Supplemental Digital Content is available in the text
Abstract
The overall survival of patients with multiple myeloma (MM) has been improved greatly over the last 2 decades with the broader use of novel drugs and autologous tandem transplantation. However, more than one tenth of myeloma patients still die shortly after diagnosis. We therefore aim to investigate the risk factors of early mortality (death within 60 days after diagnosis) in patients with MM.
We included in this study 451 consecutive patients with MM, newly diagnosed at an Asian tertiary medical center between January 1, 2002 and April 30, 2015. A total of 57 subjects who experienced early mortality were identified. Risk factors for early mortality in myeloma patients were collected and analyzed.
Early mortality occurred in 57 (12.6%) of the myeloma patients. In the multivariate analysis, being male (adjusted OR 2.93, 95% CI 1.17–7.31), serum albumin < 3.5 g/dL (adjusted OR 2.71, 95% CI 1.09–6.74), primary plasma cell leukemia (adjusted OR 17.61, 95% CI 1.01–306.05), serum albumin (adjusted OR 2.70, 95% CI 1.15–6.38), corrected serum calcium ≥ 12 mg/dL (adjusted OR 2.94, 95% CI 1.21–7.14), and LDH ≥ 250 U/L (adjusted OR 3.07, 95% CI 1.50–6.27) were identified as independent risk factors of early mortality. Pneumonia with other infections contributed most to early mortality (n = 36, 65%), followed by renal failure and cardiac failure.
The early mortality rate is high (12.6%) in patients with MM. Patients who are male and those with primary plasma cell leukemia, low serum albumin, high-corrected serum calcium, or LDH are at risk of early mortality. Nearly two thirds of the myeloma patients who experienced early mortality in our study (37 of 57, 65%) died of infection. Once a high-risk group is identified, much effort is required to target new approaches for prevention, early detection, and treatment of infections.
INTRODUCTION
Multiple myeloma (MM), a neoplasm of plasma cells, is the second most common B-cell malignancy in Western countries, accounting for more than 10% of hematologic malignancies in the United States.1 Each year, MM affects 4 to 5 per 100,000 individuals worldwide.2 The incidence of MM in Taiwan has also dramatically increased in recent years.3 Having undergone a paradigm shift with the routine use of immunomodulatory drugs and proteasome inhibitors as the standard of care for induction therapy, in combination with autologous hematopoietic stem cell transplantation as consolidation for eligible patients, the life expectancy of MM patients has been improved significantly over the last 2 decades.4 The Mayo Clinic estimated that the median survival of myeloma patients is 8 years, and improvements have occurred not only during the early stages of the disease but also throughout the disease course.5
Several studies have reported that this improvement in survival can be ascribed to the broader use of novel drugs and autologous stem cell transplantation.6 However, the impact of these interventions on early mortality is less well known. Kumar et al5 reported a high incidence of early mortality, in which approximately 25% of MM patients die within the 1st 3 years of their disease and 10% within the first year of diagnosis. Many of these patients, thus, cannot reap the benefits of novel antitumor therapies.
Current staging systems of MM are used for predicting overall survival. However, MM is a heterogeneous disease and the survival duration ranges from a few months to more than 10 years.7 Therefore, previous studies have identified several parameters as independent risk factors for predicting early mortality, instead of for predicting overall survival.8–10 Combined effects of active disease and comorbidity factors are also found to exacerbate early mortality in myeloma patients; however, they are not included in any of the myeloma scoring systems.10
There have been few studies or reports mentioning the incidence of early mortality, but no data are available for outlining factors for predicting early mortality of MM in Taiwan. In addition, neither such clinical randomized control trials nor large observational studies have been conducted. Moreover, since infection and renal failure are the leading causes of early mortality during induction therapy, accurate prediction by identifying prognostic features is urgently required.10 Therefore, it is very important to fully understand which factors contribute to early death in MM patients in order to identify risk groups of early mortality and optimize treatment accordingly. We therefore designed this large retrospective study to include patients newly diagnosed with symptomatic MM to examine this issue.
PATIENTS AND METHODS
Study Population
This study includes consecutive patients who were newly diagnosed with symptomatic MM between January 1, 2002 and April 30, 2015 at Taipei Veterans General Hospital. Follow-up was continued to June 30, 2015. MM was diagnosed using commonly accepted criteria,11 and all patients received bone marrow biopsies. Patients diagnosed with solitary plasmacytomas and smoldering myeloma were excluded.
Laboratory Studies
Data collection was performed by reviewing medical records. Clinical characteristics, including age, sex, laboratory parameters including plasma cells of bone marrow, hemoglobin, platelets, serum albumin, corrected serum calcium,5 serum creatinine, lactate dehydrogenase (LDH) and β2-microglobulin (β2M), performance status according to the Eastern Cooperative Oncology Group (ECOG) performance score,12,13 and diagnosis of primary plasma cell leukemia (PCL),14 were recorded at diagnosis. Cutoff values of serum hemoglobin, calcium, albumin, and β2M – 10 g/dL, 12 mg/dL, 3.5 g/dL, and 5.5 mg/mL, respectively – were chosen according to Durie–Salmon (DS) and International Staging System (ISS)7 criteria. Cutoff values of serum creatinine, platelets, and LDH were 2 mg/dL, 150,000/μ, and 250 U/L, respectively, which were correlated with early mortality in previous studies.10,15 Primary PCL is linked to increased risk of early mortality.4,14 The diagnosis of primary PCL is based upon the percentage (≥20%) and absolute number (≥2 × 109/L) of plasma cells in the peripheral blood.14 Clinical stages were determined based on the ISS and DS staging systems.7
Treatment regiments composed of induction treatment and bisphosphonates were collected. According to Kumar et al8, improved survival in recent years is mostly coupled with the increased use of novel agents, including thalidomide, lenalidomide, and bortezomib, as part of initial therapy. In our study, the patients were grouped into 2 diagnosis-year strata: a group from 2002 to 2008 and 2009 to 2015. The cutoff value was chosen because payment of bortezomib has been reimbursed since 2009 in Taiwan for treating myeloma patients. Our primary endpoint was early mortality, defined as death within 60 days after diagnosis.10 Retrospective review of medical records was conducted in accordance with an institutional ethics committee in agreement with the Helsinki Declaration of 1975, revised in 2008. This study has been approved by the Institutional Review Board at Taipei Veterans General Hospital (no. 2015-05-001B).
Statistical Analysis
Patients’ demographic and clinical characteristics with and without early mortality are presented as the total number (n) and proportion (%). We used Chi-square tests or Fisher exact tests to compare between-group rates of death within 60 days. Data are presented as medians and interquartile ranges for skewed data.
Odds ratios (ORs) and the 95% confidence interval (CI) were calculated using logistic regression models. We used multivariate logistic regression models to calculate ORs while adjusting for possible independent confounding factors. All risk factors with P < 0.1 in the univariate model were further entered into the multivariate analysis. To avoid immortal time bias, we used a multivariate Cox regression model with time-dependent variables to evaluate the effects of treatment on early mortality. Furthermore, this approach was used as sensitivity analysis for all-cause mortality within 30 days. Data management and all statistical analysis were performed using SAS 9.3 software (SAS Institute Inc., Cary, NC). All statistically significant levels were set at P < 0.05.
RESULTS
Clinical Characteristics of the Study Population
A total of 460 patients with MM diagnosed between January 1, 2002 and April 30, 2015 at Taipei Veterans General Hospital were identified. Patients who were diagnosed with solitary plasmacytomas (n = 5) and smoldering myeloma (n = 4) were excluded. Finally, 451 MM patients were enrolled in the study. The flow chart of patient selection is shown in Figure 1. The median age was 71 (range, 60–78 years), and 66.3% were male. According to ISS, 14.1, 37.6, and 48.3% had stages I, II, and III, respectively. Regarding another commonly used risk stratification, the DS staging system, 28.7%, 25.3%, and 46.0% of the patients had stages I, II, and III. The treatment which the studied population received in 60 days after diagnosis was categorized into cytotoxic agents, thalidomide, and bortezomib, which were 30.6%, 24.8%, and 20.2% of 451 patients, respectively. There was no significant difference of treatment approaches utilized between the patients of early mortality and the other. Clinical characteristics are shown in Table 1.
FIGURE 1.
Patient selection flow chart.
TABLE 1.
Baseline Patient Characteristics of Multiple Myeloma Patients
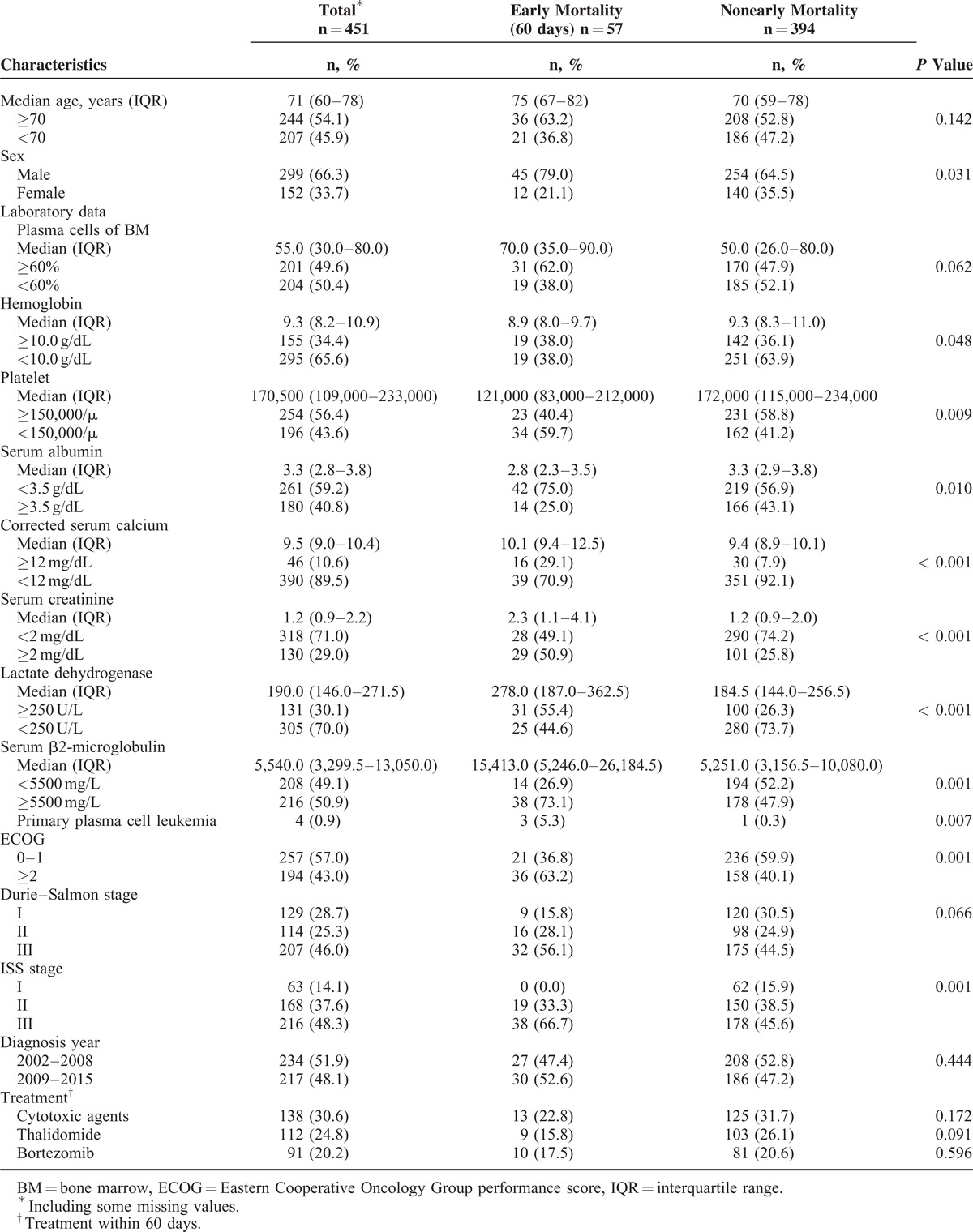
Features of Early Mortality
In this study, early mortality occurred in 57 (12.6%) of the myeloma patients. Compared with the nonearly-mortality myeloma patients, early-mortality patients had a higher probability of being male, having primary PCL, high plasma cells of BM, low hemoglobin, a low platelet count, low serum albumin, high corrected serum calcium, high serum creatinine, high LDH, high serum β2M, and high ISS stage (Table 1).
Risk Factors of Early Mortality
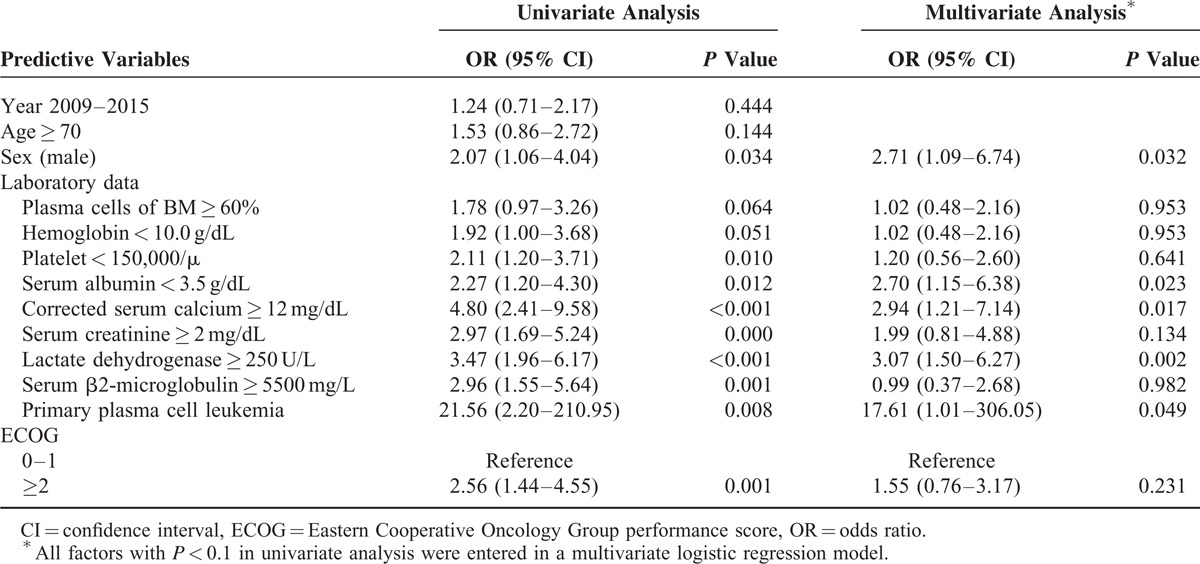
In the univariate analysis for age, sex, laboratory data, diagnosis year, primary PCL, and ECOG performance status, we found that being male, having primary PCL, plasma cells of BM ≥ 60%, hemoglobin < 10.0 g/dL, platelets < 150,000/μL, serum albumin < 3.5 g/dL, corrected serum calcium ≥ 12 mg/dL, serum creatinine ≥ 2 mg/dL, LDH ≥ 250 U/L, serum β2M ≥ 5500 mg/L, and ECOG ≥ 2 were significant predictors for early mortality in patients with MM. In the multivariate analysis, only being male (adjusted OR 2.71, 95% CI 1.09–6.74), having primary PCL (adjusted OR 17.61, 95% CI 1.01–306.05), serum albumin < 3.5 g/dL (adjusted OR 2.70, 95% CI 1.15–6.38), corrected serum calcium ≥ 12 mg/dL (adjusted OR 2.94, 95% CI 1.21–7.14), and LDH ≥ 250 U/L (adjusted OR 3.07, 95% CI 1.50–6.27) remained significant. The analysis is detailed in Table 2. We further analyzed the effects of treatment on early mortality by using a Cox regression model with therapeutic regimens as time-dependent variables. Cytotoxic agents, bortezomib, thalidomide or lenalidomide, and bisphosphonates were not independent predictors of early mortality (Supplemental Table 1).
TABLE 2.
Univariate and Multivariate Analysis of Factors Associated With Early Mortality (60 days) in Patients With Multiple Myeloma
Reasons for Early Mortality
Infection as the direct cause of early death occurred in 30 myeloma patients (52.6%) and contributed to death in nearly 65% of all cases (n = 37). Pneumonia directly caused death in 26 cases, accounting for 86% of the patients who died early of infection. Other infections related to early mortality included bacteremia (n = 4), urinary tract infections (n = 3), infectious colitis (n = 1), and intra-abdominal infections (n = 1). Although renal failure was documented as a direct cause of early mortality for only 2 cases, it, overall, was contributory to 24 early deaths, in which 21 cases (21 of 24, 87.5%) were associated with infections, and 17 patients (17 of 24, 70%) had chronic kidney disease upon diagnosis of myeloma. Bleeding occurred in 13 cases of early mortality: 10 of these involved gastrointestinal bleeding, 2 were pulmonary hemorrhage, and the remaining 1 was intracranial bleeding. Cardiac failure accounted for 8 cases (13%) of early death, and half of them (n = 4) had underlying congestive heart failure before myeloma diagnosis. Acute myocardial infarction was implicated in four patients (7%) and directly caused 3 early deaths. The causes of early mortality are shown in Figure 2.
FIGURE 2.
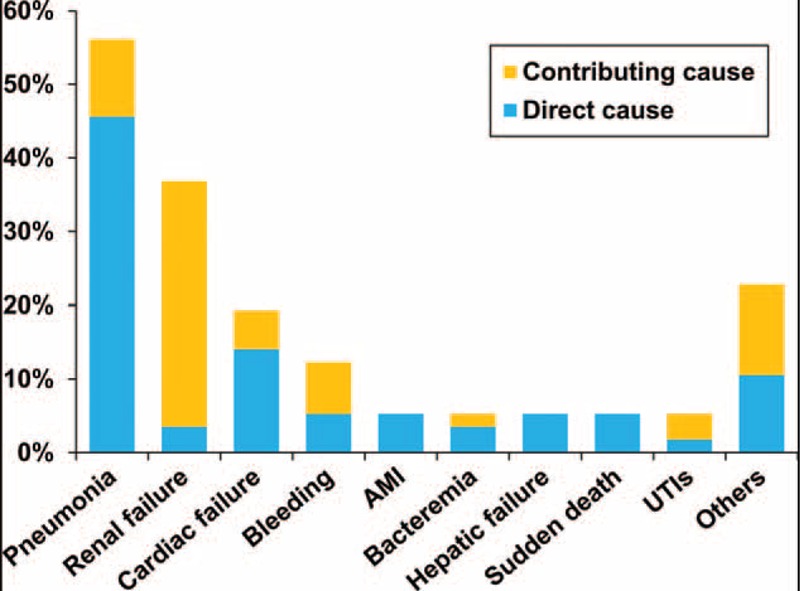
Contributing causes of early deaths (percentage of all deaths).
Sensitivity Analysis
We used a different definition of early mortality, 30-day mortality, for sensitivity analysis.8,9,16 The univariate analysis showed that being male, having primary PCL, platelets < 150,000/μL, corrected serum calcium ≥ 12 mg/dL, serum creatinine ≥ 2 mg/dL, LDH ≥ 250 U/L, and ECOG ≥ 2 were significant variables. Being male (adjusted OR 4.49, 95% CI 1.17–17.28) and LDH ≥ 250 U/L (adjusted OR 3.83, 95% CI 1.52–9.62) remained significant predictors for 30-day mortality in the multivariate analysis (Supplemental Table 2).
DISCUSSION
To the best of our knowledge, this is the 1st large cohort study to examine the risk factors associated with early mortality in patients newly diagnosed with MM in Asia, in which the diagnosis was also confirmed by thorough review of all records, and detailed information on disease characterization and primary treatment for individual patients was obtained. Our study reveals an increased risk of early mortality among male patients and those with low serum albumin, high corrected serum calcium, high LDH, and primary PCL, with an adjusted hazard ratio of 2.71, 2.70, 2.94, 3.07, and 17.61, respectively. Our results are in accordance with those from previous studies reporting on the association between early mortality and the serum level of albumin,8,17 corrected calcium,5 and LDH.8,9,18
Despite advances in supportive care, we found that up to 12.6% of MM patients died within 60 days after diagnosis. Augustson et al10 analyzed myeloma patients enrolled in variable clinical trials between 1980 and 2002 and reported a 60-day early death rate of 10%, which reflects the strict enrollment criteria of clinical trials.5 Conversely, the population included in our study provides a better real-world view since our National Health Insurance system enables patients to access treatment at tertiary medical centers in a timely fashion.
The levels of serum albumin, creatinine, and LDH reflect disease severity in symptomatic MM.7,19 Low serum albumin in MM is caused mainly by inflammatory cytokines, such as interleukin-6, secreted by the myeloma microenvironment.7,20,21 It also may reflect that myeloma patients who died early have a high degree of stress caused by severe infection, and impaired kidney and liver function.9 Increased LDH, which catalyzes the reversible transformation of pyruvate to lactate in the glycolysis pathway, denotes an aggressive disease and suggests a high proliferation rate and the presence of a tumor mass, in particular extramedullary and extraosseous disease.22–25 Several studies in the conventional chemotherapy era of myeloma treatment have shown that high LDH levels are associated with shorter overall survival.15,22,26,27 Also in the era of novel therapy, LDH still has its impact on survival.23 Costa et al4 reported that being male is a significant factor associated with early mortality in MM patients. In our study, compared to female patients, male patients were older (P = 0.01), had lower serum albumin (P = 0.022), higher serum creatinine (P = 0.028), and more advanced ECOG stages (P = 0.043) when analyzed using a Chi-square test. Being male remained significant, with an even higher OR after adjusting for age, serum albumin, serum creatinine, and ECOG.
β2M was identified in several studies as a significant risk factor associated with early mortality in myeloma patients.8–10,18 The serum concentration of β2M is related to not only renal function and disease burden but also other so-far-unknown parameters, possibly including immune function.7,20,28,29 Our study also shows, in the univariate analysis, that serum β2M is a potential prognostic marker associated with early mortality (OR = 2.96, P = 0.001). After adjusting for serum creatinine, however, the bone marrow plasma cell percentage and β2M became insignificant predictors in the multivariate analysis.
Holmstrom et al9 reported on the trend of improved survival when comparing death within 180 days in the periods 2005 to 2008 and 2009 to 2012 (P = 0.08). Kumar et al8 demonstrated that the proportion of MM patients who died within the 1st year in the period 2006 to 2010 (10%) was significantly lower than that in 2001 to 2005 (16%) (P = 0.01).8 The better outcomes were coupled closely with the use of new agents as initial therapy.30–32 The year of diagnosis (2002–2008 vs 2009–2015) showed no significance in predicting early mortality in MM patients.
We further analyzed the potential impact of front-line therapies on early mortality and found that neither cytotoxic agents nor novel drugs were associated with early death. It is probably because such deaths occurred even before cytotoxic agents or immunomodulatory drugs achieved their maximal beneficial effect of reducing tumor load. Moreover, compared with the earlier group, the latter group had a higher bone marrow plasma cell percentage (P = 0.047), lower serum albumin (P = 0.011), higher ISS stages, and suffered from more comorbidities, including diabetes mellitus (P = 0.05) and renal disease (P = 0.025), based on Chi-square analysis. These characteristics are related to early mortality,5,8,9 and they may therefore mask the improved outcome of novel therapies. Some studies have shown that subsets of myeloma patients receiving bisphosphonates saw improved overall survival.33–35 Furthermore, Morgan et al36 reported that zoledronic acid reduced mortality by 16% compared to that of clodronic acid. However, our study shows that these treatments were not independent predictors of early mortality.
PCL, a rare form of clonal plasma cell dyscrasia but the most aggressive variant of the human monoclonal gammopathies, was diagnosed in 4 patients in our cohort (0.88%), which was much lower compared with those from the Western world.37,38 A higher proportion of patients with PCL had significant leukocytosis, an elevated serum level of LDH and β2M than patients with MM.14 Costa et al4 reported that primary PCL is significantly linked to death within the 1st year after diagnosis. We also found that PCL was a risk factor for early mortality within 60 days, which might be explained by the above clinical features of PCL.
Our cohort found that pneumonia with other infections were the largest contributors to early death, followed by renal failure and cardiac failure, in myeloma patients, Teh et al39 found a bimodal peak in incidence of bacterial infection (4–6 and 70–72 months) following disease diagnosis, which coincides with progressive disease. It echoes the risk factors identified in this study that the more aggressiveness of the disease that is represented by PCL, LDH and albumin, and the higher incidence of infection-related early mortality. When a high-risk group is identified, much effort is required to target new approaches for prevention, early detection, and treatment of infections.
There is no consecutive definition for early mortality. Costa et al4 and Kumar et al5,8 defined it as 1 year, Murakami et al18 as 6 months, and Holmstrom et al9 as 30 days. In our sensitivity analysis, after the multivariate analysis, being male and having LDH ≥ 250 U/L were significant predictors for 30-day death; however, serum albumin, corrected calcium, and primary PCL were insignificant.
Our study has some limitations. The timing of initiating cancer treatment and the drugs chosen as front-line therapy depend on the judgment of clinicians in charge and thus it is difficult to analyze the impact of treatment on the development of early mortality without confounding factors or bias. It means that the role of treatment in the early death of myeloma patients is inconclusive and further study is needed to clarify it. Moreover, we did not perform interphase fluorescent in situ hybridization (iFISH) for every patient to analyze the chromosomal abnormalities, which were identified as a key element for defining biological features of MM.40 And, therefore, we did not adopt the latest published suggestion regarding revised prognostic stratification for newly diagnosed myeloma patients.41 Another limitation is the lack of ability to draw firm conclusions from these data, which is due to the retrospective nature of this study. These associations between the identified clinical parameters in this study and early mortality remain consistent after adjusting for potentially confounding factors, suggesting an independent predictive value of these serum parameters at diagnosis. Careful assessment of patient and disease characteristics, adjustment for known confounding factors, and sensitivity analysis produce a stable effect value. Nevertheless, it is impossible to completely exclude residual confounders in our study setting.
CONCLUSION
Despite the development of targeted novel therapies, MM remains incurable and significantly variable in regards to survival time due to tumor biology. It is important to find the prognostic factors and risk stratification to define treatment strategies. We herein identified the risk factors of early mortality, including being male (adjusted OR 2.71), having primary PCL (adjusted OR 17.61), serum albumin < 3.5 g/dL (adjusted OR 2.70), corrected serum calcium ≥ 12 mg/dL (adjusted OR 2.94), and LDH ≥ 250 U/L (adjusted OR 3.07), which highlights the inadequacy of the current prognostic system on the issue of early death of MM patients. Of great importance, infection contributes almost two thirds of early mortality, reflecting the immunoparesis nature of myeloma. Early intervention with antimicrobiological prophylaxis, as well as use of intravenous immunoglobulin at the very beginning of infection, should therefore be seriously considered for high-risk patients. However, more solid evidence from prospective studies is needed to support this point.
Supplementary Material
Acknowledgments
The authors thank Taipei Veterans General Hospital (V104B-023), the Taiwan Clinical Oncology Research Foundation, the Szu-Yuan Research Foundation of Internal Medicine, and the Chong Hin Loon Memorial Cancer and Biotherapy Research Center, National Yang-Ming University for the support. The funding sources had no role in the study design or conduct, or in the decision to submit it for publication.
Footnotes
Abbreviations: β2M = β2-microglobulin, CI = confidence interval, DS = Durie–Salmon, ECOG = Eastern Cooperative Oncology Group, IQR = interquartile range, ISS = International Staging System, LDH = lactate dehydrogenase, MM = multiple myeloma, OR = odds ratio, PCL = plasma cell leukemia.
HP and C-JL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. HP, Y-CL, and C-JL designed the study. C-MY, T-WL, and C-JL acquired the data and performed statistical analysis. A-SK, C-JT, H-HW, T-JC, and C-HT provided the final interpretation of the results. HP, T-WL, and C-JL drafted the manuscript. C-JT, H-HW, V-YS, Y-TC, S-HC, and L-YH made critical revisions to the manuscript for important intellectual content. C-MY, T-JC, and C-HT provided administrative, technical, and material support. T-JC, C-HT, and C-JL were the study supervisors. C-JL and Y-CL act as guarantors and accept responsibility for the integrity of the work as a whole. All authors have read and approved the final manuscript.
This study was supported by grants from Taipei Veterans General Hospital (V104B-023), the Taiwan Clinical Oncology Research Foundation, the Szu-Yuan Research Foundation of Internal Medicine, and the Chong Hin Loon Memorial Cancer and Biotherapy Research Center, National Yang-Ming University.
The funding sources had no role in the study design or conduct, or in the decision to submit it for publication
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: Cancer J Clin 2008; 58:71–96. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 3.Huang SY, Yao M, Tang JL, et al. Epidemiology of multiple myeloma in Taiwan: increasing incidence for the past 25 years and higher prevalence of extramedullary myeloma in patients younger than 55 years. Cancer 2007; 110:896–905. [DOI] [PubMed] [Google Scholar]
- 4.Costa LJ, Gonsalves WI, Kumar SK. Early mortality in multiple myeloma. Leukemia 2015. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S. Risk of early death in multiple myeloma. Clin Adv Hematol Oncol 2012; 10:172–174. [PubMed] [Google Scholar]
- 6.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111:2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23:3412–3420. [DOI] [PubMed] [Google Scholar]
- 8.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014; 28:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmstrom MO, Gimsing P, Abildgaard N, et al. Causes of early death in multiple myeloma patients who are ineligible for high-dose therapy with hematopoietic stem cell support: a study based on the nationwide Danish Myeloma Database. Am J Hematol 2015; 90:E73–E74. [DOI] [PubMed] [Google Scholar]
- 10.Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002 – Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005; 23:9219–9226. [DOI] [PubMed] [Google Scholar]
- 11.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121:749–757. [PubMed] [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655. [PubMed] [Google Scholar]
- 13.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984; 2:187–193. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez de Larrea C, Kyle RA, Durie BG, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 2013; 27:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suguro M, Kanda Y, Yamamoto R, et al. High serum lactate dehydrogenase level predicts short survival after vincristine-doxorubicin-dexamethasone (VAD) salvage for refractory multiple myeloma. Am J Hematol 2000; 65:132–135. [DOI] [PubMed] [Google Scholar]
- 16.Teng HW, Teng CJ, Wang WS, et al. High early mortality rate in elderly patients with multiple myeloma receiving a vincristine-doxorubicin-dexamethasone regimen. Am J Hematol 2010; 85:812–815. [DOI] [PubMed] [Google Scholar]
- 17.Binder M, Rajkumar SV, Gertz MA, et al. Predictors of early response to initial therapy in patients with newly diagnosed symptomatic multiple myeloma. Am J Hematol 2015. [DOI] [PubMed] [Google Scholar]
- 18.Murakami H, Hayashi K, Hatsumi N, et al. Risk factors for early death in patients undergoing treatment for multiple myeloma. Ann Hematol 2001; 80:452–455. [DOI] [PubMed] [Google Scholar]
- 19.Kim JE, Yoo C, Lee DH, et al. Serum albumin level is a significant prognostic factor reflecting disease severity in symptomatic multiple myeloma. Ann Hematol 2010; 89:391–397. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson JL, Hussein MA, Barlogie B, et al. A new staging system for multiple myeloma patients based on the Southwest Oncology Group (SWOG) experience. Br J Haematol 2003; 122:441–450. [DOI] [PubMed] [Google Scholar]
- 21.Bologa RM, Levine DM, Parker TS, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis 1998; 32:107–114. [DOI] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Barlogie B, Smith TL, et al. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Inter Med 1991; 115:931–935. [DOI] [PubMed] [Google Scholar]
- 23.Terpos E, Katodritou E, Roussou M, et al. High serum lactate dehydrogenase adds prognostic value to the international myeloma staging system even in the era of novel agents. Eur J Haematol 2010; 85:114–119. [DOI] [PubMed] [Google Scholar]
- 24.Anagnostopoulos A, Gika D, Symeonidis A, et al. Multiple myeloma in elderly patients: prognostic factors and outcome. Eur J Haematol 2005; 75:370–375. [DOI] [PubMed] [Google Scholar]
- 25.Barlogie B, Bolejack V, Schell M, et al. Prognostic factor analyses of myeloma survival with intergroup trial S9321 (INT 0141): examining whether different variables govern different time segments of survival. Ann Hematol 2011; 90:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anagnostopoulos A, Gika D, Symeonidis A, et al. Multiple myeloma in elderly patients: prognostic factors and outcome. Eur J Haematol 2005; 75:370–375. [DOI] [PubMed] [Google Scholar]
- 27.Suguro M, Kanda Y, Yamamoto R, et al. High serum lactate dehydrogenase level predicts short survival after vincristine–doxorubicin–dexamethasone(VAD) salvage for refractory multiple myeloma. Am J Hematol 2000; 65:132–135. [DOI] [PubMed] [Google Scholar]
- 28.Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol 2006; 17:546–555. [DOI] [PubMed] [Google Scholar]
- 29.Donadio C, Lucchesi A, Ardini M, et al. Cystatin C, beta 2-microglobulin, and retinol-binding protein as indicators of glomerular filtration rate: comparison with plasma creatinine. J Pharm Biomed Anal 2001; 24:835–842. [DOI] [PubMed] [Google Scholar]
- 30.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348:2609–2617. [DOI] [PubMed] [Google Scholar]
- 31.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 2013; 14:1055–1066. [DOI] [PubMed] [Google Scholar]
- 32.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 2006; 24:431–436. [DOI] [PubMed] [Google Scholar]
- 33.Berenson JR, Lichtenstein A, Porter L, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol 1998; 16:593–602. [DOI] [PubMed] [Google Scholar]
- 34.Lipton A, Cook RJ, Coleman RE, et al. Clinical utility of biochemical markers of bone metabolism for improving the management of patients with advanced multiple myeloma. Clin Lymphoma Myeloma 2007; 7:346–353. [DOI] [PubMed] [Google Scholar]
- 35.McCloskey EV, Dunn JA, Kanis JA, et al. Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. Br J Haematol 2001; 113:1035–1043. [DOI] [PubMed] [Google Scholar]
- 36.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet (Lond, Engl) 2010; 376:1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsingh G, Mehan P, Luo J, et al. Primary plasma cell leukemia: a Surveillance, Epidemiology, and End Results database analysis between 1973 and 2004. Cancer 2009; 115:5734–5739. [DOI] [PubMed] [Google Scholar]
- 38.Tiedemann RE, Gonzalez-Paz N, Kyle RA, et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia 2008; 22:1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teh BW, Harrison SJ, Worth LJ, et al. Risks, severity and timing of infections in patients with multiple myeloma: a longitudinal cohort study in the era of immunomodulatory drug therapy. Br J Haematol 2015. [DOI] [PubMed] [Google Scholar]
- 40.Ross FM, Avet-Loiseau H, Ameye G, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica 2012; 97:1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


