Abstract
The major issue in selecting patients for transarterial chemoembolization (TACE) lies in determining the optimal number of TACE sessions that may benefit patients before switching to other therapies. This is often a subjective decision not based on any standardized protocol. The ART (Assessment for Retreatment with Transarterial chemoembolization) score was recently developed to determine patients who may benefit from multiple sessions of TACE for treatment of hepatocellular carcinoma. The primary aim of the study was to validate the ART score in a Taiwanese cohort. The secondary aims were to evaluate overall survival and clinical determinants of improved survival in patients treated with multiple TACE sessions. The ART score, clinical characteristics, and outcomes of 82 patients with hepatocellular carcinoma who received multiple TACE sessions at Taipei Veterans General Hospital from September 2007 to July 2013 were analyzed. Among the 82 patients evaluated, 69.5% (n = 57) had an ART score of 0 to 1.5 and 34.1% (n = 25) had a score of ≥2.5. The median overall survival was 23.1 months and the overall mortality rate was 62.2% (n = 51). The ART score was not associated with survival (P = 0.58). Multivariate Cox regression analysis revealed that tumor size >7.2 cm (hazard ratio 4.44, P < 0.001), aspartate transaminase (AST) level above 95 IU/L (hazard ratio 2.18, P = 0.02), AST increase more than 25% (hazard ratio 2.13, P = 0.02), 2nd/1st (pre-TACE) alpha-fetoprotein ratio (hazard ratio 1.40, P = 0.001), and lack of radiological response to TACE (hazard ratio 2.21, P = 0.02) were independent clinical determinants of survival. The ART score was not found to be effective in selecting patients for TACE retreatment in our Taiwanese cohort. Large tumor size, high AST level, high 2nd/1st (pre-TACE) alpha-fetoprotein ratio, AST increase >25%, and lack of radiological response to TACE were independently associated with shorter survival after TACE therapy.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 6th most common cancer worldwide and one of the highest causes of cancer-related deaths.1,2 The distribution of HCC is highly geographic, with 80% of the world's cases found in East-Asia, followed by sub-Saharan Africa and Melanesia.3,4 Chronic viral hepatitis infection and alcoholic liver cirrhosis are the major causes of HCC, whereas other chronic liver diseases (eg, nonalcoholic steatohepatitis or inherited metabolic disorders) represent a smaller proportion of HCC etiologies.5
In Asia, excluding Japan, the most prevalent etiology is chronic hepatitis B virus (HBV) infection, whereas in Japan and the western countries, hepatitis C virus (HCV) infection predominates, together with alcohol use.4 Therefore, different staging systems have been developed in different parts of the world.6 However, the Barcelona Clinic Liver Cancer (BCLC) staging system—an algorithm integrating baseline clinical status, liver function, prognosis, and therapeutic options—remains the most widely adopted staging system and is endorsed by both American and European management guidelines.7
For the early stages of HCC, curative therapies include surgical removal, orthotopic liver transplantation (OLT), and locoregional procedures (ie, radiofrequency ablation).8 Nevertheless, only 30% to 40% of patients with HCC are diagnosed in their early stage. HCC is more often diagnosed at the intermediate (BCLC stage B) and advanced (BCLC stage C) stages, when only palliative treatments, such as transarterial chemoembolization (TACE) or target therapy, can be considered.9
Compared to western countries, use of TACE is considerably higher in the Asian-Pacific countries.10 It is the standard of care for intermediate-stage HCC, but can also be performed in selected cases of early or advanced HCC.11,12 Despite its wide usage, the technical procedure has not been standardized and the treated patient population is usually heterogeneous with variable tumor burdens, liver function status, and disease etiologies.13,14 This implies that not all patients with intermediate-stage HCC will derive similar benefit from TACE, and some patients may benefit more from other treatment options, such as sorafenib, if the liver function is preserved (Child–Pugh ≦B7) in conditions not suitable for TACE such as main portal vein thrombosis, distant metastasis, or absence of radiologic tumor response after a previous TACE.15,16
The major issue in selecting patients for TACE is determining the optimal number of TACE sessions that will benefit patients before switching to other therapies.17,18 Unfortunately, this is often a subjective decision not based on any standardized protocol. For this reason, the Assessment for Retreatment with Transarterial chemoembolization (ART) score (a point score for the assessment of retreatment with TACE) has recently been developed in Austria.19 ART score is calculated based on 3 parameters measured just before the second TACE session, including Child–Pugh score increase (+1 or +2 points) from base line; aspartate transaminase (AST) increase >25% from baseline (not from normal value); and radiologic evidence of tumor response after a previous TACE session. An ART score of at least 2.5 indicates poor prognosis, whereas a score of 0 to 1.5 suggests better prognosis and potential benefit from repeat TACE.19 Its value was also validated by the same Austrian team, just before the 3rd and 4th TACE sessions.20
The ART score has been validated in other populations with mixed results. These other cohorts have included Japanese, Italian, and French patients with HCV-related cirrhosis as the major contributing factor for HCC.21–23 On the basis of the fact that the original score was developed in a population with mostly alcoholic cirrhosis-related HCC, while the more recent validations were based on HCV-related HCC, we postulated that the discrepancy in score effectiveness may be related to differences in underlying etiologies. Since the ART score has not been tested in a population with mainly HBV-related HCC, the principal aim of this study was to verify the effectiveness of ART score in a Taiwanese cohort with predominantly HBV-related HCC. The secondary objective was to determine the clinical factors associated with HCC survival.
MATERIALS AND METHODS
Study Population
This retrospective cohort study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (IRB No: 2013-08-018BC). Inform consent was waived for this retrospective study. This study was conducted at the Taipei Veterans General Hospital—a tertiary medical center in Taiwan, where approximately 500 cases of newly diagnosed HCC are treated each year. Patients were enrolled according to the study protocol of Sieghart et al,19 who first proposed the ART score as an aid to the decision-making process regarding retreatment of patients with TACE.
A total of 711 patients treated with conventional TACE (cTACE) at Taipei Veterans General Hospital between January 2007 and July 2013 were screened for eligibility. Patients were diagnosed with HCC by histology or dynamic computed tomography (CT)/magnetic resonance imaging (MRI) according to the most recent European Association for the Study of the Liver (EASL) diagnostic criteria. Inclusion criteria were as follows: age >18 years at the time of the first TACE cycle; BCLC stage of A or B with preserved liver function (Child–Pugh stage A or B7); and had received at least 2 TACE sessions within 100 days. Patients were excluded if they received TACE as a bridging therapy before liver transplantation or resection, or had received TACE for HCC recurrence after liver transplantation. In addition, patients with Child–Pugh C cirrhosis, main portal vein thrombosis, or those who presented with a Eastern Cooperative Oncology Group (ECOG) performance status >1 were considered unsuitable for retreatment with TACE. Patients with incomplete laboratory data (involving Child–Pugh score, alpha-fetoprotein [AFP], or AST) or those who lacked CT scan evaluation after TACE therapy were also excluded from analysis. Finally, a total of 82 patients were recruited for analysis. Six of the 7 patients who lost follow-up were deceased after as per the Taiwan Cancer Registry. The flowchart for patient selection is shown in Figure 1.
FIGURE 1.
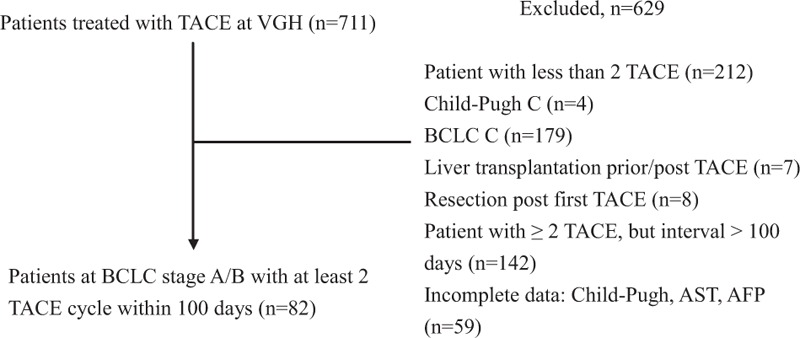
Flowchart for patient selection. AFP = alphafetaprotein, BCLC = Barcelona Clinic Liver Cancer, TACE = transarterial chemoembolization.
Data Collection
Demographic data, clinical data, and biochemical metrics (such as AFP, liver function parameters, clinical and radiological characteristics, and tumor treatment response) were collected in Taipei Veterans General Hospital. Dynamic changes in AFP, AST, and Child–Pugh scores were compared between the 2 TACE sessions. HCC was staged according to the BCLC classification.1,7 The definition of tumor size is diameter of the largest tumor. Radiologic evidence of tumor response was assessed by an independent radiologist who compared the CT/MRI scan before the second TACE session (maximal 100 days after the first TACE) to the baseline imaging performed 5 to 7 days before the first TACE session, according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria.24
Objective tumor response was classified into partial response, stable disease (SD), and progressive disease (PD). The definition of partial response was a decrease of the sum of unidimensional diameters of target lesions, which were viable and enhanced in the arterial phase, for at least 30%, using the sum of the diameters of target lesions at baseline as reference; the definition of progressive disease was an increase of the sum of the diameters of viable target lesions for at least 20% after treatment begun. Cases that did not meet the criteria for partial response or progressive disease were defined as stable disease.24 The observational period for patient survival lasted 1 year longer than the study period, that is, until July 2014.
TACE Procedure
Conventional TACE was performed by a treatment-on-demand schedule.13 Therefore, a TACE session would not be performed in the case of a complete radiologic response. To preserve as much viable liver parenchyma as possible, the feeding arteries of the tumor were catheterized superselectively. A mixture of 20�mg to 30�mg adriamycin (Carlo Erba, Milan, Italy) and 5�mL to 10�mL Lipiodol (Laboratoire Guerbet, Roissy-Charles-de-Gaulle Cedex, France) was infused into the supplying arteries. The dose of adriamycin and Lipiodol was decided depending on the size, number, and vascularity of the tumors. Under fluoroscopic guidance, the vessels were subsequently embolized with Gelfoam (Upjohn; Kalamazoo, MI) of less than 1-mm strips until complete flow stagnation was achieved. Laboratory measurements of serum biochemistry, AFP levels, and dynamic imaging studies of the liver were performed every 2 to 3 months after TACE. TACE was repeatedly performed in patients with compensated liver disease and residual viable tumor on dynamic imaging studies. The best supportive care was given if advanced liver failure occurred. Single-agent sorafenib was considered for patients with advanced HCC, including main and first branch of portal vein invasion and/or extrahepatic spread and preserved liver function (Child–Pugh class A).
Statistical Analysis
Statistical analysis was performed using R 3.1.2 software (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were expressed as mean ± standard deviation (SD) for continuous variables, and as the number of cases and percentage (%) for categorical variables. To detect nonlinear effects of continuous covariates and to identify appropriate cut-off point(s) for discretizing continuous covariates, we used the simple and multiple generalized additive models (GAMs), which added a nonparametric smoothing function on each covariate into generalized linear models (GLMs) for estimating nonlinear effects of continuous covariates.25
For univariate analysis, the Kaplan–Meier method with log-rank test was used to calculate survival and to determine significance. A 2-tailed P value <0.05 was considered statistically significant. To find the most appropriate regression model for multivariate analysis that fitted the observed data well for effect estimation and outcome prediction, basic model-fitting techniques for variable selection, goodness-of-fit (GOF) assessment, and regression diagnostics and remedies were performed. Any discrepancy between univariate and multivariate analysis results was likely due to the confounding effects of the uncontrolled covariates in univariate analysis. We arranged the GOF measures, including the coefficient of the estimated area under the concordance, and adjusted generalized R2 (for Cox proportional-hazards model) was examined to assess the GOF of the fitted regression model. The statistical tools of regression diagnostics and remedies, such as residual analysis to detect influential cases, confirmation of proportional hazards assumption, and check of multicollinearity, were applied to discover any data or model problems.
RESULTS
Patient Characteristics
Patient characteristics are summarized in Table 1. The majority of the 82 enrolled patients were BCLC stage B (n = 61, 74.4%) and all patients received conventional TACE as first-line therapy (n = 82, 100%). In our cohort, virus infection was the predominant etiology of cirrhosis, accounting for 89% (HBV, n = 42, 51.2%; HCV, n = 30, 36.6%; HCV + HBV, n = 1, 1.2%, total viral etiology, n = 73, 89%) of the population. There was no case of alcoholic liver cirrhosis-related HCC.
TABLE 1.
Patient Characteristics of the Enrolled 82 Cases
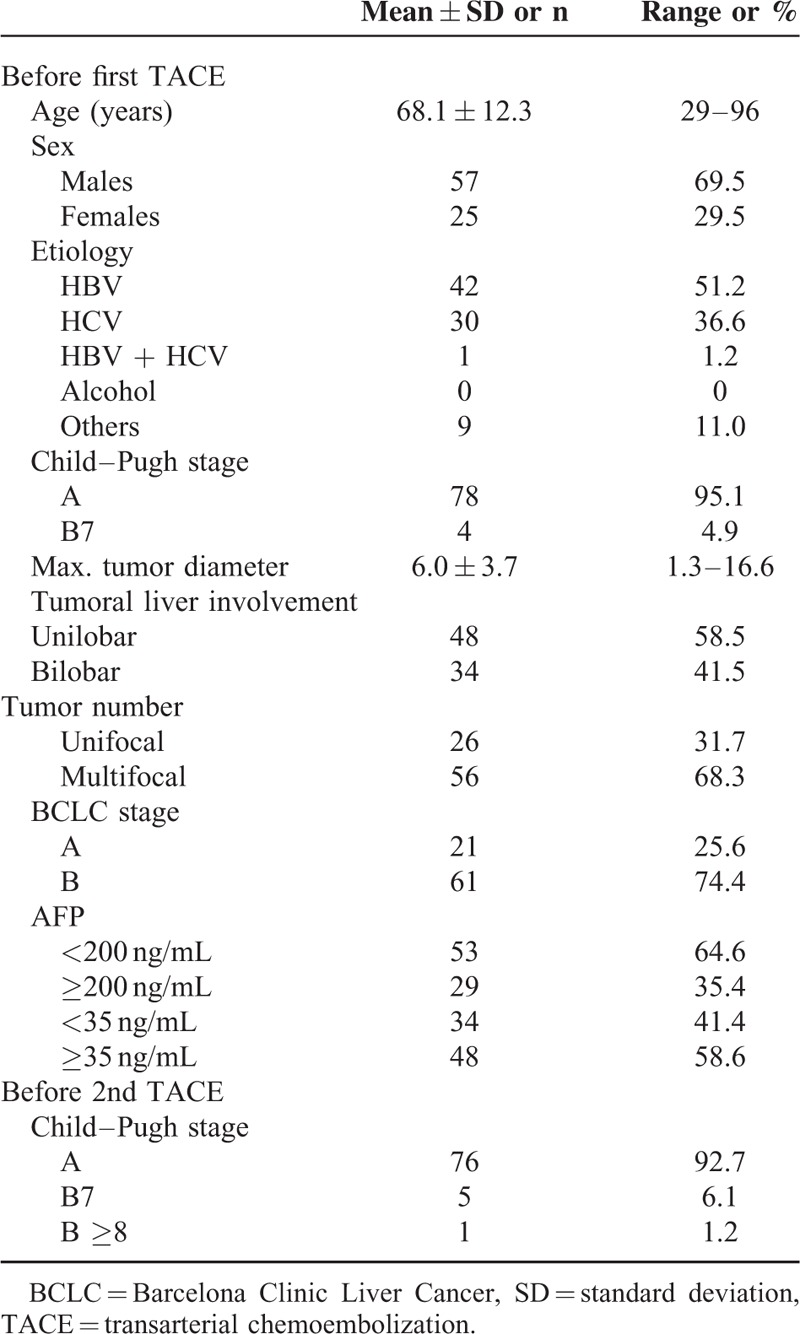
Between the 1st and 2nd TACE, 18 patients (22.0%) experienced a Child–Pugh score increase of at least 1 point, whereas 59 patients (72.0%) showed no change and 5 patients (6.0%) showed a decrease in the Child–Pugh score of at least 1 point. The median number of TACE performed was 4 (range 2–13) and the median time interval between the 1st and 2nd TACE was 70 days (range 23–98).
Overall Survival and Univariate Analysis of Prognostic Factors
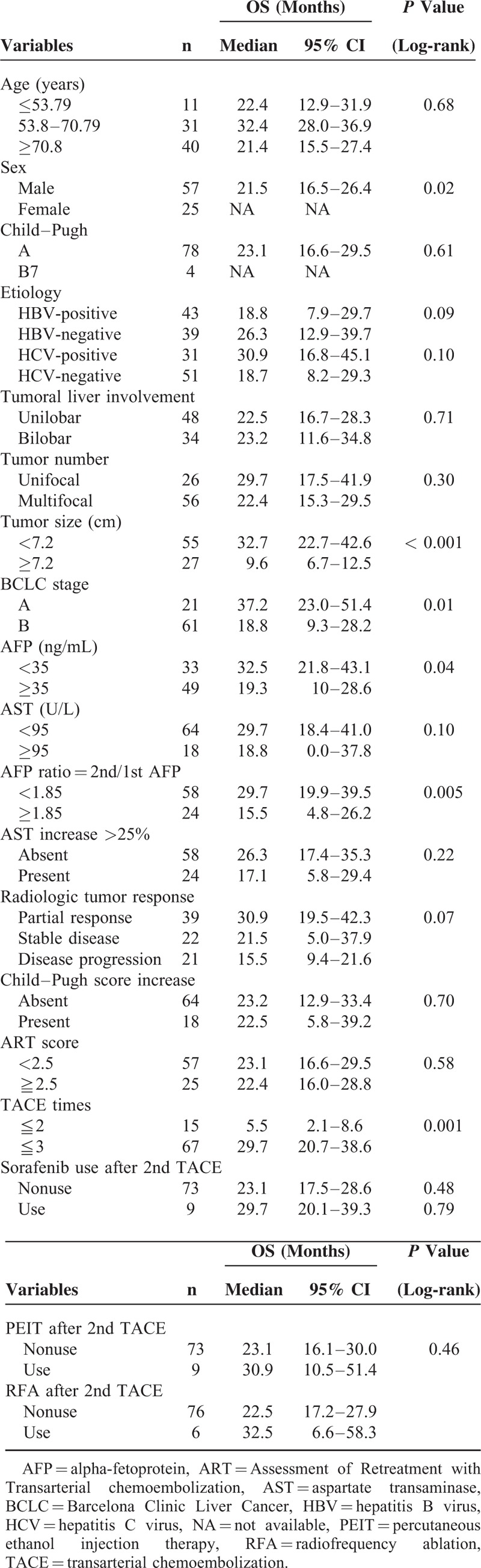
During the observational period, 51 patients (62.2%) died, 24 (29.3%) were still alive, and 7 (8.5%) were lost to follow-up. The median overall survival (OS) of the entire patient population was 23.1 months (95% confidence interval [CI] 16.3–29.7). The median follow-up was 19.8 months. Upon univariate analysis, sex (P = 0.02), tumor size (P < 0.001), BCLC stage (P = 0.01), baseline AFP level (P = 0.04), AFP ratio defined by 2nd/1st AFP (P = 0.005), and TACE more than 2 times (P = 0.001) were significantly associated with survival (Table 2). The use of sorafenib after tumor progression in 9 patients, percutaneous ethanol injection therapy in 9 patients, and radiofrequency ablation in 6 patients after 2nd TACE were all not associated with better survival (P = 0.48, 0.79, and 0.48 respectively; Table 2).
TABLE 2.
Overall Survival and Univariate Analysis of Prognostic Factors
Multivariate Analysis
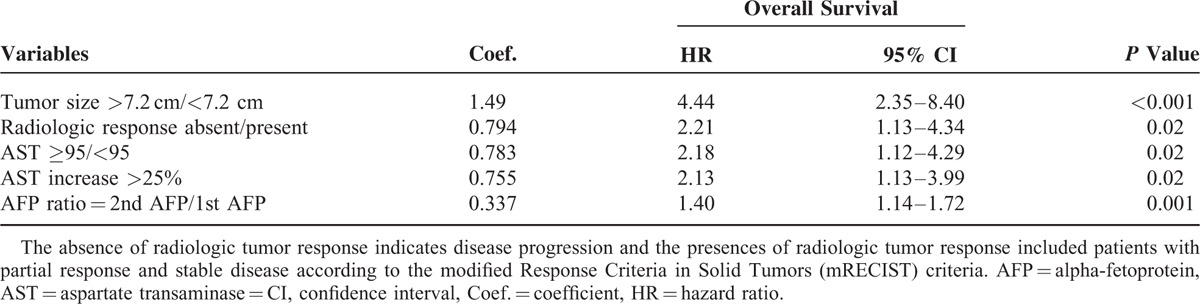
All potential relevant covariates were placed on the multivariate analysis. After one-at-a-time stepwise removal of the covariates with P value >0.05 until all regression coefficients were significantly different from 0, tumor size >7.2 cm (hazard ratio [HR] 4.44, 95% CI 2.35–8.40, P < 0.001), absence of radiologic tumor response (HR 2.21, 95% CI 1.13–4.34, P = 0.02), AST ≥95 IU/L (HR 2.18, 95% CI 1.12–4.29, P = 0.02), AST increase >25% (HR 2.13, 95% CI 1.13–3.99, P = 0.02), and AFP ratio (2nd/1st AFP, HR 1.40, 95% CI 1.14–1.72, P = 0.001) were independent factors associated with poor survival (Table 3).
TABLE 3.
Multivariate Stepwise Cox Regression Analysis of Prognostic Factors
ART Score
Among the 82 patients, 57 (69.5%) had an ART score of 0 to 1.5, and 25 (34.1%) had a score ≥2.5. The ART score was not found to be associated with survival (P = 0.58).
DISCUSSION
To the best of our knowledge, this is the first time the ART score has been tested in a population with primarily HBV-related HCC. Previous studies in Japan, Italy, and France were composed predominantly of patients with HCV-related cirrhosis, and the original creation of ART score in Austria was based on patients with alcoholic cirrhosis.19,21–23 Major study results evaluating the effectiveness of ART score are summarized in Table 4. In our study, ART score was not associated with survival.
TABLE 4.
The Effectiveness of ART Score in Different Populations
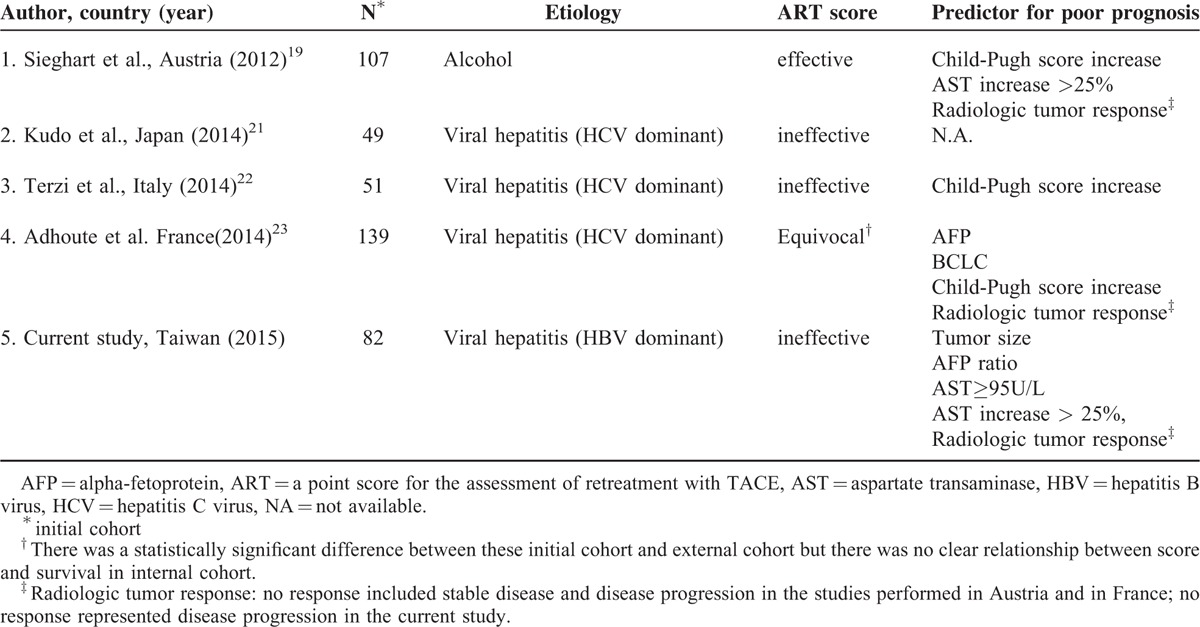
Our finding of a positive association between survival and an AST increase >25% was similar to the findings of Sieghart et al.19 A significant association between radiologic tumor response and survival was also noted in our study, similar to the results of Sieghart et al19 and Adhoute et al.23 Sieghart et al,19 Terzi et al,22 and Adhoute et al23 all showed that Child–Pugh score increase was a predictor of survival. In contrast, tumor size, AFP ratio, and high baseline AST level were found to have statistical significance in our population. The above discrepancies in study results may be related to differences in patient populations.
Upon multivariate analysis, a baseline AST level above 95 IU/L and an AST increase >25% were both significant variables based on our population. Cirrhosis-related HCC is present in 80% to 90% of patients with HCC.5 In our hospital, nearly all patients with HBV-related HCC receive antiviral agents for tertiary prophylaxis and patients undergoing TACE therapy have stable HBV control.26 In comparison, patients with alcoholic cirrhosis-related HCC or HCV-related HCC more frequently experience fluctuations in AST levels as they are usually not receiving antiviral therapy when HCC occurs and thus may have a higher prevalence of AST changes unrelated to TACE.19,22,23
Before the 2nd TACE session, Child–Pugh A patients still accounted for 92.7% of our population. Therefore, it is not surprising that Child–Pugh score increase was not a significant clinical determinant of survival in the current study. Of the 18 patients who experienced a Child–Pugh score increase of at least 1 point, only 2 of them had an increase of 2 points and 16 of them had an increase of 1 point. The possible explanation may be that doctors in our hospital tend not to arrange a second TACE under circumstances of liver function deterioration. In addition, our patients’ OS was higher than that reported by Sieghart et al (median OS 23.1 months vs 16.2 months, respectively).
In addition to our HBV-predominant patient population, another feature of our study was the method of statistical analysis. In our analysis, meaningful cut-points that affected survival were detected by simple and multiple GAMs. In contrast to the study by Sieghart et al, where only variables with P < 0.05 in the univariate analysis were entered as candidate variables into the multivariate stepwise Cox regression model,19 we included all the univariate significant and nonsignificant relevant covariates (listed in Table 1) and some of their interactions on the multivariate list to be selected. Therefore, confounding effects of the uncontrolled covariates in univariate analysis would not be missed.
This study had several limitations including its single-center, retrospective nature. All patients in our cohort received conventional TACE. Therefore, our findings may not extend to patients accepting TACE-drug eluting bead (DEB). Our patients did not undergo surgery or other therapies (such as Y-90 or sorafenib); therefore, our results may not be generalizable to patients who received such therapies. In addition, patients with segmental portal vein thrombosis were classified as BCLC stage C and were excluded from this study. However, they might still benefit from TACE, according to several recently published papers.27–30 Discrepancy between the number of patients receiving TACE (n = 711) and the number of patients included in analysis (n = 82) should also be noted. Thus, the effectiveness of the ART score in guiding TACE retreatment in this population requires further testing using larger randomized, controlled prospective studies.
In summary, the ART score did not apply to our series of patients with mainly viral infection-related HCC (mostly HBV infection) who had well preserved liver function. Whether the ART score might be effective in other patients with alcoholic-related HCC remains to be elucidated. As suggested by Adhoute et al, the most rational strategy seems to be a clinically-oriented individualized selection of patients for each course of TACE, considering each patient's clinical condition, liver function, the location and extent of viable tumor, and available treatment options.31
In conclusion, the ART score was not an effective guide for TACE retreatment in Taiwanese patients with primarily viral infection-related HCC. Large tumor size, high AST level, an AST increase >25%, high AFP ratio, and absence of radiologic tumor response were independent clinical factors associated with poor survival after TACE in our population.
Acknowledgments
We appreciate FCH for the consultation on statistical analysis.
Footnotes
Abbreviations: ART = Assessment for Retreatment with Transarterial chemoembolization, HCC = hepatocellular carcinoma, OS = overall survival, TACE = transarterial chemoembolization.
Authors’ contributions: YC planned and conducted the study, interpreted the data, drafted and revised the manuscript, approved the final draft, accepted full responsibility for the conduct of the study, and had control of the decision to publish the study. C-LT conducted the study, collected, analyzed, and interpreted the data, revised the manuscript, and approved the final draft. W-JL, C-JH, and Y-HH conducted the study, collected and interpreted the data, drafted the manuscript, and approved the final draft. C-WS, I-CL, H-ST, C-PL, R-CL, and H-CL revised the manuscript and approved the final draft.
‘Funding: This study was partially funded by the Szu-Yuan Research Foundation of Internal Medicine.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908–943. [DOI] [PubMed] [Google Scholar]
- 2.Kim do Y, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer 2012; 1:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han KH, Kudo M, Ye SL, et al. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology 2011; 81 Suppl 1:158–164. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365:1118–1127. [DOI] [PubMed] [Google Scholar]
- 5.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127 (5 Suppl 1):S35–S50. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005; 41:707–716. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM1, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19:329–338. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362:1907–1917. [DOI] [PubMed] [Google Scholar]
- 9.Hucke F, Sieghart W, Schöniger-Hekele M, et al. Clinical characteristics of patients with hepatocellular carcinoma in Austria - is there a need for a structured screening program? Wien Klin Wochenschr 2011; 123:542–551. [DOI] [PubMed] [Google Scholar]
- 10.Cheng AL, Amarapurkar D, Chao Y, et al. Re-evaluating transarterial chemoembolization for the treatment of hepatocellular carcinoma: consensus recommendations and review by an International Expert Panel. Liver Int 2014; 34:174–183. [DOI] [PubMed] [Google Scholar]
- 11.Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terzi E, Golfieri R, Piscaglia F, et al. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol 2012; 57:1258–1267. [DOI] [PubMed] [Google Scholar]
- 14.Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 2012; 32:348–359. [DOI] [PubMed] [Google Scholar]
- 15.Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011; 37:212–220. [DOI] [PubMed] [Google Scholar]
- 16.Raoul JL, Bruix J, Greten TF, et al. Relationship between baseline hepatic status and outcome, and effect of sorafenib on liver function: SHARP trial subanalyses. J Hepatol 2012; 56:1080–1088. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012; 57:821–829. [DOI] [PubMed] [Google Scholar]
- 19.Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 2013; 57:2261–2273. [DOI] [PubMed] [Google Scholar]
- 20.Hucke F, Sieghart W, Pinter M, et al. The ART-strategy: Sequential assessment of the ART scorepredicts outcome of patients with hepatocellular carcinoma re-treated with TACE. J Hepatol 2016; 60:118–126. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Arizumi T, Ueshima K. Assessment for retreatment (ART) score for repeated transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 2014; 59:2424–2425. [DOI] [PubMed] [Google Scholar]
- 22.Terzi E, Terenzi L, Venerandi L, et al. The ART score is not effective to select patients for transarterial chemoembolization retreatment in an Italian series. Dig Dis 2014; 32:711–716. [DOI] [PubMed] [Google Scholar]
- 23.Adhoute X, Penaranda G, Naude S, et al. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol 2015; 62:855–862. [DOI] [PubMed] [Google Scholar]
- 24.Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 2011; 55:1309–1316. [DOI] [PubMed] [Google Scholar]
- 25.Guisan A, Edwards TC, Jr, Hastie T. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Modell 2002; 157:89–100. [Google Scholar]
- 26.Chien RN. Can tertiary prevention of hepatocellular carcinoma be achieved by nucleos(t)ide analogs therapy of hepatitis B? J Gastroenterol Hepatol 2011; 26:1699–1701. [DOI] [PubMed] [Google Scholar]
- 27.Kim KM, Kim JH, Park IS, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol 2009; 24:806–814. [DOI] [PubMed] [Google Scholar]
- 28.Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011; 258:627–634. [DOI] [PubMed] [Google Scholar]
- 29.Xue TC, Xie XY, Zhang L, et al. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol 2013; 13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011; 18:413–420. [DOI] [PubMed] [Google Scholar]
- 31.Adhoute X, Penaranda G, Castellani P, et al. Recommendations for the use of chemoembolization in patients with hepatocellular carcinoma: usefulness of scoring system? World J Hepatol 2015; 7:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]


