Abstract
Osteogenesis imperfecta (OI) is a hereditary connective tissue disorder that leads to bone weakness and deformities, especially in the spine, which can lead to poor outcomes.
The aim of this study was to find patterns and risk factors in spinal deformities in patients with OI.
In a retrospective study, 70 patients with OI were selected. Radiographs of the spine were evaluated. We observed the presence or absence of the following changes: biconcave vertebrae, chest and vertebral deformities, unilateral rib, and thoracolumbar kyphosis. The greater curve was considered the primary one, and the secondary curve considered compensatory.
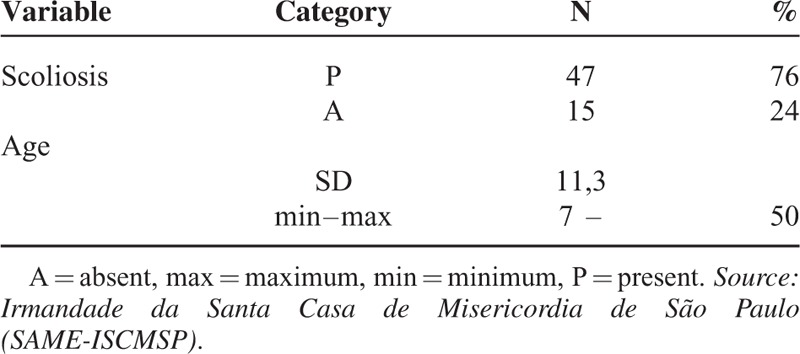
In the study sample, we observed that the patients’ ages ranged between 7 and 50 years, with a mean equal to 13 years, and 76% had scoliosis. In 68% of cases the main curve in the thoracic region was observed with the convexity to the right.
The following was found in patients with OI: scoliosis, biconcave vertebrae, vertebral and chest deformity, unilateral rib, and thoracolumbar kyphosis. The thoracolumbar kyphosis is highly associated with thoracic hypokyphosis in patients with OI.
INTRODUCTION
Osteogenesis imperfecta (OI) is a hereditary connective tissue disorder due to a qualitative or quantitative defect of type I collagen. OI is a rare disease, occurring in 1 case per 15,000 to 20,000 births and affecting 1 in every 200,000 individuals; however, there are no citations in the literature about the predominance of race or gender.1
The main features of OI are ligamentous laxity, osteopenia, short stature, fractures caused by mild trauma, progressive skeletal deformities, and additional clinical manifestations including blue sclera, dentinogenesis imperfecta, and deafness.2,3
Bone fragility in the spine usually leads to serious consequences. The vertebrae can easily break, either in an acute or in a chronic form, and they commonly present deformed and with a biconcave shape due to the pressure exerted by the intervertebral discs at the endplate of the vertebrae (Fig. 1). The prevalence of scoliosis in OI patients ranges from 39% to 100% according to the literature,4 very high when compared to the same ratio in the general population (idiopathic scoliosis), which is close to 2% according to the classic study of ref.5
FIGURE 1.
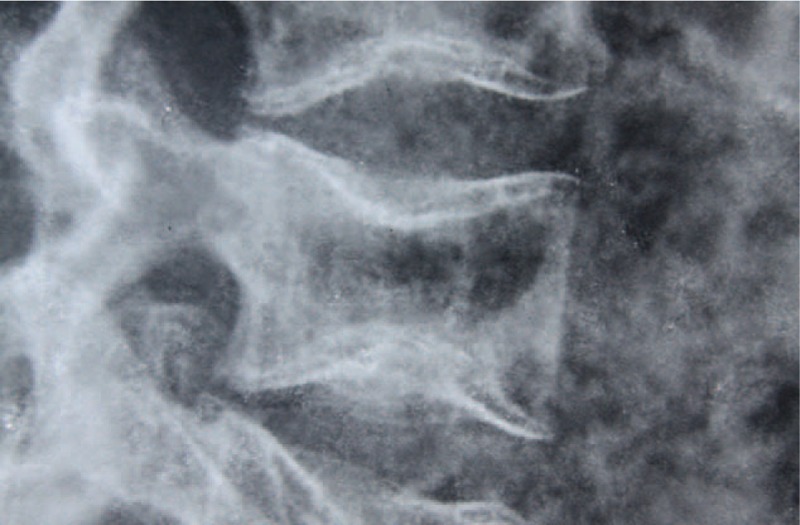
Biconcave vertebrae. Source: Irmandade da Santa Casa de Misericordia de São Paulo (SAME ISCMSP).
Axial skeleton changes can lead to substantial functional disability associated with a painful clinical condition and, in severe cases (Fig. 2), even signs of radicular spinal neurological impairment.6 In addition, patients with considerable deformities of the thoracic cage still show decreased lung ventilatory capacity, and the cardio-respiratory complications are the major cause of late morbidity and mortality.7
FIGURE 2.
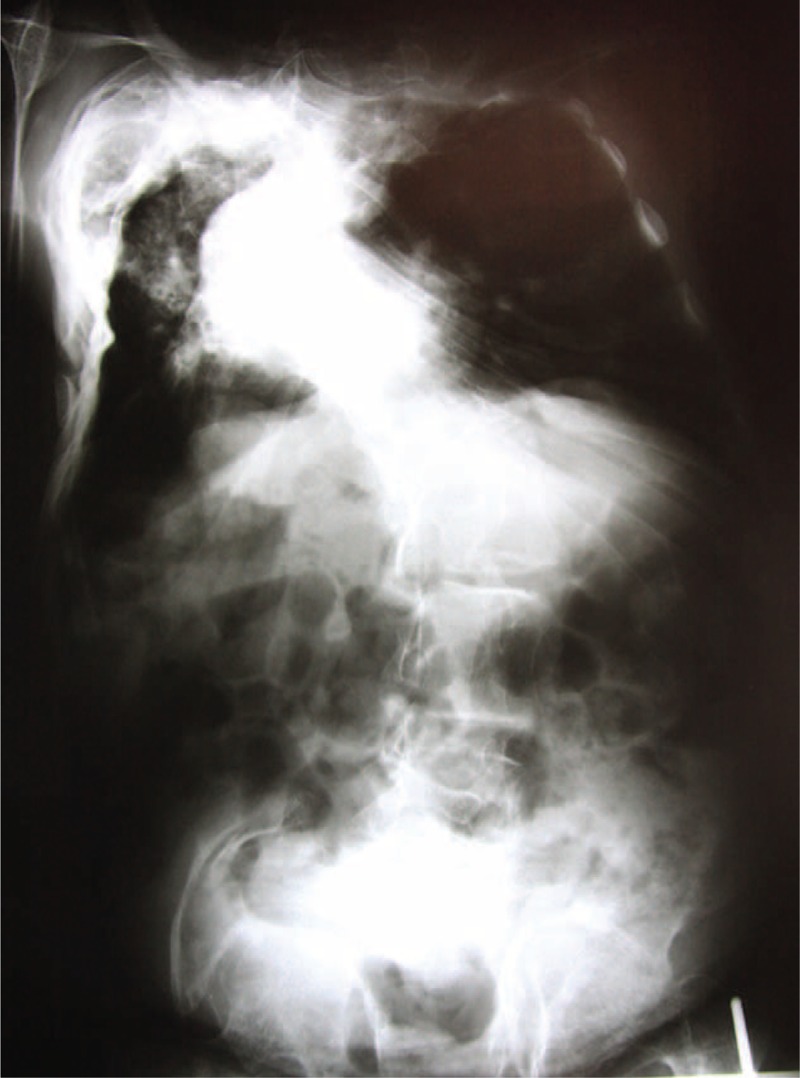
Severe scoliosis in the osteogenesis imperfecta (OI) patient. Source: Irmandade da Santa Casa de Misericordia de São Paulo (SAME-ISCMSP).
The aim of this study was to find patterns and risk factors in spinal deformities in patients with OI.
METHODS
This study was conducted at the Osteometabolic Diseases Group of Pediatric Orthopedics and Traumatology Group, Department of Orthopaedics and Traumatology from Irmandade da Santa Casa de Misericordia de São Paulo – CROI MS (OI Reference Center of the Ministry of Health).
This study was approved by the Ethics and Research Committee and when necessary, data were collected from medical records of the Department of Medical Records of the Irmandade da Santa Casa de Misericordia de São Paulo.
Inclusion criteria: to be a patient with OI diagnosis with panoramic X-rays in frontal and lateral views (coronal and sagittal) of the spine, and agree to sign the consent form (or responsible person in case of minors).
Patients with any other metabolic bone disease except OI were excluded, as were those who underwent surgery for correction of spinal deformity and did not have preoperative radiographs, or those who did not agree to sign the consent.
Radiographic Evaluation and Measurements
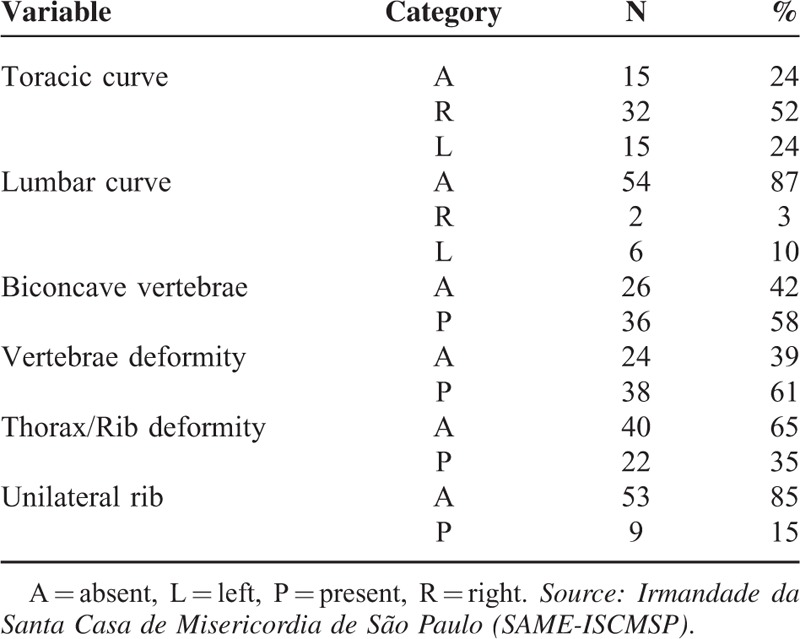
Radiographs of the spine were evaluated in the coronal (anterior–posterior) and sagittal (lateral) views, in the standing position, of all patients included in the study, except in the cases of the nonambulating patients, in which the radiographs were obtained in the sitting position. Angles were measured according to the Cobb method8 for scoliosis, lumbar lordosis, and thoracic kyphosis, which consists of measuring the angle between the line perpendicular to the upper terminal plates of the first vertebra and the lower vertebra that belongs to the curve. We observed the presence or absence of the following changes: biconcave vertebrae (Fig. 1), chest and vertebral deformities, unilateral rib (Fig. 3), and thoracolumbar kyphosis (Fig. 4). The greater curve was considered the primary one, and the secondary curve considered compensatory. The thoracolumbar transition, defined as a neutral zone, was limited proximally by the eleventh thoracic vertebra and the second lumbar vertebra distally (ie, included the T11, T12, L1, and L2 vertebrae).
FIGURE 3.
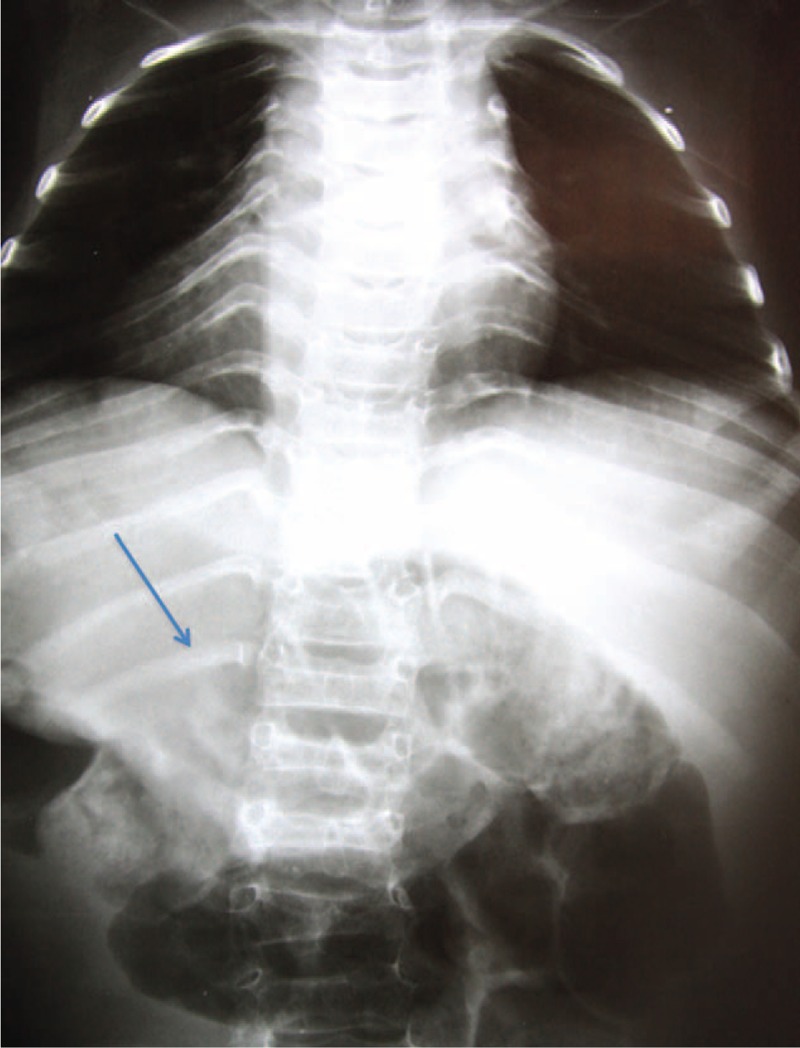
Thorax deformity and unilateral rib (arrow). Source: Irmandade da Santa Casa de Misericordia de São Paulo (SAME-ISCMSP).
FIGURE 4.
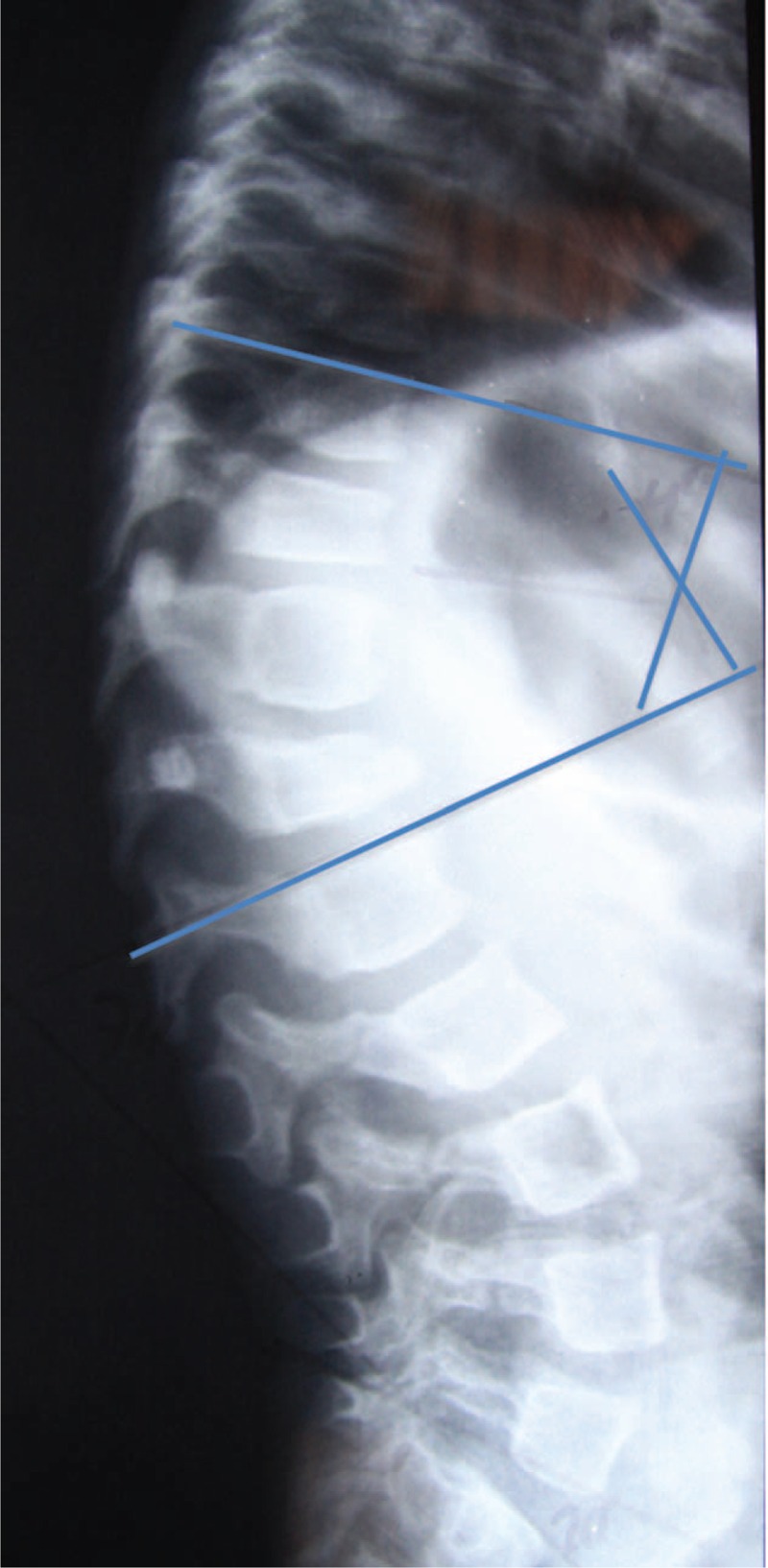
Thoracolumbar kyphosis. (Cobb T10-L2: 40°) Source: Irmandade da Santa Casa de Misericordia de São Paulo (SAME–ISCMSP).
Statistical Analysis
For descriptive analysis, categorical measures were presented on a gross basis and percentage; regarding continuous measures, means, and standard deviations were presented.
Inferentially, to test the association between severity of thoracic kyphosis and lumbar lordosis and angular deformity severity, Spearman correlation was used since violation of the assumption of normal distribution (assessed by Kolmogov–Smirnov test) in the angles, level of measurement did not have intervals between categories of severity (ie, increased, decreased, and physiological). To interpret the magnitude of the correlations, we consider the study of Cohen.9
To evaluate the association between 2 dichotomous measures (ie, presence/absence, as in the case of the association between the presence of thoracolumbar kyphosis and scoliosis), we used the Chi-square test. If values less than 5 were observed in the blanks, we used the Fisher exact test.
A P-value lower than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 17.
RESULTS
Seventy patients with OI who attended said service between 2009 and 2010 were selected. Eight patients were excluded because they underwent surgery to correct deformities of the spine and lacked adequate preoperative radiographs. Of the total 62 patients included in the study, 34 were female (54.83%) and 28 male.
In the study sample (62 patients), we observed that the patients’ ages ranged between 7 and 50 years, with a mean equal to 13 years, and 76% had scoliosis. The socio-demographic characteristics of the participants are described in Table 1.
TABLE 1.
Scoliosis Rate and Age
In the radiographic variables (Table 2), we note that 52% had right thoracic curve and 13% a secondary or compensatory lumbar curve. The biconcave vertebrae (Fig. 4) appeared in 58% of radiographs. The vertebral deformity (Figs. 2 and 3) was present in 61% of cases. Thirty-five percent had deformity of the chest and ribs, and 15% showed the presence of unilateral rib.
TABLE 2.
Radiographic Variables
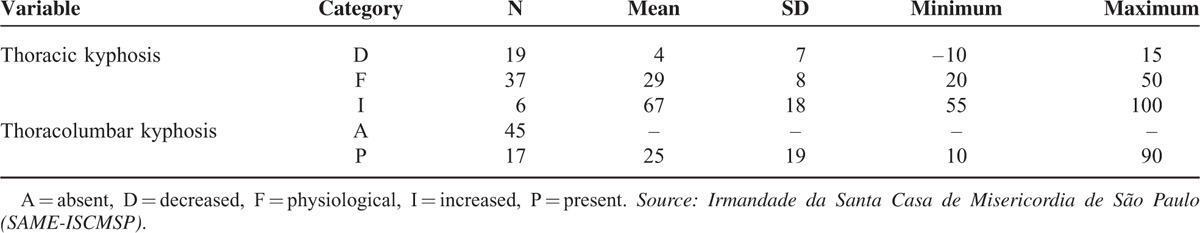
In Table 3, we note that the greater the severity of thoracic kyphosis, the greater the average intensity of angular deformity; the magnitude of this correlation was statistically significant and strong (r = 0.785, P < 0:01). After the physiological thoracic kyphosis, we note that the decreased thoracic kyphosis was the most frequent (31%). For the 17 patients with thoracolumbar kyphosis (Figure 6), the lordosis angle ranged between 10° and 90°, with an average equal to 25°.
TABLE 3.
Thoracic and Thoracolumbar Kyphosis Main Measures
It is noteworthy that one cannot infer causality in such associations, that is, one cannot infer the direction of causality between the 2 pairs of measurements.
Considering the associations between 2 dichotomous measurements, we observed that thoracolumbar kyphosis is associated with: Scoliosis (Fisher exact test P-value = 0.048), biconcave vertebrae (Fisher exact test P-value = 0.004), deformity of the vertebra (Fisher exact test P-value = 0.001), and deformity of the chest (χ2 (1) = 5.543, P = 0.018).
DISCUSSION
The prevalence of scoliosis in patients with OI varies between 25% and 100% according to the literature (Watanabe et al, 2007).10 In our study, 47 patients (76% of cases) had a deformity. In 68% of cases, the main curve in the thoracic region was observed with the convexity to the right, similar to the pattern that is most commonly found in idiopathic scoliosis.10–12
Ishikawa et al4 identified the presence of biconcave vertebrae as a risk factor for developing severe spinal deformity. Indeed, in our study, 58% of patients had this deformity. Yet our evaluation showed that the vertebral deformity is present in 61% of cases, the deformation of the chest in 35%, and the presence of unilateral rib in 15% of cases. Renshaw et al13 had also identified these radiographic risk factors for negative outcomes (Fig. 3).
Hanscom et al6 evaluated 43 patients with OI and spinal deformity, which were placed in 6 well-defined groups based on a set of radiological alterations. The radiographic criteria used for classification include the shape, dimensions, and appearance of long bones, pelvis in the presence of clover and acetabular protrusion, and the shape of the vertebrae. This study, however, considers the pelvis and long bones, not only the spine. Patients could be classified more accurately when the dynamic nature of radiographic changes were recorded. Furthermore, the authors observed a difficulty in that progressive X-rays are needed to facilitate classification. Because of that it is only effective in cases where periodic radiographic records for some years are present, then in those cases it would be possible to distinguish the groups more efficiently, otherwise it is poorly reproducible.
Abelin et al14 evaluated the appearance of deformities of the spine and changes of physiological sagittal plane curves that can be explained by multiple fractures of the spine compression, reducing the height of the bodies. These progressive changes of the trunk are responsible for global sagittal imbalance. However, only 18 patients with OI participated in that study. In these patients, thoracic kyphosis was statistically significantly higher in the control group. The sagittal balance was positive in patients with OI and the negative control group. This statistically significant difference between the displacements in the sagittal plane of the groups indicated that patients with OI have a center of gravity that allows them to balance the trunk displaced anteriorly, when compared with the normal population.
Our radiographic thoracic kyphosis followed the recommendation of the Scoliosis Research Society (SRS),15,16 who consider normal values for thoracic kyphosis as a range between 20° and 50°, with the curve measured by the method of Cobb modified between the vertebrae T3 to T12. Furthermore, the SRS defines a normal absolute value of 40° in thoracic kyphosis. Even in 37 patients who had thoracic kyphosis within the normal range (between 20° and 50°), the average was 29° below the normal absolute value of 40°. This drew attention to the high number of cases with hypokyphosis, 19, in relation to cases with kyphosis, 6. However, as opposed to the study of Abelin et al,14 a tendency toward decreased thoracic kyphosis was observed in the normal population.
Koerber et al17 introduced a new classification of spinal abnormalities in patients with OI, but used only lateral view X-rays. As in our study, they identified the high incidence of thoracolumbar kyphosis as a risk factor.
The presence of thoracolumbar kyphosis is always pathologic18 (Fig. 4). The SRS itself also defined the region as a neutral zone without angulation in the coronal and sagittal planes. We observed that the presence of thoracolumbar kyphosis showed a statistically significant relationship with the presence of decreased thoracic kyphosis. Deformities of the spine in these patients are caused in part by microfractures. The thoracolumbar region is burdened by the rib cage and the trend is to increase the kyphosis, as in normal people, where the fracture of the thoracolumbar spine usually defaults to segmental kyphosis.19 With the thoracolumbar kyphosis acquired chronically leads us to suspect that the thoracic hypokyphosis is a consequence of the need to equalize the overall sagittal balance, which rectifies the segment in order to offset the deformity of the adjacent distal segment.
CONCLUSIONS
These factors were found in patients with OI: scoliosis, biconcave vertebrae, vertebral and chest deformity, unilateral rib, and thoracolumbar kyphosis.
The thoracolumbar kyphosis is highly associated with thoracic hypokyphosis in patients with OI.
Footnotes
Abbreviations: OI = osteogenesis imperfecta, SRS = Scoliosis Research Society.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Byers PH, Steiner RD. Osteogenesis imperfect. Annu Rev Med 1992; 43:269–282. [DOI] [PubMed] [Google Scholar]
- 2.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfect. J Med Genet 1979; 16:101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vetter U, Pontz B, Zauner E, et al. Osteogenesis imperfecta: a clinical study of the first ten years of life. Calcif Tissue Int 1992; 50:36–41. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa S, Kumar SJ, Takahashi HE, et al. Vertebral body shape as a predictor of spinal deformity in osteogenesis imperfect. J Bone Joint Surg Am 1996; 78:212–219. [DOI] [PubMed] [Google Scholar]
- 5.Shands A, Eisberg H. The incidence of scoliosis in the state of Delaware; a study of 50,000 minifilms of the chest made during a survey for tuberculosis. J Bone Joint Surg Am 1995; 37:1243–1249. [PubMed] [Google Scholar]
- 6.Hanscom DA, Winter RB, Lutter L, et al. Osteogenesis imperfecta. Radiographic classification, natural history, and treatment of spinal deformities. J Bone Joint Surg Am 1992; 74/4:598–616. [PubMed] [Google Scholar]
- 7.Widmann RG, Bitan FD, Laplaza FJ, et al. Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfect. Spine (Phila Pa 1976) 1999; 24:1673–1678. [DOI] [PubMed] [Google Scholar]
- 8.Cobb JR. The American Academy of Orthopedic Surgeons Instructional Course Lectures. 1948; Ann Arbor, MI: Edwards, Vol. 5. [Google Scholar]
- 9.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed.New York: Academic Press; 1988. [Google Scholar]
- 10.Watanabe G, Kawaguchi S, Matsuyama T, et al. Correlation of scoliotic curvature with Z-score bone mineral density and body mass index in patients with osteogenesis imperfect. Spine (Phila Pa 1976) 2007; 32-E:488–494. [DOI] [PubMed] [Google Scholar]
- 11.King HA, Moe JH, Bradford DS, et al. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am 1983; 65-A:1302–1313. [PubMed] [Google Scholar]
- 12.Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am 2001; 83-A:1169–1181. [PubMed] [Google Scholar]
- 13.Renshaw TS, Cook RS, Albright JA. Scoliosis in osteogenesis imperfecta. Clin Orthop Relat Res 1979; 145:163–167. [PubMed] [Google Scholar]
- 14.Abelin K, Vialle R, Lenoir T, et al. The sagittal balance of the spine in children and adolescents with osteogenesis imperfect. Eur Spine J 2008; 17:1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roussouly P, Nnadi C. Sagittal plane deformity: an overview of interpretation and management. Eur Spine J 2010; 19:1824–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scoliosis Research Society, SRS Terminology Committee and Working Group on Spinal Classification Revised Glossary of Terms, Working Group on 3-D Classification (Chair Larry Lenke, MD), and the Terminology Committee, March 2000. http://www.srs.org/professionals/resources/sagittal_plane_white_paper.pdf (accessed May 12, 2011). [Google Scholar]
- 17.Koerber F, Semler O, Demant AW, et al. Standardized x-ray reports of the spine in osteogenesis imperfect. Rofo 2011; 183/5:462–469. [DOI] [PubMed] [Google Scholar]
- 18.Janus GF, Finidori G, Engelbert RH, et al. Operative treatment of severe scoliosis in osteogenesis imperfecta: results of 20 patientsafter halo traction and posterior spondylodesis with instrumentation. Eur Spine J 2000; 9:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernhardt M, Bridwell KH. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine (Phila Pa 1976) 1989; 14:717–721. [DOI] [PubMed] [Google Scholar]


