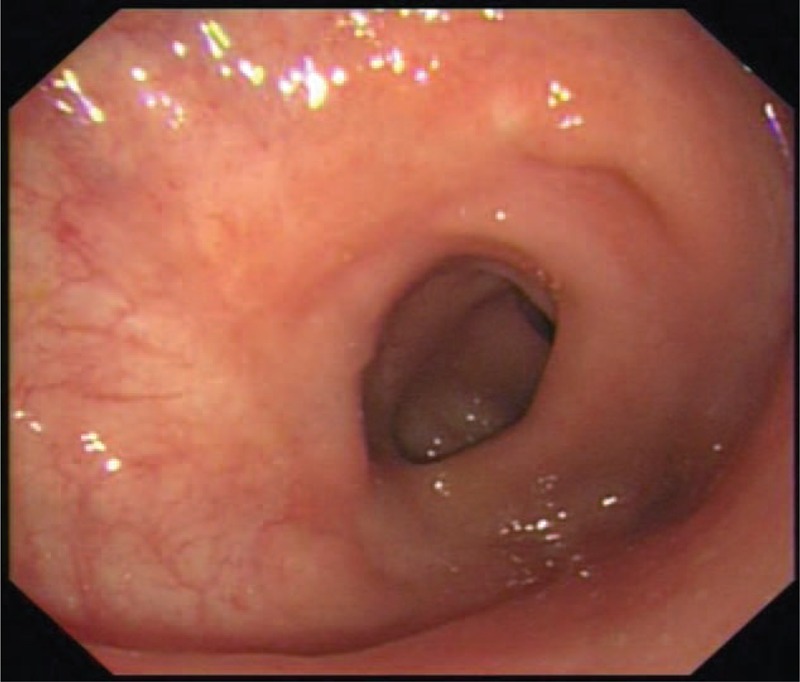
Representative endoscopic finding of the anastomosis after surgery. This was taken from a patient (No. 6) 3 months after surgery. A routine endoscopic examination was performed before the closure of his loop ileostomy. It revealed that the anastomotic wall appeared to be smooth, slightly stenosis but unobstructed.
DISCUSSION
Laparoscopic low and ultralow anterior resection coupled with the eversion technique has been used for decades and increases the possibility of sphincter preservation for patients with distal rectal cancer. Here, we report a combination of these techniques using a well-designed commercial stapling set. Our preliminary data confirmed the surgical safety and technical feasibility of achieving both an oncologically safe distal margin, and a sound end-to-end anastomosis without dog ear formation for patients with distal rectal cancer.
Owing to the potential risk of microscopic involvement of the rectal wall below the tumor, the distance from the lower edge of the rectal lesion to the anal verge has been considered to be the key factor in the decision-making process for sphincter-saving resection. According to general consensus, distal retrograde lymphatic spread is rare and a 1-cm distal margin is acceptable for small lesions without unfavorable histopathologic features.29,37–40 The transanal eversion and prolapse techniques can facilitate the identification of the resection line to ensure an adequate distal margin for ultralow rectal neoplasms.
At the lateral intersecting margins in the DST anastomosis, staples formed by linear cutter may be cut by circular cutter. This has been verified on swine models under radiography by Roumen et al.35 The authors also tested the burst pressures (BP) in 2 types of anastomoses (Group A: n = 35, circular anastomoses without dog ear formation; Group B: n = 32, double-stapled anastomoses with bilateral dog ears). Group A demonstrated significantly higher burst pressures than Group B (median, 90 versus 60 mmHg; P < 0.001). Remarkably, in Group B, 13 (40.6%) patients experienced their first disruption at the corner of a dog ear. In a subgroup of patients with low-lying rectal cancer, Zong et al41 also observed a significantly higher leakage rate when using DST compared with the improved Bacon procedure.
During open surgery, the dog ears formed by DST anastomoses can be safely sutured under direct vision, although it is technically impossible for patients with a narrow pelvis or an ultralow anastomosis.25 This repair may decrease the risk of developing an anastomotic event. It, however, is not feasible to perform a regular repair in laparoscopic surgery. During Lap LAR the multiple firings on the resection line are technically inevitable.42 This may cause tissue ischemia and contribute to anastomotic leakage.35,43–45
An end-to-end (nondog ears) anastomosis may decrease this risk caused by traditional DST anastomosis. Method that could avoid dog ear formation had been tested in a porcine model by Kvasha et al using a circular stapler (Autosuture, EEA, Covidien, CT). The authors demonstrated the feasibility of endolumenal excision of a substantial length of bowel and transanal natural orifice specimen extraction without rectal stump opening. A mass associated with the resected bowel, however, could hinder the completion of eversion procedure on human subjects.46,47 The double housing volume of KOL® stapler design ensures the complete resection of the 2 suture lines (of the proximal colon and the rectal stump). Therefore it eliminates the potential “dog ears” following full-thickness end-to-end anastomosis (Figure 6).
Meng et al48 reported a successful synchronous transanal endoscopic microsurgery-TME procedure on patients with narrow pelvic opening and bulky rectal tumors by using the KOL® staple set. The intralumenal purse-string installation of the rectal stump via a circular anal dilator seemed to be time consuming. Recently, Crafa et al49 also presented their experience in avoiding dog ear formation in low colorectal anastomoses using the same staple set. After resection of the rectum distally (not proximally) to the lesion, transanal placement of 4 sutures on the rectal stump was performed intracorporeally. Then the rectum was everted and the remaining steps were similar to ours. The procedure they described, however, seemed to be technically challenging in laparoscopy setting and not applicable to patients with an ultralow rectal tumor.
For patients with mid to distal rectal cancer with deep tumor infiltration (T3–4) or positive lymph node involvement (N1–2), radiation or chemoradiation therapy before surgery is recommended. The neoadjuvant therapy can reduce the locoregional recurrence10,50 and enhance the possibility of sphincter preservation.51,52 In the current study, 5 patients were cT3 or LN-positive (N1a or 1b). Four of them were treated with neoadjuvant therapy, and most patients underwent this combined procedure successfully. Moreover, the eversion procedure helps to detect the reduced lesion more clearly under direct vision for patients after neoadjuvant therapy. This is especially important for those who only has a “scar-liking” lesion or clinical complete response after neoadjuvant therapy (ycCR).
In the current report, most patients had a normal BMI value (Table 1). Our preliminary data indicates that this combination technique is a safe procedure. This technique may be applicable for patients with a narrow pelvis, difficult anastomosis location, or even patients requiring a salvage technique following stapling failure. The eversion operation, however, may not be applicable to obese patients with hypertrophic mesorectum or patients with bulky tumors.
One of the disadvantage of the eversion technique and sphincter saving surgery for patients with an ultralow rectal cancer is that it may lead to poor defecation control after surgery. Furthermore, during prolapse procedure, the mucosal epithelium 1 to 2 cm above the dentate line should be retained carefully because many epithelial sensory nerve endings in this area. Importantly, elderly patients or patients with preoperative anal dysfunction should not undergo sphincter preservation.
One of the limitations of this study was the lack of endoscopic ultrasound for T staging. Instead, we used magnetic resonance imaging examinations for both T and N preoperative staging. This staging approach underestimates (4/11) the T stage and overall TNM staging (No. 7, 8, 10, and 11, see Table 3). Guillem et al,53 however, suggested that although some patients may be overstaged (and thus overtreated), more patients would be understaged and require postoperative chemoradiation therapy. Another limitation was that the current preliminary study was underpowered with only 11 patients and definitive conclusions were not possible. We are establishing a randomized clinical trial to investigate the risk of leaks between anastomoses with and without dog ears, and we look forward to reporting these results in the near future.
In summary, Lap LAR with TME coupled with the synchronous pull-through technique are well-established procedures for patients with low or ultralow rectal neoplasms. We combined these currently available techniques with a commercial stapling set to achieve an oncologically safe distal margin. Our preliminary data also confirmed the technical feasibility of achieving a sound end to end anastomosis without dog ear formation.
Footnotes
Abbreviations: ASA = the American Society of Anesthesiologists, DST = double-stapling technique, Lap LAR = laparoscopic low anterior resection.
CZ and LL have contributed equally to this work.
This research was supported by grants from the Medical Innovation Program of Fujian Province (No. 2015-CXB-6 to Changhua Zhuo), the National Natural Science Foundation of China (No. 51377024 to Xinxiang Li), and the Research Project of Shanghai Science and Technology Commission (No.13JC1407202 and No. 15411968100 to Xinxiang Li).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no conflict of interest.
REFERENCES
- 1.Morino M, Risio M, Bach S, et al. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc 2015. 755–773. [DOI] [PubMed] [Google Scholar]
- 2.Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002; 45:200–206. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto S, Watanabe M, Hasegawa H, et al. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepato-gastroenterology 2004; 51:998–1000. [PubMed] [Google Scholar]
- 4.Smith KJ, Jones PF, Burke DA, et al. Lymphatic vessel distribution in the mucosa and submucosa and potential implications for T1 colorectal tumors. Dis Colon Rectum 2011; 54:35–40. [DOI] [PubMed] [Google Scholar]
- 5.Saraste D, Gunnarsson U, Janson M. Predicting lymph node metastases in early rectal cancer. Eur J Cancer (Oxford, England: 1990) 2013; 49:1104–1108. [DOI] [PubMed] [Google Scholar]
- 6.Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. Br J Surg 1995; 82:1297–1299. [DOI] [PubMed] [Google Scholar]
- 7.Ridgway PF, Darzi AW. The role of total mesorectal excision in the management of rectal cancer. Cancer Control 2003; 10:205–211. [DOI] [PubMed] [Google Scholar]
- 8.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345:638–646. [DOI] [PubMed] [Google Scholar]
- 9.Murty M, Enker WE, Martz J. Current status of total mesorectal excision and autonomic nerve preservation in rectal cancer. Semi Surg Oncol 2000; 19:321–328. [DOI] [PubMed] [Google Scholar]
- 10.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991; 1:144–150. [PubMed] [Google Scholar]
- 12.Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010; 11:637–645. [DOI] [PubMed] [Google Scholar]
- 13.van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013; 14:210–218. [DOI] [PubMed] [Google Scholar]
- 14.Arezzo A, Passera R, Scozzari G, et al. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc 2013; 27:1485–1502. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy RH, Francis EA, Wharton R, et al. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol 2014; 32:1804–1811. [DOI] [PubMed] [Google Scholar]
- 16.Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 2013; 100:75–82. [DOI] [PubMed] [Google Scholar]
- 17.Vennix S, Pelzers L, Bouvy N, et al. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 2014; 4:Cd005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372:1324–1332. [DOI] [PubMed] [Google Scholar]
- 19.Corman ML. Classic articles in colonic and rectal surgery. A new method of excising the two upper portions of the rectum and the lower segment of the sigmoid flexure of the colon: by H. Widenham Maunsell. Dis Colon Rectum 1981; 24:649–654. [DOI] [PubMed] [Google Scholar]
- 20.Bennett RS. The place of pull-through operations in treatment of carcinoma of the rectum. Dis Colon Rectum 1976; 19:420–424. [DOI] [PubMed] [Google Scholar]
- 21.Di Betta E, D’Hoore A, Filez L, et al. Sphincter saving rectum resection is the standard procedure for low rectal cancer. Int J Colorectal Dis 2003; 18:463–469. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Choi G-S, Park J, et al. Comparison of intracorporeal single-stapled and double-stapled anastomosis in laparoscopic low anterior resection for rectal cancer: a case-control study. Int J Colorectal Dis 2013; 28:149–156. [DOI] [PubMed] [Google Scholar]
- 23.Varma JS, Chan AC, Li MK, et al. Low anterior resection of the rectum using a double stapling technique. Br J Surg 1990; 77:888–890. [DOI] [PubMed] [Google Scholar]
- 24.Fu CG, Muto T, Masaki T. Results of the double stapling procedure in colorectal surgery. Surg Today 1997; 27:706–709. [DOI] [PubMed] [Google Scholar]
- 25.Sato H, Maeda K, Hanai T, et al. Modified double-stapling technique in low anterior resection for lower rectal carcinoma. Surg Today 2006; 36:30–36. [DOI] [PubMed] [Google Scholar]
- 26.Heald RJ. The “Holy Plane” of rectal surgery. J R Soc Med 1988; 81:503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe M, Teramoto T, Hasegawa H, et al. Laparoscopic ultralow anterior resection combined with per anum intersphincteric rectal dissection for lower rectal cancer. Dis Colon Rectum 2000; 43:S94–97. [DOI] [PubMed] [Google Scholar]
- 28.Fukunaga M, Kidokoro A, Iba T, et al. Laparoscopy-assisted low anterior resection with a prolapsing technique for low rectal cancer. Surg Today 2005; 35:598–602. [DOI] [PubMed] [Google Scholar]
- 29.Fu CG, Gao XH, Wang H, et al. Treatment for early ultralow rectal cancer: pull-through intersphincteric stapled transection and anastomosis (PISTA) versus low anterior resection. Tech Coloproctol 2013; 17:283–291. [DOI] [PubMed] [Google Scholar]
- 30.Schiessel R, Karner-Hanusch J, Herbst F, et al. Intersphincteric resection for low rectal tumours. Br J Surg 1994; 81:1376–1378. [DOI] [PubMed] [Google Scholar]
- 31.Ito M, Saito N, Sugito M, et al. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis Colon Rectum 2009; 52:64–70. [DOI] [PubMed] [Google Scholar]
- 32.Han JG, Wei GH, Gao ZG, et al. Intersphincteric resection with direct coloanal anastomosis for ultralow rectal cancer: the experience of People's Republic of China. Dis Colon Rectum 2009; 52:950–957. [DOI] [PubMed] [Google Scholar]
- 33.Hohenberger W, Merkel S, Matzel K, et al. The influence of abdomino-peranal (intersphincteric) resection of lower third rectal carcinoma on the rates of sphincter preservation and locoregional recurrence. Colorectal Dis 2006; 8:23–33. [DOI] [PubMed] [Google Scholar]
- 34.Yamada K, Ogata S, Saiki Y, et al. Long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum 2009; 52:1065–1071. [DOI] [PubMed] [Google Scholar]
- 35.Roumen RM, Rahusen FT, Wijnen MH, et al. Dog ear formation after double-stapled low anterior resection as a risk factor for anastomotic disruption. Dis Colon Rectum 2000; 43:522–525. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe M. Milsom JW, Böhm B, Nakajima K, Tonohira Y. Laparoscopic Anterior Resection for Rectal Cancer. Laparoscopic colorectal surgery 2nd ed.New York: Springer; 2006. 170–187. [Google Scholar]
- 37.Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients’ survival. Br J Surg 1983; 70:150–154. [DOI] [PubMed] [Google Scholar]
- 38.Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg 1983; 198:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rullier E, Laurent C, Bretagnol F, et al. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg 2005; 241:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tjandra JJ, Kilkenny JW, Buie WD, et al. Practice parameters for the management of rectal cancer (revised). Dis ColonRectum 2005; 48:411–423. [DOI] [PubMed] [Google Scholar]
- 41.Zong L, Chen P, Kitano S, et al. Clinical experience and analysis of laparoscopic total mesorectal excision combined with improved bacon for the treatment of lower rectal cancer. Hepato-gastroenterology 2011; 58:1538–1544. [DOI] [PubMed] [Google Scholar]
- 42.Kuroyanagi H, Oya M, Ueno M, et al. Standardized technique of laparoscopic intracorporeal rectal transection and anastomosis for low anterior resection. Surgical Endosc 2008; 22:557–561. [DOI] [PubMed] [Google Scholar]
- 43.Leroy J, Jamali F, Forbes L, et al. Laparoscopic total mesorectal excision (TME) for rectal cancer surgery: long-term outcomes. Surgical Endosc 2004; 18:281–289. [DOI] [PubMed] [Google Scholar]
- 44.Ito M, Sugito M, Kobayashi A, et al. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis 2008; 23:703–707. [DOI] [PubMed] [Google Scholar]
- 45.Kawada K, Hasegawa S, Hida K, et al. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surgical Endosc 2014; 28:2988–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kvasha A, Kvasha V, Hadary A, et al. Endolumenal rectal resection and transanal natural orifice specimen extraction (NOSE) without rectal stump opening: a novel, clean surgical technique in a porcine model. Surg Innov 2013; 20:454–458. [DOI] [PubMed] [Google Scholar]
- 47.Kvasha A, Hadary A, Biswas S, et al. Novel totally laparoscopic endolumenal rectal resection with transanal natural orifice specimen extraction (NOSE) without rectal stump opening: a modification of our recently published clean surgical technique in a porcine model. Surgical Innov 2015; 22:245–251. [DOI] [PubMed] [Google Scholar]
- 48.Meng W, Lau K. Synchronous laparoscopic low anterior and transanal endoscopic microsurgery total mesorectal resection. MITAT 2014; 23:70–73. [DOI] [PubMed] [Google Scholar]
- 49.Crafa F, Megevand J, Romano G, et al. New double-stapled anastomotic technique to avoid crossing staple lines. Techniques Coloproctol 2015; 19:319–320. [DOI] [PubMed] [Google Scholar]
- 50.Rahbari NN, Elbers H, Askoxylakis V, et al. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol 2013; 20:4169–4182. [DOI] [PubMed] [Google Scholar]
- 51.Wagman R, Minsky BD, Cohen AM, et al. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Phys 1998; 42:51–57. [DOI] [PubMed] [Google Scholar]
- 52.Luna-Perez P, Rodriguez-Ramirez S, Hernandez-Pacheco F, et al. Anal sphincter preservation in locally advanced low rectal adenocarcinoma after preoperative chemoradiation therapy and coloanal anastomosis. J Surg Oncol 2003; 82:3–9. [DOI] [PubMed] [Google Scholar]
- 53.Guillem JG, Diaz-Gonzalez JA, Minsky BD, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol 2008; 26:368–373. [DOI] [PubMed] [Google Scholar]


