Abstract
Modifiable cardiovascular risk factors such as obesity, hypertension, dyslipidemia, smoking, diabetes mellitus, and metabolic syndrome can easily give rise to coronary heart disease (CHD). However, due to the existence of the so-called “obesity paradox” and “smoking paradox,” the impact of these modifiable cardiovascular risk factors on mortality after percutaneous coronary intervention (PCI) is still not clear.
Therefore, in order to solve this issue, we aim to compare mortality between patients with low and high modifiable cardiovascular risk factors after PCI.
Medline and EMBASE were searched for studies related to these modifiable cardiovascular risk factors. Reported outcome was all-cause mortality after PCI. Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated, and the pooled analyses were performed with RevMan 5.3 software.
A total of 100 studies consisting of 884,190 patients (330,068 and 514,122 with high and low cardiovascular risk factors respectively) have been included in this meta-analysis. Diabetes mellitus was associated with a significantly higher short and long-term mortality with RR 2.11; 95% CI: (1.91–2.33) and 1.85; 95% CI: (1.66–2.06), respectively, after PCI. A significantly higher long-term mortality in the hypertensive and metabolic syndrome patients with RR 1.45; 95% CI: (1.24–1.69) and RR 1.29; 95% CI: (1.11–1.51), respectively, has also been observed. However, an unexpectedly, significantly lower mortality risk was observed among the smokers and obese patients.
Certain modifiable cardiovascular risk subgroups had a significantly higher impact on mortality after PCI. However, mortality among the obese patients and the smokers showed an unexpected paradox after coronary intervention.
INTRODUCTION
Coronary heart disease, also known as coronary artery disease (CAD), is the most common type of heart disease in the elderly. Almost all over the world, it is the number 1 cause of death in both men and women. From the year 1990 to 2013, there has been a rise from 5.74 to 8.14 million deaths from CAD globally.1 There are many risk factors associated with CAD. These risk factors include hypertension, dyslipidemia, smoking, obesity, old age, family history, diabetes mellitus (DM), and metabolic syndrome (MS).2 These risk factors can still be subdivided into modifiable and nonmodifiable risk factors. Modifiable risk factors are those that can be changed; or simply, if careful precautions are taken, the risk for developing CAD will be lower in the susceptible population. For example, eating a healthy diet, doing regular exercises, avoiding smoking, and maintaining a healthy weight are all safety measures which can help to prevent CAD.3,4 Except old age and a family history with cardiovascular disorders, factors such as a high body mass index (BMI), hypertension, dyslipidemia, smoking, DM, and MS are all considered as modifiable cardiovascular risk factors.
Unfortunately, because of the unhealthy lifestyle adopted by people nowadays, they finally end up with conditions which expose them to a high risk for CAD. When symptoms become more severe, or intolerable, and when medications become ineffective, percutaneous coronary intervention (PCI) proves to be the most common invasive treatment in these patients.5 However, due to the presence of the so-called phenomenon “obesity paradox” and “smoking paradox” whereby the mortality rate in the obese patients and smokers is unexpectedly lower compared to the normal weight patients and nonsmokers, respectively, the impact of these modifiable cardiovascular risk factors on mortality after PCI is still not clear. Therefore, in order to solve this issue, we aim to compare the short- and long-term mortality in patients with low and high modifiable cardiovascular risk factors after PCI.
METHODS
Data Sources and Search Strategy
Medline and EMBASE were searched for randomized controlled trials (RCTs) and observational studies by typing the words or phrases “X and percutaneous coronary intervention/PCI” whereby X was interchangeable with these modifiable cardiovascular risk factors such as smoking, overweight/obesity/high BMI, hypertension, hyperlipidemia/hypercholesterolemia/high-density lipoprotein (HDL)/low-density lipoprotein (LDL), DM, and MS. To further enhance this search, the term “angioplasty” has also been used to replace PCI and the words “smoking paradox” and “obesity paradox” have been used to replace smoking and obesity, respectively. No language restriction was applied.
Inclusion and Exclusion Criteria
Studies were included if:
They were RCTs or observational studies relating these modifiable cardiovascular risk factors with PCI.
They reported mortality among their clinical endpoints.
They included data for both the experimental and the control groups. For example, DM and non-DM, smokers and nonsmokers, overweight/obese and nonobese/normal weight, MS and non-MS, hypertensive and normotensive patients, increased LDL and normal/low LDL, or decreased high density lipoprotein (HDL) and increased HDL.
Studies were excluded if:
They did not include these modifiable cardiovascular risk factors.
They were meta-analyses, case studies, or letter to editors.
No control group was present.
Mortality was not among the reported endpoints.
Duplicates.
Types of Participants
All the patients were >18 years old and suffered from CAD. Enrolled patients in the experimental group had at least 1 modifiable cardiovascular risk factor (diabetes, MS, high BMI, dyslipidemia, cigarette smoking, or hypertension) whereas those patients in the control group did not suffer from the risk factor being analyzed in the corresponding subgroups. All patients underwent PCI.
Definitions, Outcomes, and Follow-Up Periods
Modifiable Cardiovascular Risk Factors: defined as cardiovascular risk factors that can be controlled or if prevented, can result in a lower risk of suffering from CAD. In our studies, these patients were considered as high risk patients. Low risk patients, who acted as controls for this meta-analysis, were those without these modifiable cardiovascular risk factors.
DM: defined as a fasting blood glucose (FBG) level of >7.0 mmol/L or an oral glucose tolerance test (OGTT) >11.1 mmol/L observed at least on 2 different occasions.
Overweight and obese: BMI of >25 and >30 kg/m2, respectively.
Hypertension: a blood pressure of >130/80 mmHg on at least 2 separate occasions.
Dyslipidemia: defined as an LDL level of (>130 mg/dL) or an HDL level of (<40 mg/dL). A borderline value was already considered as dyslipidemia in this study.
MS: a condition diagnosed if at least 3 of the followings were present: central obesity, high blood pressure, high fasting plasma glucose, high serum triglyceride, and low-high-density lipoproteins.
Smoking: included current smokers and late nonsmokers. Quitters, former smokers, pre- and post-PCI smoking quitters have not been included in the study.
In-hospital mortality: included all-cause deaths during the hospital stay (≤30 days).
Short-term mortality: included all-cause deaths during a follow-up period from 30 days to 1 year after PCI.
Long-term mortality: included all-cause deaths during a follow-up period at 1 year or more than 1 year after PCI.
Data Extraction and Quality Assessment
Two authors (PKB and ZW) independently reviewed the data and assessed the eligibility and methodological quality of each eligible study. Information and data regarding the number of patients and patient characteristics, associated risk factors, intervention strategies, and the clinical outcomes, and respective follow-up periods (in-hospital, short-term, and long-term) were systematically extracted. If any of the 2 authors disagreed about the information or data extracted, disagreements were discussed between the authors, and if the authors could not reach a final decision, disagreements were resolved by the 3rd author (MHC). The bias risk of trials was assessed with the components recommended by the Cochrane Collaboration.6
Methodological Quality and Statistical Analysis
Study selection, data collection, analysis, and reporting of the results were performed using the recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. Heterogeneity across trials was assessed using the Cochrane Q-statistic (P ≤ 0.05 was considered significant) and I2-statistic. I2 described the percentage of total variation across studies, that is, due to heterogeneity rather than chance. A value of 0% indicated no heterogeneity, and larger values indicated increased heterogeneity. If I2 was <50%, fixed effect model was used. However, if I2 was >50%, a random effect model was used. Publication bias was visually estimated by assessing funnel plots. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for categorical variables. The pooled analyses were performed with RevMan 5·3 software. Since this is a systematic review and meta-analysis, ethical approval was not required.
RESULTS
Selection of Studies for This Meta-Analysis
A total of 7456 articles were identified from search databases, and 32 articles were identified from references. After excluding the 1120 duplicates, 6030 articles were excluded by title and abstract since they were not related to our topic. Among the remaining articles, 79 were related to obesity, 142 to diabetes, 25 to MS, 36 to dyslipidemia, 29 to smoking, and 27 were related to hypertension. A total of 338 full text articles were assessed for eligibility. More articles were excluded since they were meta-analyses, case studies, data for the control group were not available, outcomes of interest were not reported and also dichotomous data which were very important for our statistical analysis were not provided. The flow diagram for the selection of studies has been represented in Figure 1.
FIGURE 1.
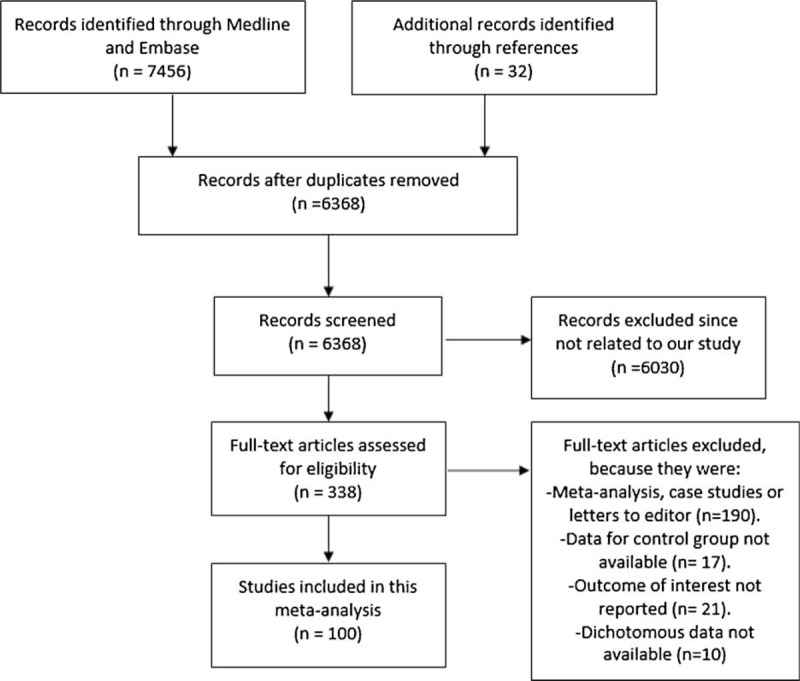
Shows the flow diagram for the study selection.
A total number of 100 articles from randomized controlled trials and observational studies have been included in this meta-analysis with a total number of 844,190 patients to be analyzed; among which, 330,068 patients were in the experimental group while 514,122 were in the control group. The total number of patients associated with the corresponding risk factors from this whole study have been given in Tables 1 and 2 shows the total number of patients in all the different subgroups (for both the experimental and control groups) as well as their follow-up periods.
TABLE 1.
An Approximation∗ of the number of patients corresponding to these modifiable risk factors throughout this whole meta-analysis
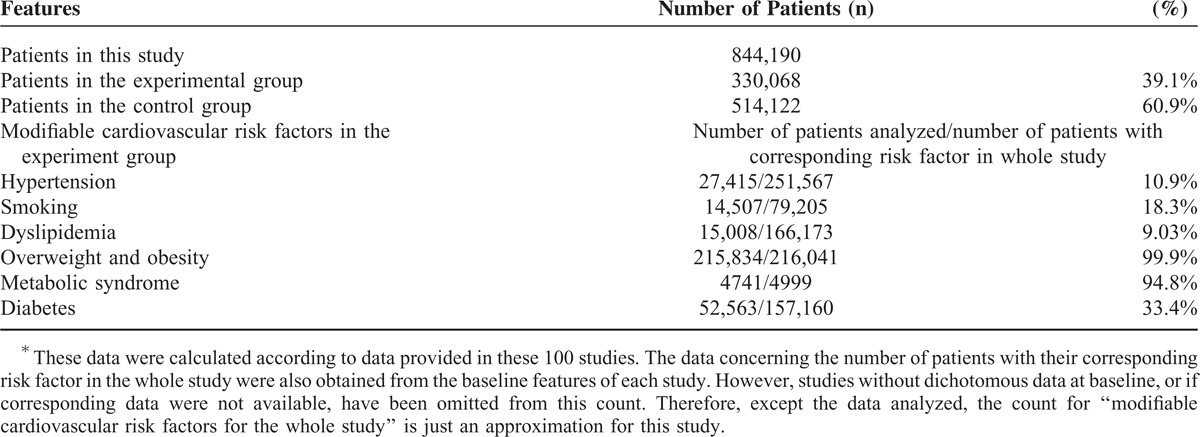
TABLE 2.
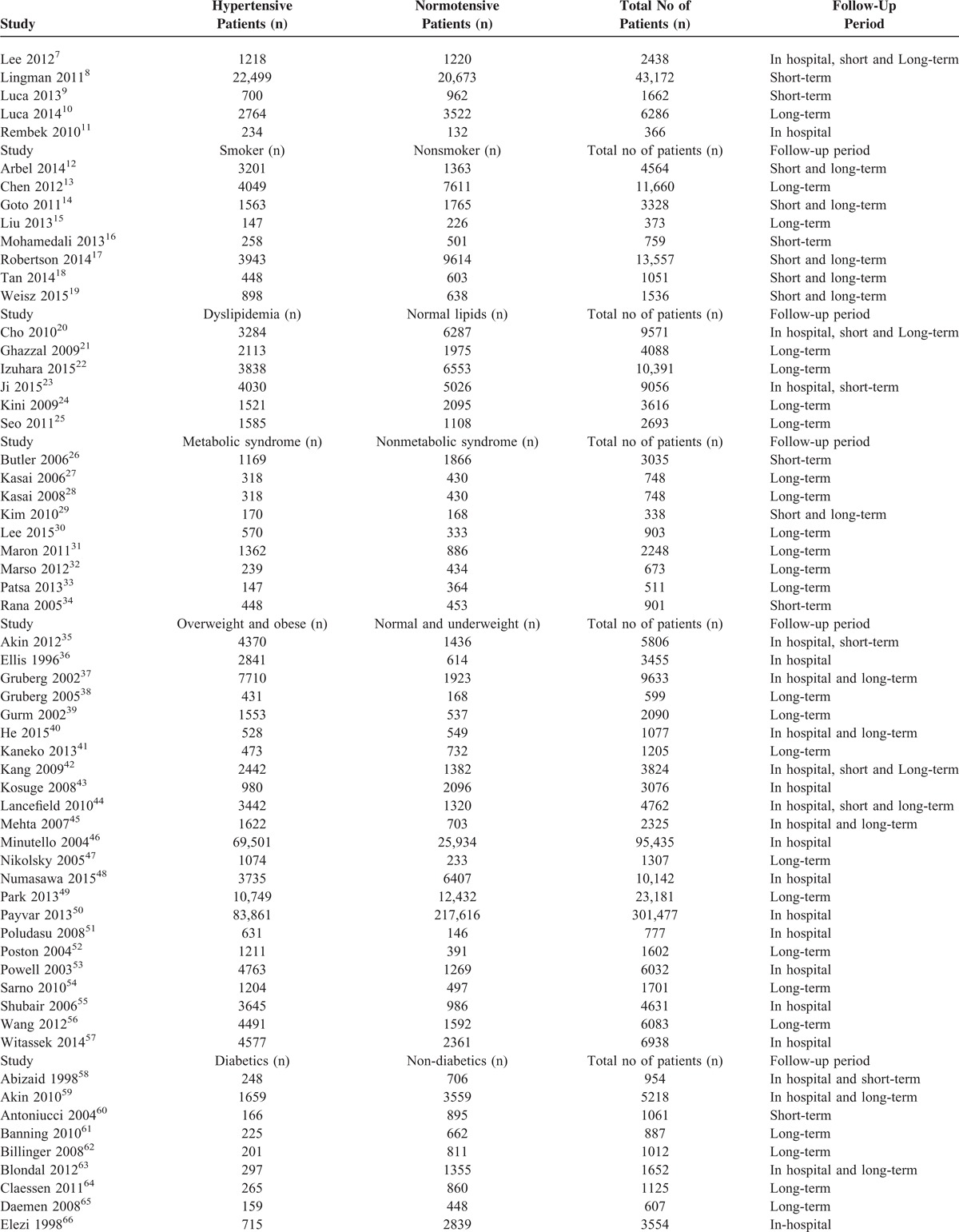
The Number of Patients in These Different Subgroups and the Corresponding Follow-Up Periods
Among these 330,068 patients analyzed in the experimental group, 76.2% had hypertension, 24% were smokers, 50.3% had dyslipidemia, 65.5% were overweight or obese, 1.5% had MS, and 47.6% had DM.
Considering this whole study, pure data for only 27,415 (10.9%) hypertensive patients, 14,507 (18.3%) smokers, 15,008 (9.03%) patients with dyslipidemia, 215,834 (99.9%) patients with high BMI, 4741 (94.8%) patients with MS, and 52,563 (33.4%) patients with DM were available for subgroup analysis. Note that data which were not available in the original articles have been omitted.
Table 2 has been divided into different subgroups of modifiable cardiovascular risk factors. Total number of patients in the experimental group, control group as well as the total number of patients in each study with their follow-up periods have been given in Table 2 . Five studies dealt with hypertension, 8 studies dealt with smoking, 6 studies dealt with dyslipidemia, 9 studies dealt with MS, 23 studies dealt with high BMI, and 51 studies dealt with DM. Two studies were common in both the hypertension and the diabetic groups since they analyzed both diabetic and hypertensive patients together. Follow-up periods were classified as in-hospital, short-term, and long-term follow-ups as mentioned in the “definition” section.
The baseline characteristics of all the included studies have been represented in Table 3 .
TABLE 2 (Continued).
The Number of Patients in These Different Subgroups and the Corresponding Follow-Up Periods
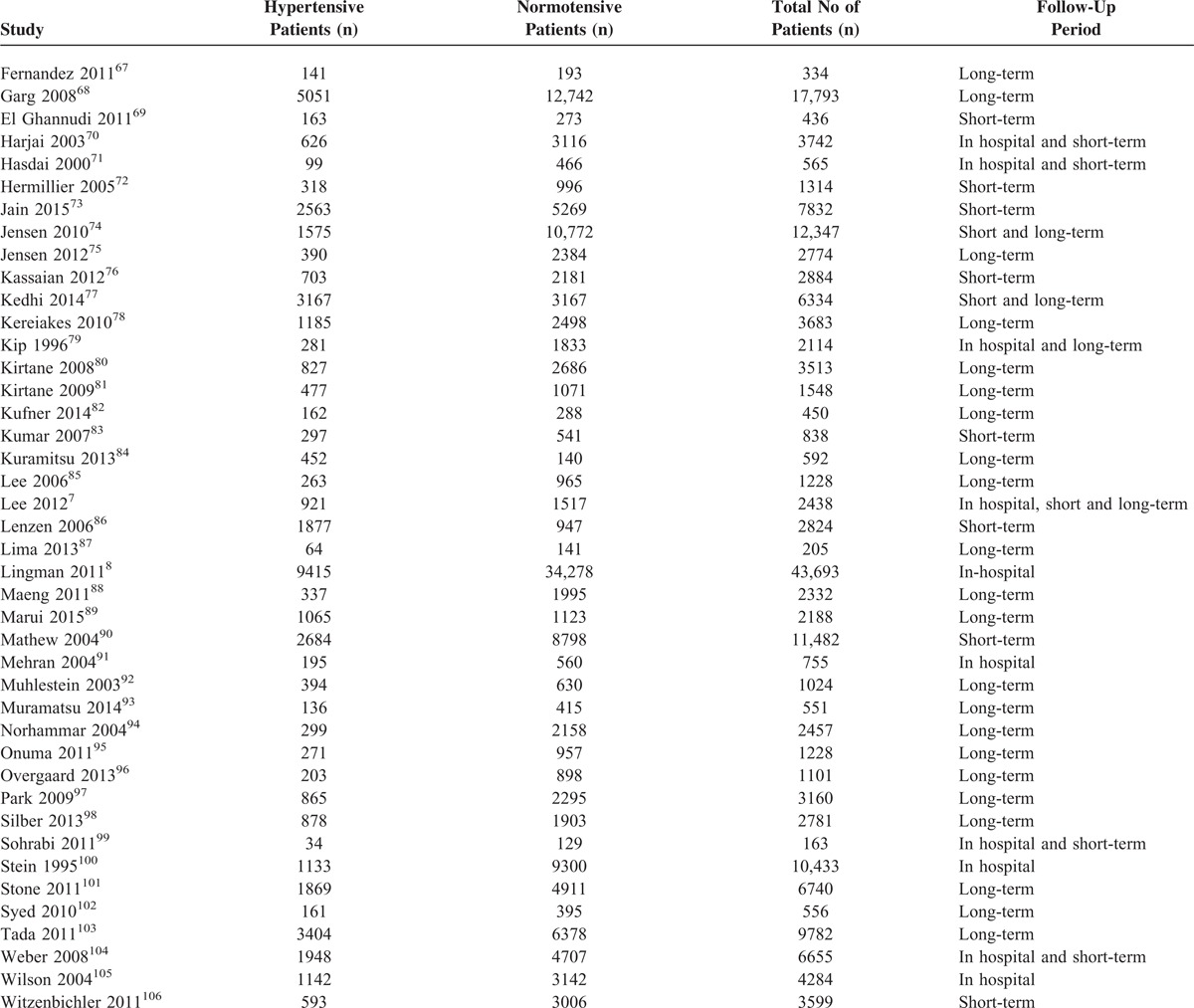
TABLE 3 (Continued).
Shows the Baseline Features of Each of the Included Studies
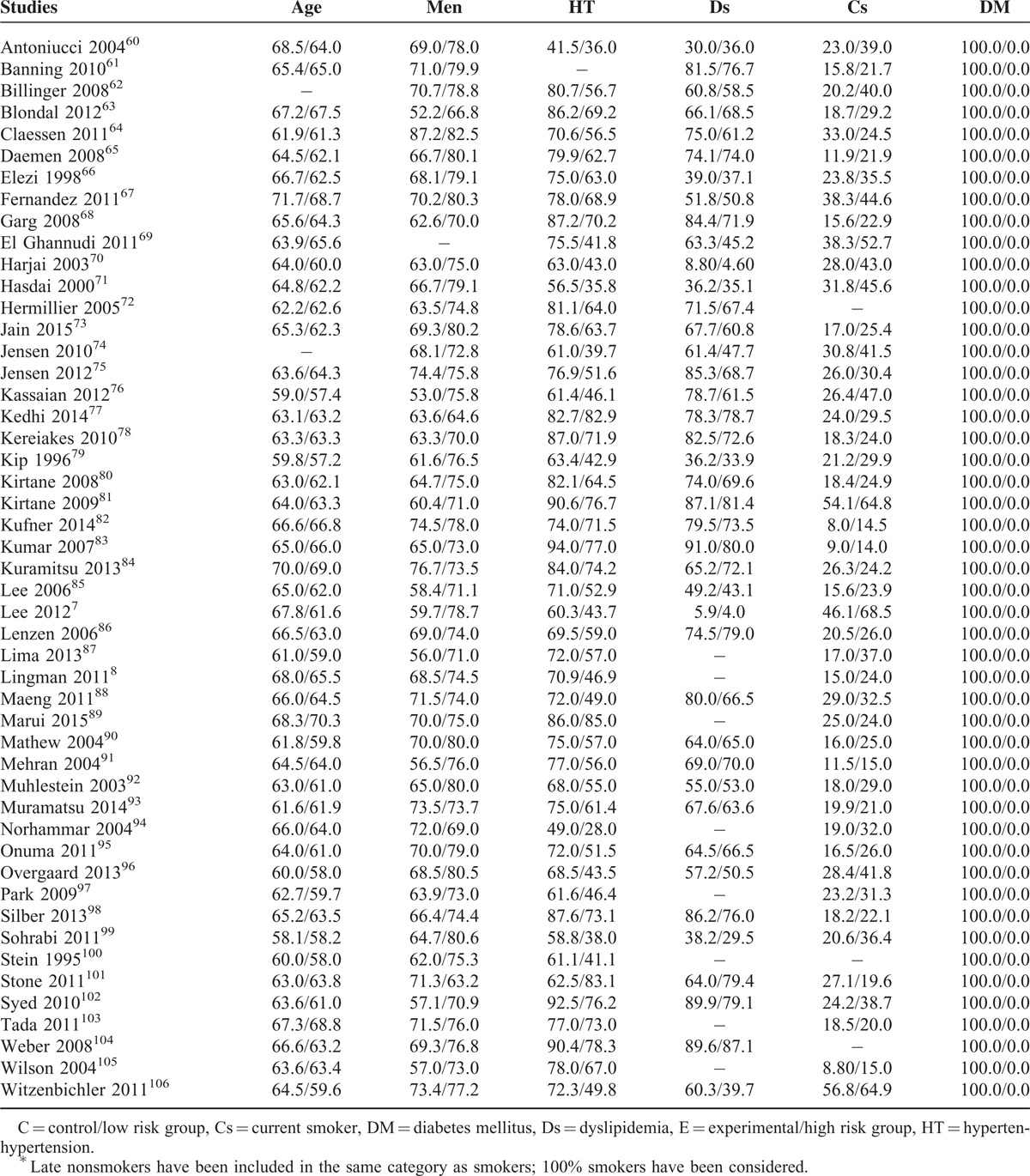
Patients in the hypertensive group were older than the normotensive patients. There were more male patients in the control group compared to the experimental group. DM and dyslipidemia were more prominent among the hypertensive patients whereas cigarette smoking was more common in the control group.
Majority of the smokers were males and they were younger than the nonsmokers. Apart from 1 study, hypertension was more prominent among the nonsmokers. Most of the nonsmokers suffered from DM too.
Patients from both the experimental and the control groups were almost similar in age. If analyzed as a whole, there was no significant differences between genders, hypertension, and smoking between these 2 groups. However, except from 1 study, DM was more prominent among those with dyslipidemia.
There was no significant difference in age between these 2 groups. Majority of those patients in the control group were males. Hypertension was more prominent in the experimental group. Smoking was almost similar in both groups. Except from 1 study which had no diabetic patients and 1 which had less patients with DM, DM was more common in the MS group.
The overweight and obese patients were younger than the normal weight and underweight patients. There were more males than females in the experimental group. Hypertension, dyslipidemia, and DM were more prominent in the experimental group. Most of the patients in the high BMI category were nonsmokers.
There was no significant difference in age between the diabetic and nondiabetic patients. Most of the patients in the control group were males. Hypertension and dyslipidemia were more prominent in the DM group. Most of the patients in the experimental group were nonsmokers.
Result of the Main Analysis
Results from this meta-analysis showed that during the in-hospital follow-up, mortality in the hypertensive and DM patients were significantly higher with RR 1.43; 95% CI: (1.05–1.94); P = 0.02 and RR 1.86; 95% CI: (1.68–2.06); P < 0.00001, respectively. The in-hospital mortality for the patients with dyslipidemia did not reach statistical significance RR 1.39; 95% CI: (0.32–5.94); P = 0.66. However, surprizingly, the in-hospital mortality significantly favored patients with high BMI with RR 0.61; 95% CI: (0.58–0.64); P < 0.00001.
Short-term mortality was significantly higher in the DM group with RR 2.11; 95% CI: (1.91–2.33); P < 0.00001. The result was not significant in the hypertensive group with RR 1.40; 95% CI: (0.95–2.06); P = 0.09; dyslipidemia group with RR 0.91; 95% CI: (0.47–1.76); P = 0.77 and MS group with RR 1.05; 95% CI: (0.88–1.25); P = 0.61. Unexpectedly, the short-term mortality significantly favored the smokers and high BMI groups with RR 0.53; 95% CI: (0.45–0.62); P < 0.00001 and 0.67; 95% CI: (0.52–0.86); P = 0.002, respectively.
Long-term mortality was significantly higher in the DM, hypertensive, and MS groups with RR 1.85; 95% CI: (1.66–2.06); P < 0.00001, 1.45, 95% CI: (1.24–1.69); P < 0.00001, and 1.29; 95% CI: (1.11–1.51); P = 0.0009, respectively. The result for dyslipidemia was still not significant. However, the long-term mortality still significantly favored the smokers and high BMI patients with RR 0.49; 95% CI: (0.39–0.63); P < 0.00001 and 0.64; 95% CI: (0.54–0.75), P < 0.00001, respectively. The mortality risks within these subgroups have been summarized in Table 4, and the detailed results for mortality among these different subgroups have been shown in Figures 2–7.
TABLE 3.
Shows the Baseline Features of Each of the Included Studies
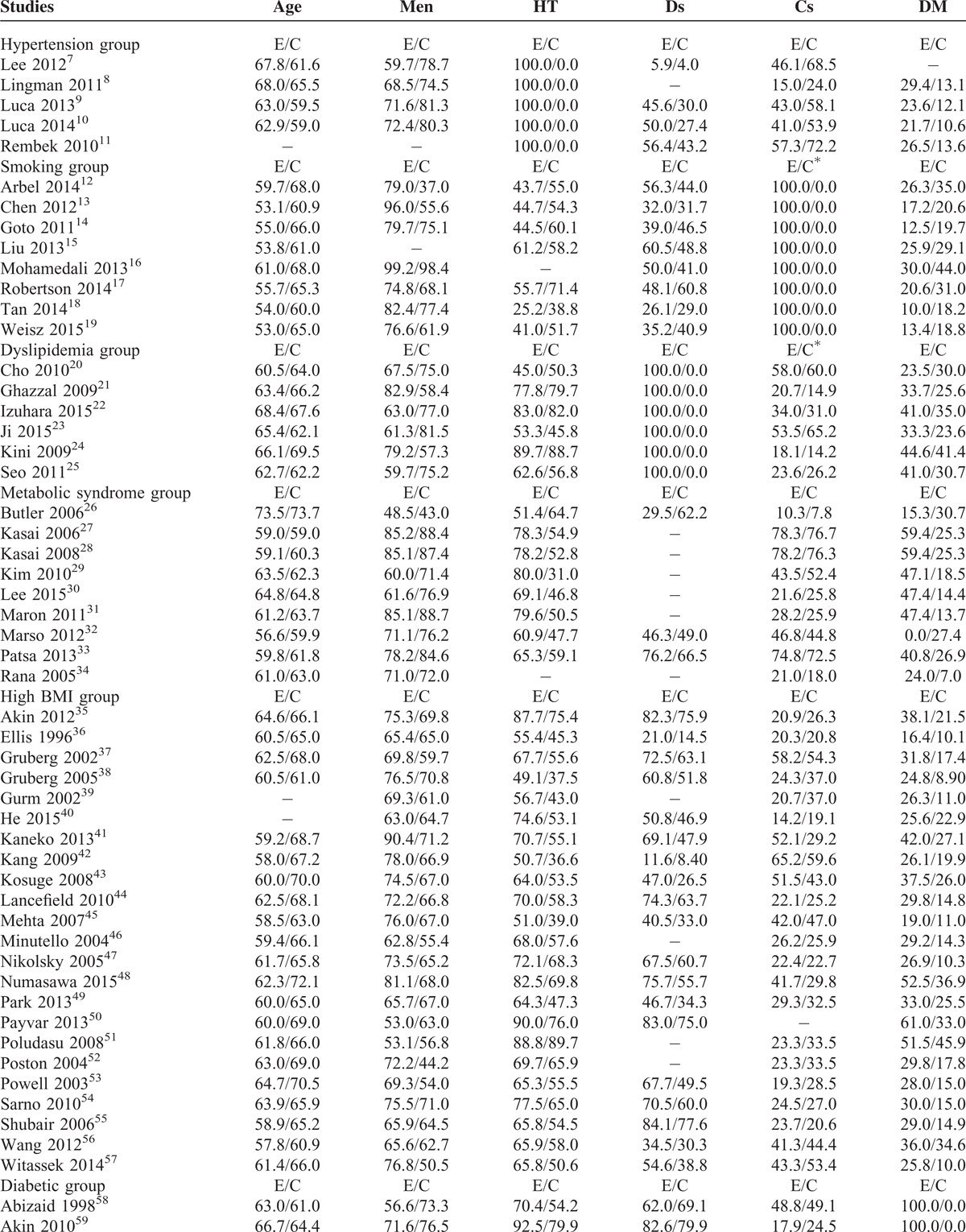
FIGURE 2.
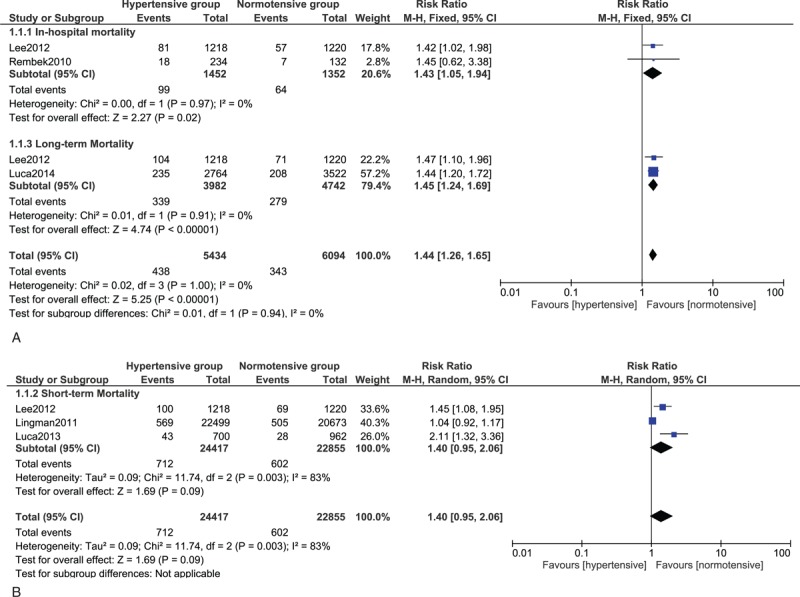
(A) Forest plot showing the in-hospital and long-term mortality risk in Hypertensive patients. (B) Forest plot showing the short-term mortality risk in hypertensive patients.
FIGURE 7.
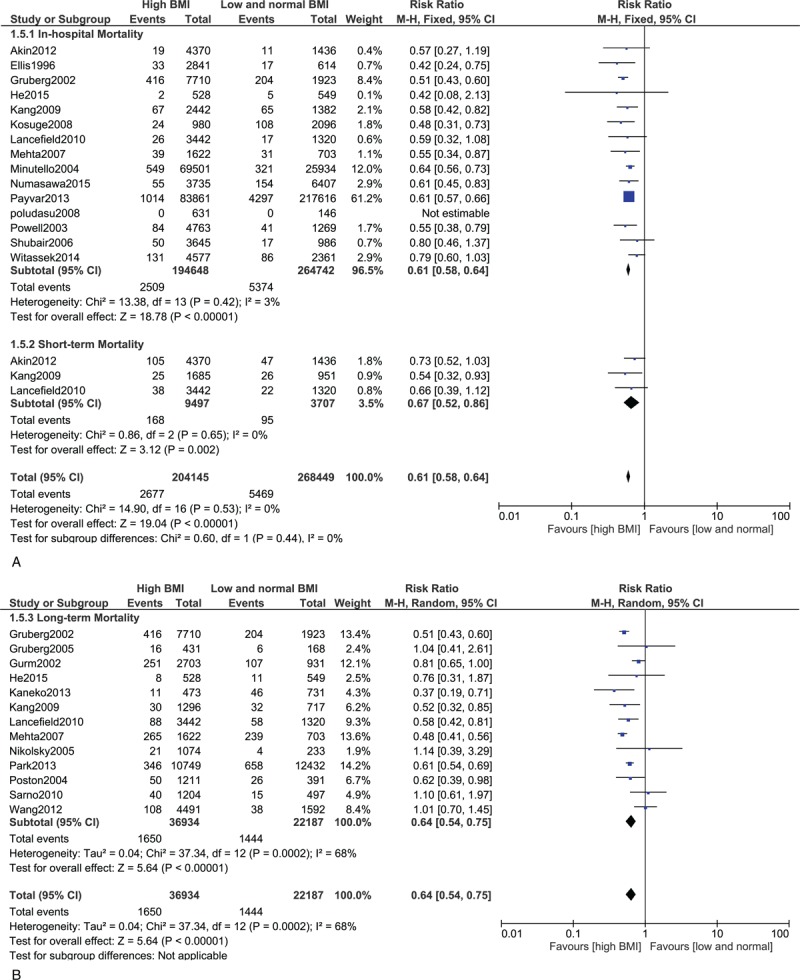
(A) Forest plot showing the in-hospital and short-term mortality in overweight and obese patients. (B) Forest plot showing the long-term mortality in overweight and obese patients.
TABLE 4.
Summarizes the Results of This Meta-Analysis
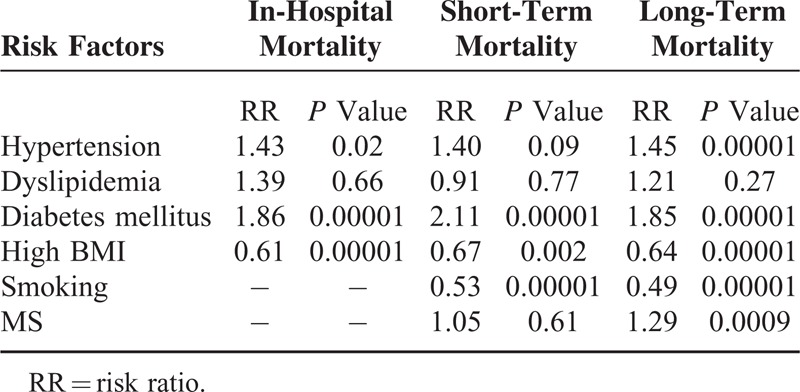
FIGURE 3.
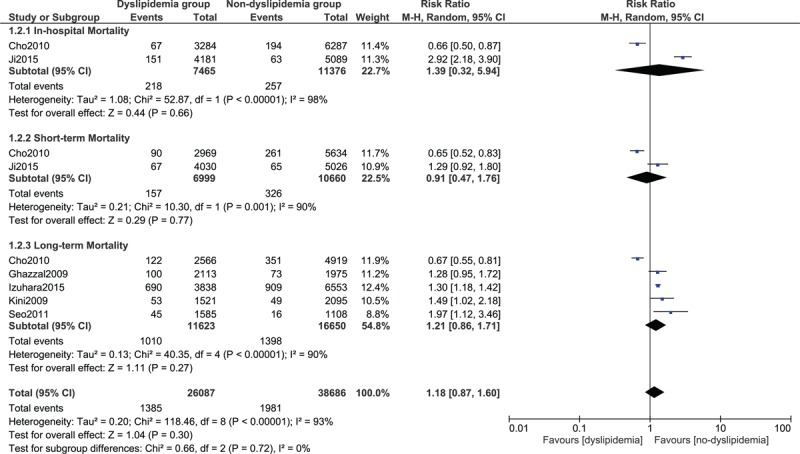
Forest plot showing the mortality in dyslipidemia patients.
FIGURE 4.
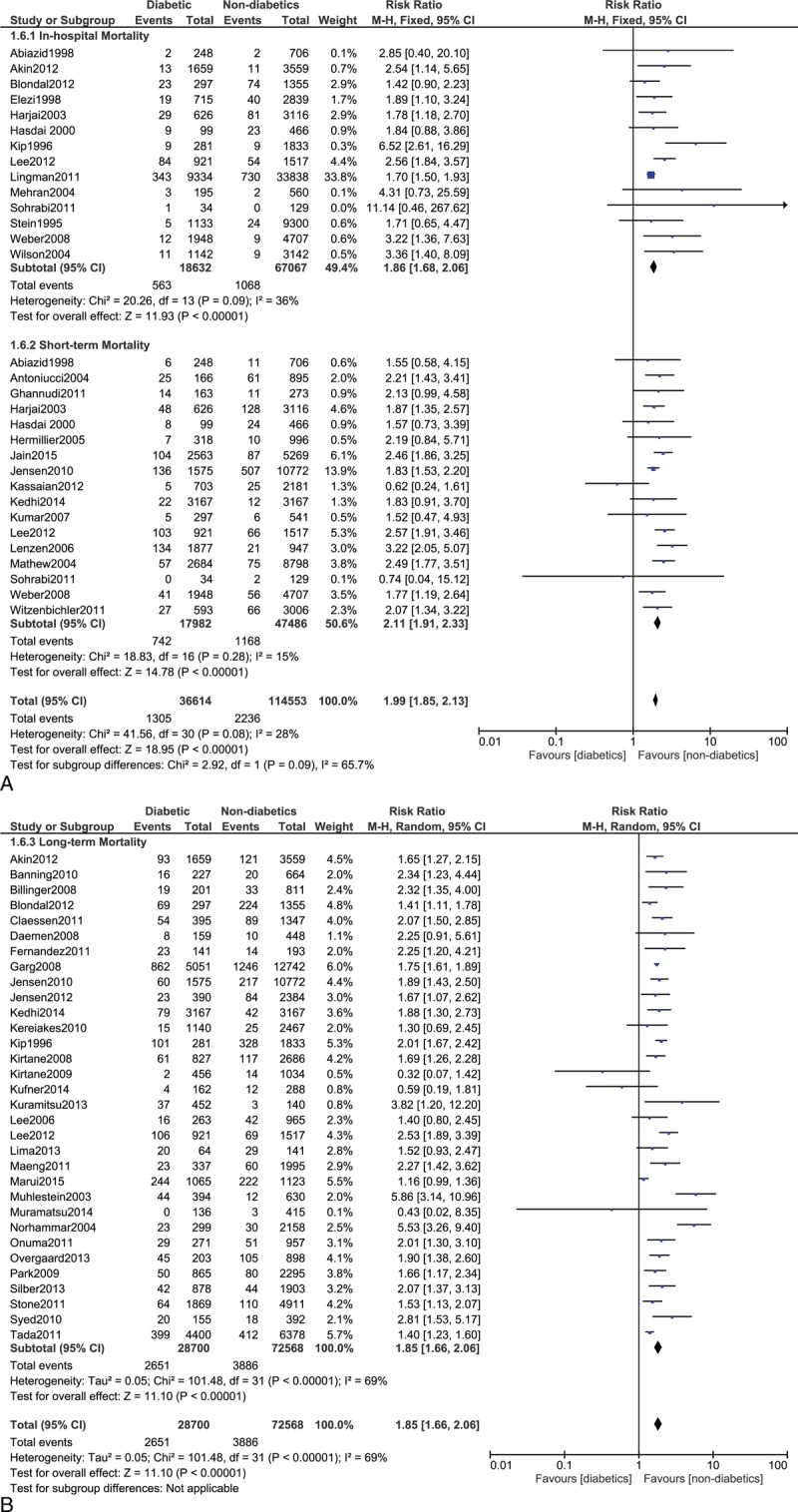
(A) Forest plot showing the in-hospital and short-term mortality in diabetic patients. (B) Forest plot showing the long-term mortality in diabetic patients.
FIGURE 5.
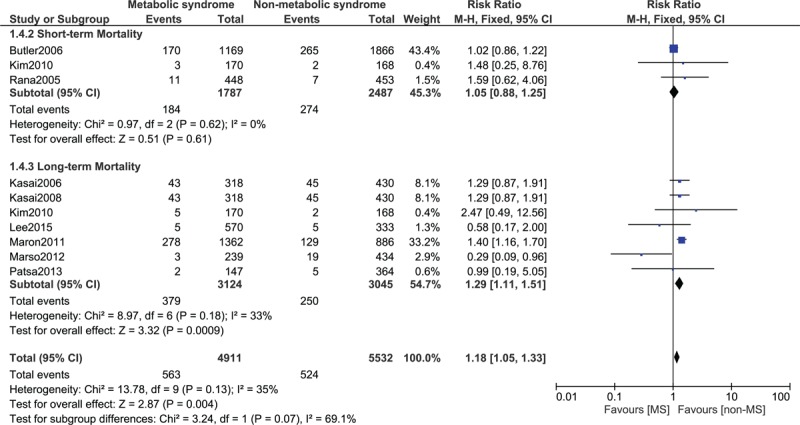
Forest plot showing the mortality in patients with metabolic syndrome.
FIGURE 6.
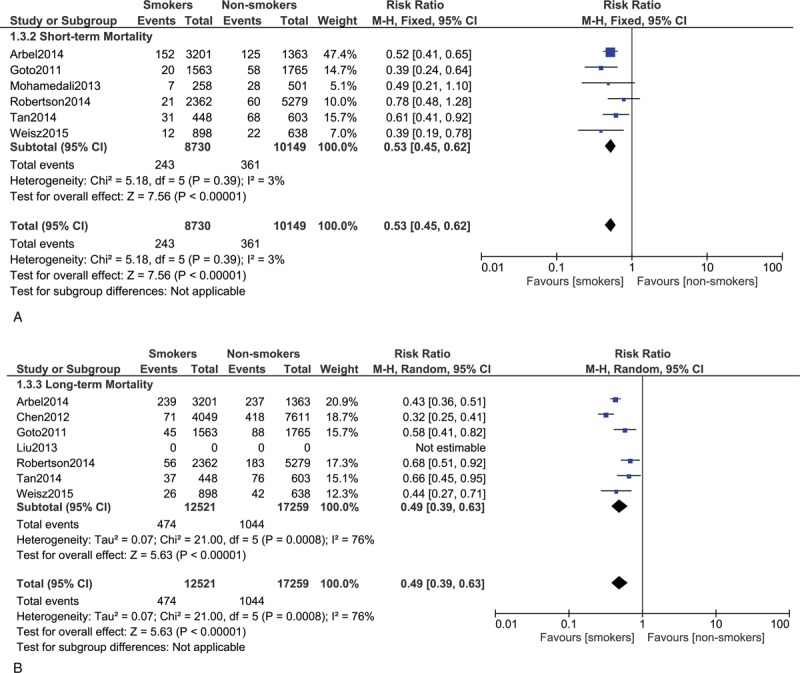
(A) Forest plot showing the short-term mortality in smokers. (B) Forest plot showing the long-term mortality in smokers.
For all of the above analyses, sensitivity analyses yielded consistent results. Based on a visual inspection of the funnel plots, there has been no evidence of publication bias for the included studies that assessed the subgroup mortality risk. Figure 8 shows the corresponding funnel plots.
FIGURE 8.
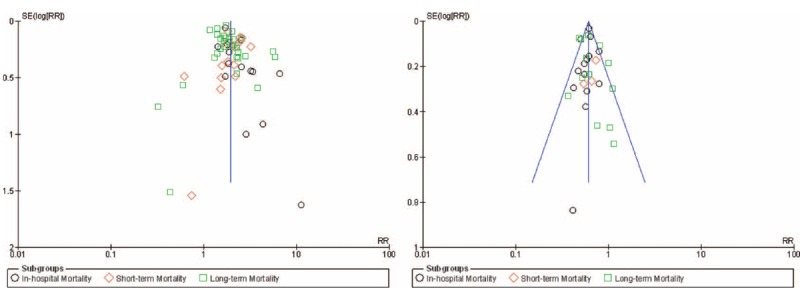
Funnel plots for the subgroup analysis.
DISCUSSION
Among these 844,190 patients who participated in this meta-analysis, an unexpected result has been obtained in certain subgroups of patients. A significantly higher mortality risk has been observed among the DM patients. A significantly higher in-hospital and long-term mortality risks have also been observed among the hypertensive patients. Moreover, a significantly higher long-term mortality has been observed in patients with MS whereas an almost similar mortality rate has been observed in patients with and without dyslipidemia.
However, smokers and those patients with high BMI had an unexpectedly lower short and long-term mortality risk compared to non-smokers and low-BMI/normal weight patients, respectively, after PCI. Several possible reasons could be responsible for such an outcome.
DM is associated with a higher risk of mortality after PCI.59 A total of 3.02%, 4.12%, and 9.24% in-hospital, short-, and long-term deaths, respectively, occurred in these DM patients compared to 1.59%, 2.46%, and 5.35% in-hospital, short-, and long-term deaths in nondiabetics patients in our study. These patients have worse adverse clinical outcomes including mortality due to severe stent thrombosis, stroke, silent myocardial infarction, or other major adverse cardiac effects. Conditions such as multicoronary vessel diseases and chronic total occlusion which are associated with DM patients partly contribute to these worse clinical outcomes after PCI. The risk of restenosis after stent implantation is also higher in diabetic patients. DM patients also have platelet dysfunction which contribute to this expected increased risk of mortality in these patients.107 A poor response to antiplatelet agents such as aspirin and clopidogrel after drug eluting stents implantation could be another reason for such a result.108 The use of insulin could also be another reason for this higher mortality risk in these diabetic patients.109 Comorbidities and severe diabetic complications are associated with these insulin-treated diabetic patients which finally result in a higher mortality in this category of patients after PCI.
MS which is considered to be a modifiable cardiovascular risk factor, includes patients who can be obese, may have diabetes, may suffer from hypertension, and may also have dyslipidemia. The long-term mortality in these patients was significantly higher compared to those without MS after PCI. A significant increase in long-term mortality from 8.21% in non-MS to 12.1% in MS has been found in our study. Its association with comorbidities such as DM and hypertension maybe one of the reasons that lead to a higher mortality in these patients after PCI.31
Hypertension is another major modifiable risk factor for CAD and acute coronary syndrome. Hypertensive patients had a higher mortality risk compared to normotensive patients after PCI. A significant long-term mortality of 8.51% has been observed in the hypertensive group, compared to the normotensive group which was only 5.88% after PCI. The reasons associated with this result could be an increased in diastolic dysfunction in these hypertensive patients which could lead to severe heart failure. Moreover, by hypertension, we refer to essential hypertension which is a disease that occurs in advanced age. Other comorbid conditions such as diabetic mellitus may be present in these hypertensive patients thus, strengthening/increasing the mortality risk in these patients after PCI.8 Patients with high blood pressure are even prone to cerebral hemorrhage if their antiplatelet dosages are not adjusted after PCI. This can also contribute to death in these patients.
Dyslipidemia is another well-known modifiable risk factor for coronary heart disease. It was expected to be associated with a higher mortality after PCI but however, the results were not significant in our study. A few studies have shown the existence of a “cholesterol paradox” whereby the mortality rate in hypercholesterolemia patients was lower compared to those with normal cholesterol levels.20 The reasons for such a phenomenon is still not clear. However, even such a result was not evident in our study. Several factors could have been responsible for this insignificant result. The use of statin (lipid-lowering drugs) has not been studied in our meta-analysis.110
Obesity is another modifiable risk factor for cardiovascular diseases. Surprizingly, our study showed an unexpectedly, significantly decreased mortality in these high BMI patients in all the follow-up categories after PCI. A significant 1.79% overall death has been observed in the overweight and obese patients whereas a higher overall mortality of 2.38% was observed among the combined normal weight/underweight patients. Several studies have shown the existence of an “obesity paradox” in such patients after cardiovascular intervention.44 The baseline features in this study showed a higher rate of diabetes, dyslipidemia, and hypertension among the overweight and obese patients. Intensive medications and aggressive medical therapies, regular counselling about health benefits, younger age, and having a good storage for nutrients after PCI could all be responsible for such a phenomenon. Size of the coronary blood vessels could also be considered as one of the reasons for this “obesity paradox.”44 However, a few studies also showed different results. The study by Akin et al35 in 2012 revealed no evidence of such a phenomenon. In his study, normal body weight patients and obese patients had similar rates of all-cause mortality. Such a different result in his study could be due to the fact that his study dealt with the comparison of different types of drug eluting stents and their corresponding adverse clinical outcomes after PCI. However, it is not clear whether or not this increased mortality risk could also have been more prominent among the underweight which could not be compensated by the normal weight population.
Smoking, which is another modifiable cardiovascular risk factor, has proved to be associated with cardiovascular disorders. Unexpectedly, results from our study showed a significantly decreased risk of overall mortality in these smokers (3.37%) compared to nonsmokers (5.13%) after PCI. According to the baseline features in this study, most of the nonsmokers were diabetics and suffered from high blood pressure. The existence of a “smoking paradox” has also been observed in other studies. For example, the study by Hasdai and Holmes found lower adverse outcomes in smokers compared to nonsmokers after PCI.111 The question about why smokers have a lower mortality rate compared to nonsmokers after PCI is more interesting than its answer. Reasons suggested for this smoking paradox could be younger age, a more favorable clinical and angiographic profile among these smokers, and less damage to microvascular function in these patients after PCI. However, many other studies had different results compared to our meta-analysis. The study Jang et al112 showed that individuals who continue smoking after PCI experienced significantly poorer outcomes compared to patients who have never smoked. Another study by Castela et al showed a higher rate of vascular complications, but a similar mortality rate between smokers and nonsmokers at 1 year. However, a smaller population size and a different definition of smoker could be responsible for this different result in his study.
Apart from these cardiovascular risk factors, an increased mortality in these patients after PCI could also have been due to factors such as drug eluting stents, which are associated with a higher long-term risk of stent thrombosis. Also, glucose-lowering drugs in DM patients have been associated with an increased risk of mortality in this modifiable cardiovascular risk group.113,114 Moreover, a study by Yusuf et al115 showed no difference in cardiovascular mortality even with intensive lifestyle intervention in DM patients indicating that there may be other factors such as socio-economic status which contribute to this increase in mortality in these high risk patients.
This meta-analysis with a large number of patients is the one and only meta-analysis comparing mortality between patients with low and high modifiable cardiovascular risk factors after PCI. Including 100 studies consisting of 844,190 patients with several modifiable cardiovascular risk factors such as DM, high BMI, hypertension, dyslipidemia, smoking and MS, and their impact on mortality after PCI makes this meta-analysis a completely new research in the field of interventional cardiology.
Several limitations in this meta-analysis were as follows: in a few studies, all-cause mortality was not among the clinical endpoints, however, being part of it, data concerning cardiac death has been considered. One study included mortality and myocardial infarction together. Because these data could not be separated, we have included them together in our meta-analysis. One study about smokers and PCI included data for the smokers undergoing fibrinolysis and PCI together. This may affect the result of our study to a certain extent. Moreover, in 1 study, overweight patients and obese patients were classified as a BMI >23.5 and 27.5 kg/m2 instead of 25 and 30 kg/m2, respectively. Another study classified a BMI of 25–35 kg/m2 to be considered as overweight and >35 kg/m2 to be considered as obese patients. Data for the baseline characteristics in several studies were not provided in the original article or could not be converted to dichotomous variables. Therefore, these data have been omitted in our meta-analysis. The baseline features of all the 100 studies have been analyzed and, the data concerning the number of patients with their corresponding risk factor in the whole study were obtained from the baseline features of each study. However, studies without dichotomous data at baseline, or if corresponding data were not available, have been omitted from this count. Therefore, except for the data being analyzed, the count for “modifiable cardiovascular risk factors for the whole study” is just an approximation for this study. However, despite these limitations, our data point to the urgent need for comprehensive comparison between these 2 groups of patients.
CONCLUSION
Certain modifiable cardiovascular risk subgroups had a significantly higher impact on mortality after PCI. However, mortality among the obese patients and the smokers showed an unexpected paradox after coronary intervention.
Footnotes
Abbreviations: BMI = body mass index, CAD = coronary artery disease, DM = diabetes mellitus, LDL = low-density lipoprotein, MS = metabolic syndrome, PCI = percutaneous coronary intervention.
There was no external source of funding for this research.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.GBD 2013 Mortality, Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta PK, Wei J, Wenger NK. Ischemic heart disease in women: a focus on risk factors. Trends Cardiovasc Med 2015; 25:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 2007; 356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 4.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014; 2:474–480. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JPTAD. Cochrane Handbook for Systematic Reviews of Interventions. Wiley 2008. [Google Scholar]
- 7.Lee MG, Jeong MH, Lee KH, et al. Prognostic impact of diabetes mellitus and hypertension for mid-term outcome of patients with acute myocardial infarction who underwent percutaneous coronary intervention. J Cardiol 2012; 60:257–263. [DOI] [PubMed] [Google Scholar]
- 8.Lingman M, Albertsson P, Herlitz J, et al. The impact of hypertension and diabetes on outcome in patients undergoing percutaneous coronary intervention. Am J Med 2011; 124:265–275. [DOI] [PubMed] [Google Scholar]
- 9.De Luca G, van’t Hof AW, Huber K, et al. Impact of hypertension on distal embolization, myocardial perfusion, and mortality in patients with ST segment elevation myocardial infarction undergoing primary angioplasty. Am J Cardiol 2013; 112:1083–1086. [DOI] [PubMed] [Google Scholar]
- 10.De Luca G, Dirksen MT, Spaulding C, et al. Impact of hypertension on clinical outcome in STEMI patients undergoing primary angioplasty with BMS or DES: insights from the DESERT cooperation. Int J Cardiol 2014; 175:50–54. [DOI] [PubMed] [Google Scholar]
- 11.Rembek M, Goch A, Goch J. The clinical course of acute ST-elevation myocardial infarction in patients with hypertension. Kardiol Pol 2010; 68:157–163. [PubMed] [Google Scholar]
- 12.Arbel Y, Matetzky S, Gavrielov-Yusim N, et al. Temporal trends in all-cause mortality of smokers versus non-smokers hospitalized with ST-segment elevation myocardial infarction. Int J Cardiol 2014; 176:171–176. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Li W, Wang Y, et al. Smoking status on outcomes after percutaneous coronary intervention. Clin Cardiol 2012; 35:570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto K, Nikolsky E, Lansky AJ, et al. Impact of smoking on outcomes of patients with ST-segment elevation myocardial infarction (from the HORIZONS-AMI Trial). Am J Cardiol 2011; 108:1387–1394. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Zhu ZY, Gao CY, et al. Long-term effect of persistent smoking on the prognosis of Chinese male patients after percutaneous coronary intervention with drug-eluting stent implantation. J Cardiol 2013; 62:283–288. [DOI] [PubMed] [Google Scholar]
- 16.Mohamedali B, Shroff A. Impact of smoking status on cardiovascular outcomes following percutaneous coronary intervention. Clin Cardiol 2013; 36:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson JO, Ebrahimi R, Lansky AJ, et al. Impact of cigarette smoking on extent of coronary artery disease and prognosis of patients with non-ST-segment elevation acute coronary syndromes: an analysis from the ACUITY Trial (Acute Catheterization and Urgent Intervention Triage Strategy). JACC Cardiovasc Interv 2014; 7:372–379. [DOI] [PubMed] [Google Scholar]
- 18.Tan NS, Goodman SG, Cantor WJ, et al. Comparison of the efficacy of pharmacoinvasive management for ST-segment elevation myocardial infarction in smokers versus non-smokers (from the Trial of Routine Angioplasty and Stenting After Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction). Am J Cardiol 2014; 114:955–961. [DOI] [PubMed] [Google Scholar]
- 19.Weisz G, Cox DA, Garcia E, et al. Impact of smoking status on outcomes of primary coronary intervention for acute myocardial infarction – the smoker's paradox revisited. Am Heart J 2005; 150:358–364. [DOI] [PubMed] [Google Scholar]
- 20.Cho KH, Jeong MH, Ahn Y, et al. Low-density lipoprotein cholesterol level in patients with acute myocardial infarction having percutaneous coronary intervention (the cholesterol paradox). Am J Cardiol 2010; 106:1061–1068. [DOI] [PubMed] [Google Scholar]
- 21.Ghazzal ZB, Dhawan SS, Sheikh A, et al. Usefulness of serum high-density lipoprotein cholesterol level as an independent predictor of one-year mortality after percutaneous coronary interventions. Am J Cardiol 2009; 103:902–906. [DOI] [PubMed] [Google Scholar]
- 22.Izuhara M, Ono K, Shiomi H, et al. High-density lipoprotein cholesterol levels and cardiovascular outcomes in Japanese patients after percutaneous coronary intervention: a report from the CREDO-Kyoto registry cohort-2. Atherosclerosis 2015; pii: S0021-915001311-8. [DOI] [PubMed] [Google Scholar]
- 23.Ji MS, Jeong MH, Ahn YK, et al. Impact of low level of high-density lipoprotein-cholesterol sampled in overnight fasting state on the clinical outcomes in patients with acute myocardial infarction (difference between ST-segment and non-ST-segment-elevation myocardial infarction). J Cardiol 2015; 65:63–70. [DOI] [PubMed] [Google Scholar]
- 24.Kini AS, Muntner P, Moreno PR, et al. Relation of high-density lipoprotein cholesterol to mortality after percutaneous coronary interventions in patients with low-density lipoprotein <70 mg/dl. Am J Cardiol 2009; 103:350–354. [DOI] [PubMed] [Google Scholar]
- 25.Seo SM, Choo EH, Koh YS, et al. High-density lipoprotein cholesterol as a predictor of clinical outcomes in patients achieving low-density lipoprotein cholesterol targets with statins after percutaneous coronary intervention. Heart 2011; 97:1943–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler J, Rodondi N, Zhu Y, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol 2006; 47:1595–1602. [DOI] [PubMed] [Google Scholar]
- 27.Kasai T, Miyauchi K, Kurata T, et al. Prognostic value of the metabolic syndrome for long-term outcomes in patients undergoing percutaneous coronary intervention. Circ J 2006; 70:1531–1537. [DOI] [PubMed] [Google Scholar]
- 28.Kasai T, Miyauchi K, Kurata T, et al. Impact of metabolic syndrome among patients with and without diabetes mellitus on long-term outcomes after percutaneous coronary intervention. Hypertens Res 2008; 31:235–241. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Lee HC, Choi BK, et al. Impact of metabolic syndrome on in-stent restenosis and clinical outcomes after percutaneous coronary stent implantation. Diabetes Res Clin Pract 2010; 88:e38–e41. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Lim YH, Shin JH, et al. The impact of metabolic syndrome on clinical outcomes after everolimus-eluting stent implantation. AJC 21208. PII: S0002-914901425-3. DOI: 10.1016/j.amjcard.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 31.Maron DJ, Boden WE, Spertus JA, et al. Impact of metabolic syndrome and diabetes on prognosis and outcomes with early percutaneous coronary intervention in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial. J Am Coll Cardiol 2011; 58:131–137. [DOI] [PubMed] [Google Scholar]
- 32.Marso SP, Mercado N, Maehara A, et al. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging 2012; 5 (3 Suppl):S42–S52. [DOI] [PubMed] [Google Scholar]
- 33.Patsa C, Toutouzas K, Tsiamis E, et al. Impact of metabolic syndrome on clinical outcomes after new generation drug-eluting stent implantation: the “obesity paradox” phenomenon is still apparent. Nutr Metab Cardiovasc Dis 2013; 23:307–313. [DOI] [PubMed] [Google Scholar]
- 34.Rana JS, Monraats PS, Zwinderman AH, et al. Metabolic syndrome and risk of restenosis in patients undergoing percutaneous coronary intervention. Diabetes Care 2005; 28:873–877. [DOI] [PubMed] [Google Scholar]
- 35.Akin I, Tolg R, Hochadel M, et al. No evidence of “obesity paradox” after treatment with drug-eluting stents in a routine clinical practice: results from the prospective multicenter German DES. DE (German Drug-Eluting Stent) Registry. JACC Cardiovasc Interv 2012; 5:162–169. [DOI] [PubMed] [Google Scholar]
- 36.Ellis SG, Elliott J, Horrigan M, et al. Low-normal or excessive body mass index: newly identified and powerful risk factors for death and other complications with percutaneous coronary intervention. Am J Cardiol 1996; 78:642–646. [DOI] [PubMed] [Google Scholar]
- 37.Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 2002; 39:578–584. [DOI] [PubMed] [Google Scholar]
- 38.Gruberg L, Mercado N, Milo S, et al. Impact of body mass index on the outcome of patients with multivessel disease randomized to either coronary artery bypass grafting or stenting in the ARTS trial: the obesity paradox II? Am J Cardiol 2005; 95:439–444. [DOI] [PubMed] [Google Scholar]
- 39.Gurm HS, Whitlow PL, Kip KE. The impact of body mass index on short- and long-term outcomes in patients undergoing coronary revascularization Insights from the bypass angioplasty revascularization investigation (BARI). J Am Coll Cardiol 2002; 39:834–840. [DOI] [PubMed] [Google Scholar]
- 40.He PY, Yang YJ, Qiao SB, et al. Impact of body mass index on the clinical outcomes after percutaneous coronary intervention in patients >/=75 years old. Chin Med J 2015; 128:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaneko H, Yajima J, Oikawa Y, et al. Obesity paradox in Japanese patients after percutaneous coronary intervention: an observation cohort study. J Cardiol 2013; 62:18–24. [DOI] [PubMed] [Google Scholar]
- 42.Kang WY, Jeong MH, Ahn YK, et al. Obesity paradox in Korean patients undergoing primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. J Cardiol 2010; 55:84–91. [DOI] [PubMed] [Google Scholar]
- 43.Kosuge M, Kimura K, Kojima S, et al. Impact of body mass index on in-hospital outcomes after percutaneous coronary intervention for ST segment elevation acute myocardial infarction. Circ J 2008; 72:521–525. [DOI] [PubMed] [Google Scholar]
- 44.Lancefield T, Clark DJ, Andrianopoulos N, et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv 2010; 3:660–668. [DOI] [PubMed] [Google Scholar]
- 45.Mehta L, Devlin W, McCullough PA, et al. Impact of body mass index on outcomes after percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol 2007; 99:906–910. [DOI] [PubMed] [Google Scholar]
- 46.Minutello RM, Chou ET, Hong MK, et al. Impact of body mass index on in-hospital outcomes following percutaneous coronary intervention (report from the New York State Angioplasty Registry). Am J Cardiol 2004; 93:1229–1232. [DOI] [PubMed] [Google Scholar]
- 47.Nikolsky E, Kosinski E, Mishkel GJ, et al. Impact of obesity on revascularization and restenosis rates after bare-metal and drug-eluting stent implantation (from the TAXUS-IV trial). Am J Cardiol 2005; 95:709–715. [DOI] [PubMed] [Google Scholar]
- 48.Numasawa Y, Kohsaka S, Miyata H, et al. Impact of body mass index on in-hospital complications in patients undergoing percutaneous coronary intervention in a Japanese real-world multicenter registry. PloS One 2015; 10:e0124399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park DW, Kim YH, Yun SC, et al. Association of body mass index with major cardiovascular events and with mortality after percutaneous coronary intervention. Circ Cardiovasc Interv 2013; 6:146–153. [DOI] [PubMed] [Google Scholar]
- 50.Payvar S, Kim S, Rao SV, et al. In hospital outcomes of percutaneous coronary interventions in extremely obese and normal-weight patients: findings from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol 2013; 62:692–696. [DOI] [PubMed] [Google Scholar]
- 51.Poludasu S, Cavusoglu E, Khan W, et al. Impact of body mass index on long-term all-cause mortality after percutaneous coronary intervention in African-Americans. J Invasive Cardiol 2009; 21:20–25. [PubMed] [Google Scholar]
- 52.Poston WS, Haddock CK, Conard M, et al. Impact of obesity on disease-specific health status after percutaneous coronary intervention in coronary disease patients. Int J Obesity Relat Metab Dis 2004; 28:1011–1018. [DOI] [PubMed] [Google Scholar]
- 53.Powell BD, Lennon RJ, Lerman A, et al. Association of body mass index with outcome after percutaneous coronary intervention. Am J Cardiol 2003; 91:472–476. [DOI] [PubMed] [Google Scholar]
- 54.Sarno G, Garg S, Onuma Y, et al. The impact of body mass index on the one year outcomes of patients treated by percutaneous coronary intervention with Biolimus- and Sirolimus-eluting stents (from the LEADERS Trial). Am J Cardiol 2010; 105:475–479. [DOI] [PubMed] [Google Scholar]
- 55.Shubair MM, Prabhakaran P, Pavlova V, et al. The relationship of body mass index to outcomes after percutaneous coronary intervention. J Interv Cardiol 2006; 19:388–395. [DOI] [PubMed] [Google Scholar]
- 56.Wang ZJ, Zhou YJ, Zhao YX, et al. Effect of obesity on repeat revascularization in patients undergoing percutaneous coronary intervention with drug-eluting stents. Obesity (Silver Spring, MD) 2012; 20:141–146. [DOI] [PubMed] [Google Scholar]
- 57.Witassek F, Schwenkglenks M, Erne P, et al. Impact of body mass index on mortality in swiss hospital patients with ST-elevation myocardial infarction: does an obesity paradox exist? Swiss Med Wly 2014; 144:w13986. [DOI] [PubMed] [Google Scholar]
- 58.Abizaid A, Kornowski R, Mintz GS, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol 1998; 32:584–589. [DOI] [PubMed] [Google Scholar]
- 59.Akin I, Bufe A, Schneider S, et al. Clinical outcomes in diabetic and non-diabetic patients with drug-eluting stents: results from the first phase of the prospective multicenter German DES. DE registry. Clin Res Cardiol 2010; 99:393–400. [DOI] [PubMed] [Google Scholar]
- 60.Antoniucci D, Valenti R, Migliorini A, et al. Impact of insulin requiring diabetes mellitus on effectiveness of reperfusion and outcome of patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2004; 93:1170–1172. [DOI] [PubMed] [Google Scholar]
- 61.Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075. [DOI] [PubMed] [Google Scholar]
- 62.Billinger M1, Beutler J, Taghetchian KR, et al. Two-year clinical outcome after implantation of sirolimus-eluting and paclitaxel-eluting stents in diabetic patients. Eur Heart J 2008; 29:718–725. [DOI] [PubMed] [Google Scholar]
- 63.Blöndal M1, Ainla T, Marandi T, et al. Sex-specific outcomes of diabetic patients with acute myocardial infarction who have undergone percutaneous coronary intervention: a register linkage study. Cardiovasc Diabetol 2012; 11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Claessen BE, Dangas GD, Godino C, et al. Long-term clinical outcomes of percutaneous coronary intervention for chronic total occlusions in patients with versus without diabetes mellitus. Am J Cardiol 2011; 108:924–931. [DOI] [PubMed] [Google Scholar]
- 65.Daemen J, Garcia-Garcia HM, Kukreja N, et al. The long-term value of sirolimus- and paclitaxel-eluting stents over bare metal stents in patients with diabetes mellitus. Eur Heart J 2007; 28:26–32. [DOI] [PubMed] [Google Scholar]
- 66.Elezi S, Kastrati A, Pache J, et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol 1998; 32:1866–1873. [DOI] [PubMed] [Google Scholar]
- 67.Fernández JF1, González CS, Navarro MJ, et al. High-risk diabetic patients with unprotected left main coronary artery disease: characteristics and medium-term outcomes of percutaneous revascularization with drug-eluting stents. Tex Heart Inst J 2011; 38:386–391. [PMC free article] [PubMed] [Google Scholar]
- 68.Garg P, Normand SLT, Silbaugh TS, et al. Drug-eluting or bare-metal stenting in patients with diabetes mellitus: results from the Massachusetts data analysis center registry. Circulation 2008; 118:2277–2285. [DOI] [PubMed] [Google Scholar]
- 69.El Ghannudi S, Ohlmann P, Jesel L, et al. Impaired inhibition of P2Y (12) by clopidogrel is a major determinant of cardiac death in diabetes mellitus patients treated by percutaneous coronary intervention. Atherosclerosis 2011; 217:465–472. [DOI] [PubMed] [Google Scholar]
- 70.Harjai KJ1, Stone GW, Boura J, et al. Comparison of outcomes of diabetic and nondiabetic patients undergoing primary angioplasty for acute myocardial infarction. Am J Cardiol 2003; 91:1041–1045. [DOI] [PubMed] [Google Scholar]
- 71.Hasdai D1, Granger CB, Srivatsa SS, et al. Diabetes mellitus and outcome after primary coronary angioplasty for acute myocardial infarction: lessons from the GUSTO-IIb angioplasty substudy. Global use of strategies to open occluded arteries in acute coronary syndromes. J Am Coll Cardiol 2000; 35:1502–1512. [DOI] [PubMed] [Google Scholar]
- 72.Hermiller JB, Raizner A, Cannon L, et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol 2005; 45:1172–1179. [DOI] [PubMed] [Google Scholar]
- 73.Jain AK, Lotan C, Meredith IT, et al. Twelve- month outcomes in patients with diabetes implanted with a zotarolimus-eluting stent: results from the E-Five Registry. Heart 2010; 96:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jensen LO1, Maeng M, Thayssen P, et al. Long-term outcomes after percutaneous coronary intervention in patients with and without diabetes mellitus in Western Denmark. Am J Cardiol 2010; 105:1513–1519. [DOI] [PubMed] [Google Scholar]
- 75.Jensen LO1, Thayssen P, Junker A, et al. Comparison of outcomes in patients with versus without diabetes mellitus after revascularization with everolimus- and sirolimus-eluting stents (from the SORT OUT IV trial). Am J Cardiol 2012; 110:1585–1591. [DOI] [PubMed] [Google Scholar]
- 76.Kassaian SE1, Goodarzynejad H, Boroumand MA, et al. Glycosylated hemoglobin (HbA1c) levels and clinical outcomes in diabetic patients following coronary artery stenting. Cardiovasc Diabetol 2012; 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kedhi E1, Généreux P2, Palmerini T3, et al. Impact of coronary lesion complexity on drug-eluting stent outcomes in patients with and without diabetes mellitus: analysis from 18 pooled randomized trials. J Am Coll Cardiol 2014; 63:2111–2118. [DOI] [PubMed] [Google Scholar]
- 78.Kereiakes DJ1, Cutlip DE, Applegate RJ, et al. Outcomes in diabetic and nondiabetic patients treated with everolimus- or paclitaxel-eluting stents: results from the SPIRIT IV clinical trial (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System). J Am Coll Cardiol 2010; 56:2084–2089. [DOI] [PubMed] [Google Scholar]
- 79.Kip KE1, Faxon DP, Detre KM, et al. Coronary angioplasty in diabetic patients. The National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. Circulation 1996; 94:1818–1825. [DOI] [PubMed] [Google Scholar]
- 80.Kirtane AJ, Ellis SG, Dawkins KD, et al. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J Am Coll Cardiol 2008; 51:708–715. [DOI] [PubMed] [Google Scholar]
- 81.Kirtane AJ1, Patel R, O'Shaughnessy C, et al. Clinical and angiographic outcomes in diabetics from the ENDEAVOR IV trial: randomized comparison of zotarolimus- and paclitaxel-eluting stents in patients with coronary artery disease. JACC Cardiovasc Interv 2009; 2:967–976. [DOI] [PubMed] [Google Scholar]
- 82.Kufner S, Byrne RA, de Waha A, et al. Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2, (ISAR-DESIRE 2) Investigators. Sirolimus-eluting versus paclitaxel-eluting stents in diabetic and non-diabetic patients within sirolimus-eluting stent restenosis: results from the ISAR-DESIRE 2 trial. Cardiovasc Revasc Med 2014; 15:69–75. [DOI] [PubMed] [Google Scholar]
- 83.Kumar R, Lee TT, Jeremias A, et al. Comparison of outcomes using sirolimus-eluting stenting in diabetic versus nondiabetic patients with comparison of insulin versus non-insulin therapy in the diabetic patients. Am J Cardiol 2007; 100:1187–1191. [DOI] [PubMed] [Google Scholar]
- 84.Kuramitsu S1, Yokoi H, Domei T, et al. Impact of post-challenge hyperglycemia on clinical outcomes in Japanese patients with stable angina undergoing percutaneous coronary intervention. Cardiovasc Diabetol 2013; 12:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee TT, Feinberg L, Baim DS, et al. Effect of diabetes mellitus on five-year clinical outcomes after singlevessel coronary stenting (a pooled analysis of coronary stent clinical trials). Am J Cardiol 2006; 98:718–721. [DOI] [PubMed] [Google Scholar]
- 86.Lenzen M1, Ryden L, Ohrvik J, et al. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur Heart J 2006; 27:2969–2974. [DOI] [PubMed] [Google Scholar]
- 87.Lima EG1, Hueb W, Garcia RM, et al. Impact of diabetes on 10-year outcomes of patients with multivessel coronary artery disease in the Medicine, Angioplasty, or Surgery Study II (MASS II) trial. Am Heart J 2013; 166:250–257. [DOI] [PubMed] [Google Scholar]
- 88.Maeng M1, Jensen LO, Tilsted HH, et al. Outcome of sirolimus-eluting versus zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a SORT OUT III Substudy). Am J Cardiol 2011; 108:1232–1237. [DOI] [PubMed] [Google Scholar]
- 89.Marui A, Kimura T, Nishiwaki N, et al. Five year outcomes of percutaneous versus surgical coronary revascularization in patients with diabetes mellitus (from the CREDO Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol 2015; 115:1063–1072. [DOI] [PubMed] [Google Scholar]
- 90.Mathew V1, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: are port from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480. [DOI] [PubMed] [Google Scholar]
- 91.Mehran R, Dangas GD, Kobayashi Y, et al. Short- and long-term results after multivessel stenting in diabetic patients. J Am Coll Cardiol 2004; 43:1348–1354. [DOI] [PubMed] [Google Scholar]
- 92.Muhlestein JB1, Anderson JL, Horne BD, et al. Effect of fasting glucose levels on mortality rate in patients with and without diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention. Am Heart J 2003; 146:351–358. [DOI] [PubMed] [Google Scholar]
- 93.Muramatsu T, Onuma Y, van Geuns RJ, et al. 1-year clinical outcomes of diabetic patients treated with everolimus-eluting bioresorbable vascular scaffolds: a pooled analysis of the ABSORB and the SPIRIT trials. JACC Cardiovasc Interv 2014; 7:482–493. [DOI] [PubMed] [Google Scholar]
- 94.Norhammar A, Malmberg K, Diderholm E, et al. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol 2004; 43:585–591. [DOI] [PubMed] [Google Scholar]
- 95.Onuma Y1, Wykrzykowska JJ, Garg S, et al. ARTS I and II Investigators. 5-Year follow-up of coronary revascularization in diabetic patients with multivessel coronary artery disease: insights from ARTS (arterial revascularization therapy study)-II and ARTS-I trials. JACC Cardiovasc Interv 2011; 4:317–323. [DOI] [PubMed] [Google Scholar]
- 96.Overgaard CB1, Džavík V, Buller CE, et al. Percutaneous revascularization and long term clinical outcomes of diabetic patients randomized in the Occluded Artery Trial (OAT). Int J Cardiol 2013; 168:2416–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park DW1, Flaherty JD, Davidson CJ, et al. Prognostic influence of diabetes mellitus on long-term clinical outcomes and stent thrombosis after drug-eluting stent implantation in Asian patients. Am J Cardiol 2009; 103:646–652. [DOI] [PubMed] [Google Scholar]
- 98.Silber S1, Serruys PW, Leon MB, et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global RESOLUTE program. JACC Cardiovasc Interv 2013; 6:357–368. [DOI] [PubMed] [Google Scholar]
- 99.Sohrabi B1, Ghaffari S, Habibzadeh A, et al. Outcome of diabetic and non-diabetic patients undergoing successful percutaneous coronary intervention of chronic total occlusion. J Cardiovasc Thorac Res 2011; 3:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stein B1, Weintraub WS, Gebhart SP, et al. Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation 1995; 91:979–989. [DOI] [PubMed] [Google Scholar]
- 101.Stone GW1, Kedhi E, Kereiakes DJ, et al. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation 2011; 124:893–900. [DOI] [PubMed] [Google Scholar]
- 102.Syed AI1, Ben-Dor I, Li Y, et al. Outcomes in diabetic versus nondiabetic patients who present with acute myocardial infarction and are treated with drug-eluting stents. Am J Cardiol 2010; 105:819–825. [DOI] [PubMed] [Google Scholar]
- 103.Tada T1, Kimura T, Morimoto T, et al. Comparison of three-year clinical outcomes after sirolimus-eluting stent implantation among insulin-treated diabetic, non-insulin-treated diabetic, and non-diabetic patients from j-Cypher registry. Am J Cardiol 2011; 107:1155–1162. [DOI] [PubMed] [Google Scholar]
- 104.Weber FD1, Schneider H, Wiemer M, et al. Sirolimus eluting stent (Cypher) in patients with diabetes mellitus: results from the German Cypher Stent Registry. Clin Res Cardiol 2008; 97:105–109. [DOI] [PubMed] [Google Scholar]
- 105.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 106.Witzenbichler B, Mehran R, Guagliumi G, et al. Impact of diabetes mellitus on the safety and effectiveness of bivalirudin in patients with acute myocardial infarction undergoing primary angioplasty: analysis from the HORIZONS-AMI (Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv 2011; 4:760–768. [DOI] [PubMed] [Google Scholar]
- 107.Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care 2003; 26:2181–2188. [DOI] [PubMed] [Google Scholar]
- 108.Geisler T, Anders N, Paterok M, et al. Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care 2007; 30:372–374. [DOI] [PubMed] [Google Scholar]
- 109.Dangas GD, Farkouh ME, Sleeper LA, et al. Long-term outcome of PCI versus CABG in insulin and non-insulin-treated diabetic patients: results from the FREEDOM trial. J Am Coll Cardiol 2014; 64:1189–1197. [DOI] [PubMed] [Google Scholar]
- 110.Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010; 363:2406–2415. [DOI] [PubMed] [Google Scholar]
- 111.Hasdai D, Holmes DR., Jr Smoking and outcome after PTCA. Eur Heart J 1997; 18:1520–1522. [DOI] [PubMed] [Google Scholar]
- 112.Jang JS, Buchanan DM, Gosch KL, et al. Association of smoking status with health-related outcomes after percutaneous coronary intervention. Circ Cardiovasc Interv 2015; 8:e002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Udell JA, Cavender MA, Bhatt DL, et al. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2015; 3:356–366. [DOI] [PubMed] [Google Scholar]
- 114.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471. [DOI] [PubMed] [Google Scholar]
- 115.Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014; 371:818–827. [DOI] [PubMed] [Google Scholar]


