Abstract
There is growing evidence that chemerin, a novel adipokine elevated in obesity and metabolic syndromes, plays a crucial role in advanced atherosclerosis. This study aimed to determine the chemerin levels in diabetes and evaluate the effects of increased chemerin on early atherosclerosis.
A total of 245 newly diagnosed diabetic patients and 148 age-matched, healthy, normal glucose tolerant (NGT) controls were enrolled. Anthropometric measurements and plasma parameters were examined, including body mass index (BMI), waist circumference, blood pressure, glucose, lipid profiles, inflammation markers, adipokines, and cell adhesion molecules. Vascular healthy was measured with brachial flow-mediated dilatation (FMD) and carotid intima-media thickness (IMT).
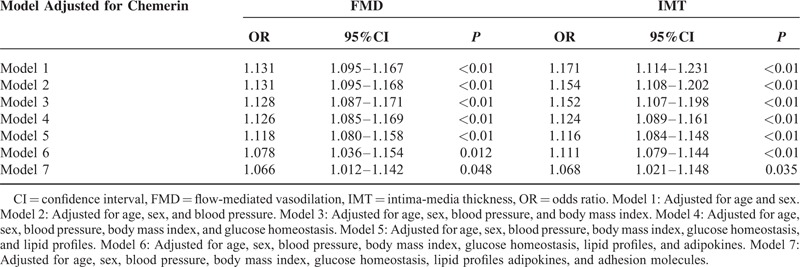
Compared with NGT controls, plasma chemerin levels were higher in diabetic patients (P < 0.01) and higher chemerin level was an independent risk factor of occurrence of diabetes even after metabolic profiles were adjusted (odds ratio [OR] = 1.352, 95% CI: 1.181–1.543, P < 0.01). In patients with type 2 diabetes, chemerin was positively associated with intercellular adhesion molecule-1 (ICAM-1), E-selectin, but not vascular adhesion molecule-1 (VCAM-1) and P-selectin. We also explored that plasma chemerin level was negatively associated with brachial FMD and positively with carotid IMT. Chemerin also retained a strong association with ICAM-1, FMD, and IMT even after adjusted for age, sex, and other risk factors (ICAM-1: r = 0.150, P = 0.024; FMD: r = −0.126, P = 0.001; IMT: r = 0.325, P < 0.001). By multiple linear regression analysis, plasma chemerin levels were related to ICAM-1 even after adjustments for conventional cardiovascular risk factors (β = 0.192, P = 0.017). Moreover, logistic regression analysis showed that high chemerin level was an independent predictive variable for impaired endothelial function (OR = 1.066, 95% CI: 1.012–1.142, P = 0.048) and enhanced carotid vessel thickness (OR = 1.068, 95% CI: 1.021–1.148, P = 0.035) in diabetic patients.
In summary, chemerin levels are independently associated with endothelial activation and early atherosclerosis in newly diagnosed type 2 diabetes.
INTRODUCTION
Type 2 diabetes mellitus (T2D) is associated with accelerated atherosclerosis and increased incidence of cardiovascular morbidity and mortality. Despite our growing understanding on the pathophysiology of coronary heart disease (CHD) in subjects with T2D, a large number of diabetic subjects still experience cardiovascular disease (CVD).1,2 Therefore, early detection of asymptomatic atherosclerosis in diabetes is a promising strategy to reduce the risk of diabetic macrovascular complications and improve the prognosis as well. Many studies have shown that changes in vascular structure, such as carotid intimal thickening,3 arterial compliance and stiffness,4 and endothelial dysfunction,1,5,6 occur in the early course of T2D and lead to accelerated atherosclerosis.7,8 In addition, several vascular adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), E-selectin, and P-selectin, which are well established vascular inflammatory markers and predictors of atherosclerosis, are also elevated during the early stage of T2D.9,10 However, the underlying mechanisms linking T2D with these vascular abnormalities remain poorly understood.
Adipose tissue, previously regarded as a passive depot for lipid storage and release of energy-rich substrates, is now considered a major active endocrine organ. Adipocytes respond to metabolic and inflammatory stimuli by secreting a variety of molecules known as adipokines. They serve as effectors to modulate atherosclerosis and are candidate risk factors for CVD.11 Chemerin, first described in 2007, was found to be highly expressed in adipose tissue,12 liver, and cells of the innate immune system as well, where it modulates the function of innate immune cells and may further link obesity and inflammation.13Like many other adipokines, chemerin is dysregulated in obesity. In human, the serum level of chemerin is associated with several key factors of metabolic syndromes. Retrospective and cross-sectional studies suggested that T2D patients had significantly higher chemerin levels than normal subjects.14,15
Notably, the pathogenic role of chemerin in CAD has been increasingly recognized. Yan et al16 have demonstrated that elevating serum chemerin levels have been observed in patients with CAD, as well, in association with the severity of coronary atherosclerosis. Lehrke et al17 reported systemic chemerin levels was related to coronary plaque burden in Caucasian subjects, although the association was lost after adjusting for established risk factors of CAD.17 Furthermore, a study examining the differential expression of multiple adipokines in human periaortic and pericoronary adipose tissue reported that chemerin expression in both of these adipose depots was highly correlated with atherosclerosis in their respective vessels.18 Chemerin is expressed in inflamed tissues and is involved in chemotactic recruitment of macrophages and other antigen presenting cells. These findings suggest a role of this adipokine in inflammatory status and atherosclerosis.19
Although the association of chemerin with established atherosclerosis has been investigated, limited data are available regarding the relationship between chemerin and early atherosclerosis. To our knowledge, only Yoo et al20 reported that in obesity, serum chemerin level was an independent predictive variable for increased brachial-ankle PWV (baPWV), even after adjusted for other cardiovascular risk factors. However, the correlation of chemerin with subclinical atherosclerosis in subjects with T2D has not been investigated.
The present study was designed to determine the importance of chemerin as a novel marker for endothelial activation and subclinical atherosclerosis in T2D, by comparing it with other markers of metabolic syndrome.
SUBJECTS AND METHODS
Study Design and Subjects Enrolled in the Study
The study used was approved by the Committees on Ethics of Nanjing General Hospital of Nanjing Command, and all subjects gave informed consent in this study.
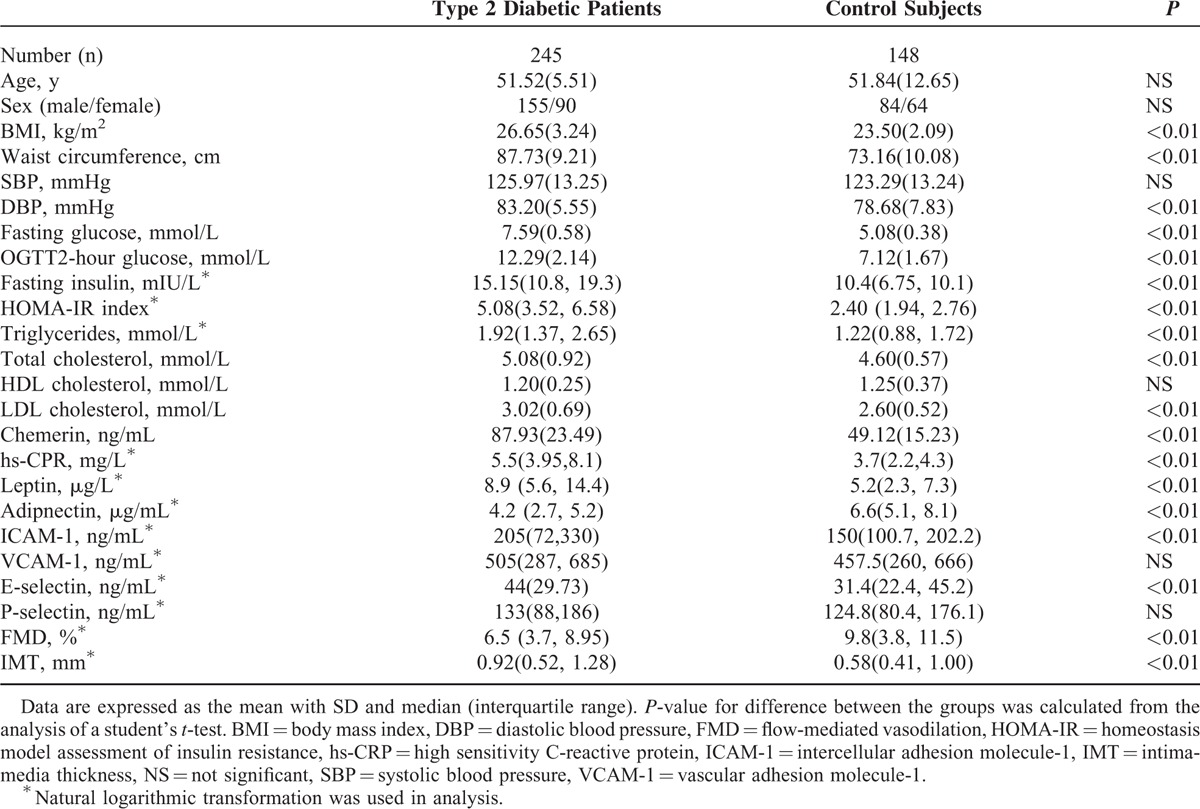
A total of 245 subjects with newly diagnosed T2D (155 men and 90 women; mean [SD] age, 51.52 [5.84] years: T2D group), and 148 subjects with normal glucose tolerance (NGT) (84 men and 64 women; mean [SD] age, 51.84 [12.65] years: NGT group) participated in the study. The baseline characteristics of the patients were shown in Table 1. Diabetes was diagnosed according to current WHO criteria. All patient conditions were newly diagnosed, and they were not treated with oral hypoglycemic agents or diet control. Subjects were excluded if they met any of the following criteria: type 1 diabetes; a history of ketoacidosis; macrovascular (CVD, congestive heart failure, myocardial infarction, stroke, or cerebrovascular conditions) or microvascular complications (retinopathy, neuropathy, or nephropathy); severe renal or hepatic disease; and malignant or chronic inflammatory diseases. None of the control subjects were taking medications known to affect glucose tolerance.
TABLE 1.
Baseline Characteristics in Diabetic and Control Group
Physical Examination and Laboratory Tests
A physical examination was performed in all study participants. Height (m) and weight (kg) were measured to calculate body mass index (BMI) as weight/height squared (kg/m2). Blood pressure was measured twice using a cuff sphygmomanometer after sitting at least 15 minutes. Waist circumference, an index of visceral fatness, was performed at the midpoint between the lower rib margin and the iliac crest.
After overnight fasting, blood samples were collected between 8:00 and 10:00 am in the morning, and plasma was obtained by centrifugation at 4 °C for 30 minutes. Glucose was measured shortly after blood collection and samples for other assays were stored at −70 °C. The plasma levels of glucose, lipid profiles, high sensitivity C-reactive protein (hs-CRP), insulin, and adipokines including chemerin, leptin, and adiponectin were determined as previously described.21 The inter- and intraassay variability of adiponectin and chemerin were 6.4% and 5.1%, 8.1% and 7.2%, respectively. Endothelial activation molecule concentrations were measured including soluble E-selectin, P-selectin, VCAM-1, and ICAM-1 using ELISA assay (Millipore, MC) and inter- and intraassay variability were 6.0 and 5.4, 6.5 and 4.8, 7.7 and 4.1, 5.2 and 3.1, respectively. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as the product of the insulin reading (mIU/L) and the plasma glucose value (mmol/L) divided by 22.5.22
Flow-Mediated Dilation (FMD) and Intima-Media Thickness (IMT) Measurement
Detailed protocols for subclinical atherosclerosis assessment have been previously described:21 Endothelial function assessed as FMD of the brachial artery by an ultrasound machine (HP Sonos5500, United States of America ), and carotid atherosclerosis measured as carotid IMT using a high-resolution ultrasound scanner (Acuson Sequoia 512; Siemens Medical Solutions, USA).
Statistical Analysis
Statistical analyses were performed using SPSS version 13.0 (Chicago, IL). Continuous variables are expressed as means ± standard deviation or median (interquartile range). The log values of variables that failed the normality test were calculated before analysis. Comparisons between the groups were assessed by ANOVA, and the correlation analysis was performed by calculating the Pearson correlation coefficient. Multivariate linear regression and logistic regression modeling were performed to evaluate association between variables. A P-value < 0.05 was considered to represent statistically significant.
RESULTS
Baseline Characteristics, Metabolic Risk Factors, Adipokines, Adhesive Molecules, and Early Atherosclerosis Parameters in Diabetic and NGT Control Groups
Basic characteristics of the patients were shown in Table 1. Age and gender distribution were comparable between both groups. Compared with NGT subjects, BMI, waist circumference, diastolic blood pressure (DBP), fasting glucose, Oral glucose tolerance test (OGTT) 2-hour glucose, plasma insulin, HOMA-IR, total cholesterol, triglyceride, LDL-C, and plasma hs-CRP levels were higher in diabetic patients (P < 0.01 in all comparisons). No significant difference was observed in systolic blood pressure and HDL-C levels between the 2 groups.
As expected, compared with NGT control subjects, the plasma chemerin and leptin levels were significantly higher whereas adiponectin levels were lower in diabetic patients (P < 0.01). There were also striking differences in plasma ICAM-1 and E-selectin in diabetic patients (P < 0.01), but VCAM-1 and P-selectin were not different. In addition, diabetic patients had lower FMD and thicker carotid IMT (P < 0.01).
For the whole study population, those with elevated chemerin levels were more likely to have T2D. Following adjustment for age, gender, and metabolic risk factors, chemerin was independently associated with T2D (odds ratio [OR] = 1.352, 95% CI: 1.181–1.543, P < 0.01).
Chemerin in Type 2 Diabetic Subjects
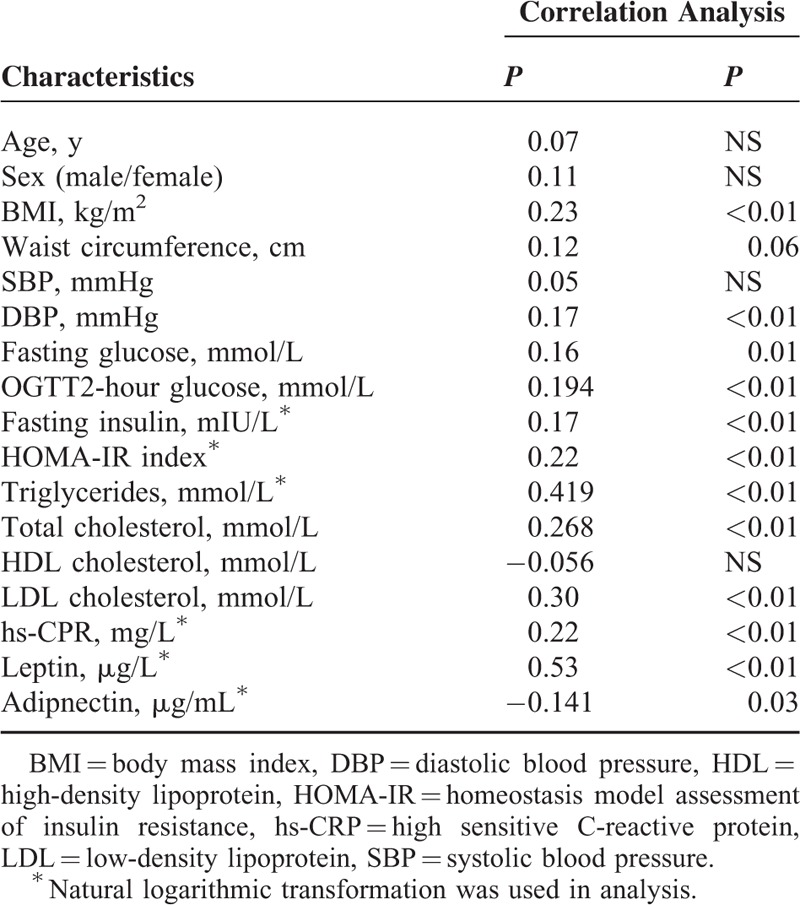
The correlations of the plasma chemerin concentration with metabolic risk factors in T2D group were shown in Table 2. After adjustment for age and sex, chemerin level was significantly positively associated with BMI, DBP, fasting glucose, fasting insulin, OGTT2-hour glucose, triglyceride, total cholesterol, LDL-cholesterol, hs-CRP, and letpin, and negatively associated with adiponectin, but not with systolic blood pressure, waist circumference, and HDL-cholesterol in diabetic patients.
TABLE 2.
Simple Correlation of Plasma Levels of Chemerin With Metabolic Syndrome-Related Phenotypes in Type 2 Diabetic Patients
A linear correlation of chemerin with adhesion molecules and subclinical atherosclerosis parameters in patients with T2D were shown in Figures 1 and 2. Chemerin did not correlate with VCAM-1 and P-selectin (Figure 1B and D), but there was a strong positive association with ICAM-1 and E-selectin (see Figure 1A and C). We also explored that plasma chemerin level was negatively associated with brachial FMD and positively with carotid IMT (see Figure 2A and B). Although the above-mentioned variables were strongly intercorrelated, the unadjusted associations of chemerin with adhesive molecules and earlier atherogenesis may be confounded. Therefore, we applied partial correlation analysis for each of the investigated variables to adjust for the aforementioned potential confounders. Even after adjustment for various confounders, ICAM-1, FMD, and IMT also exhibited significant associations with plasma chemerin concentration in diabetic patients, but not E-selectin. (ICAM-1: r = 0.150, P = 0.024; FMD: r = −0.126, P = 0.001; IMT: r = 0.325, P < 0.001).
FIGURE 1.
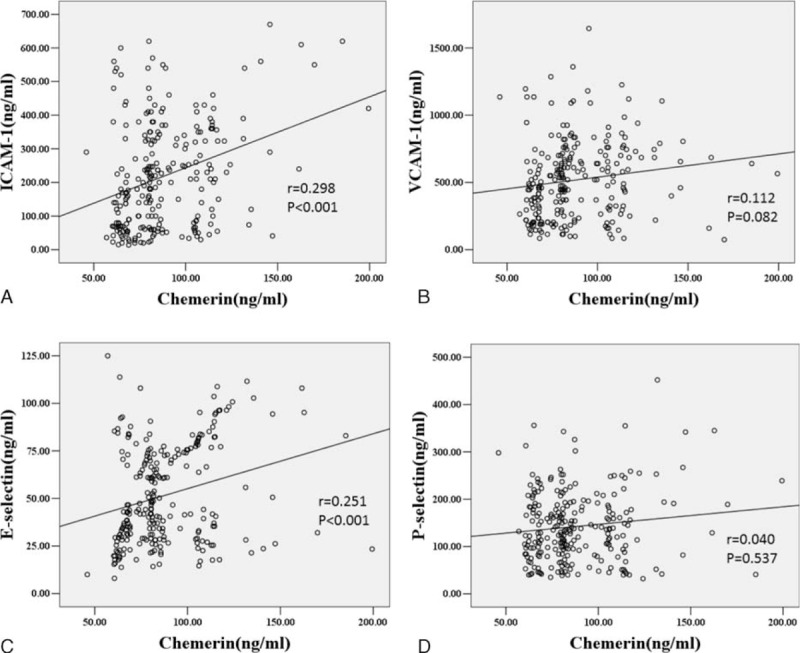
Scatterplot showing the correlation of plasma levels of chemerin with ICAM-1 (A), VCAM-1(B), E-selectin (C), and P-selectin (D) in diabetic patients. Scatter plot with regression line showing positive relationship between chemerin levels and ICAM-1, VCAM-1, E-selectin, and P-selectin in diabetic patients. Solid lines indicate regression lines. Pearson correlation was calculated in the correlation analyses. ICAM-1 = intercellular adhesion molecule-1, VCAM-1 = vascular adhesion molecule-1.
FIGURE 2.
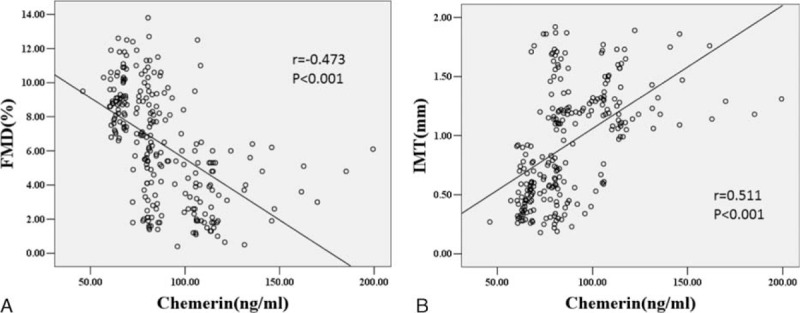
Scatterplot showing the correlation of plasma chemerin levels with endothelial function (FMD) and carotid atherosclerosis (IMT) in diabetic patients. Scatter plot with regression line showing negative relationship between chemerin and FMD, and the positive relationship with IMT in diabetic patients. Solid lines indicate regression lines. Pearson correlation was calculated in the correlation analyses. FMD = flow-mediated dilation, IMT = intimal-media thickness.
Multiple Regression Analysis for Predictors of Elevated Adhesive Molecules in Diabetic Patients
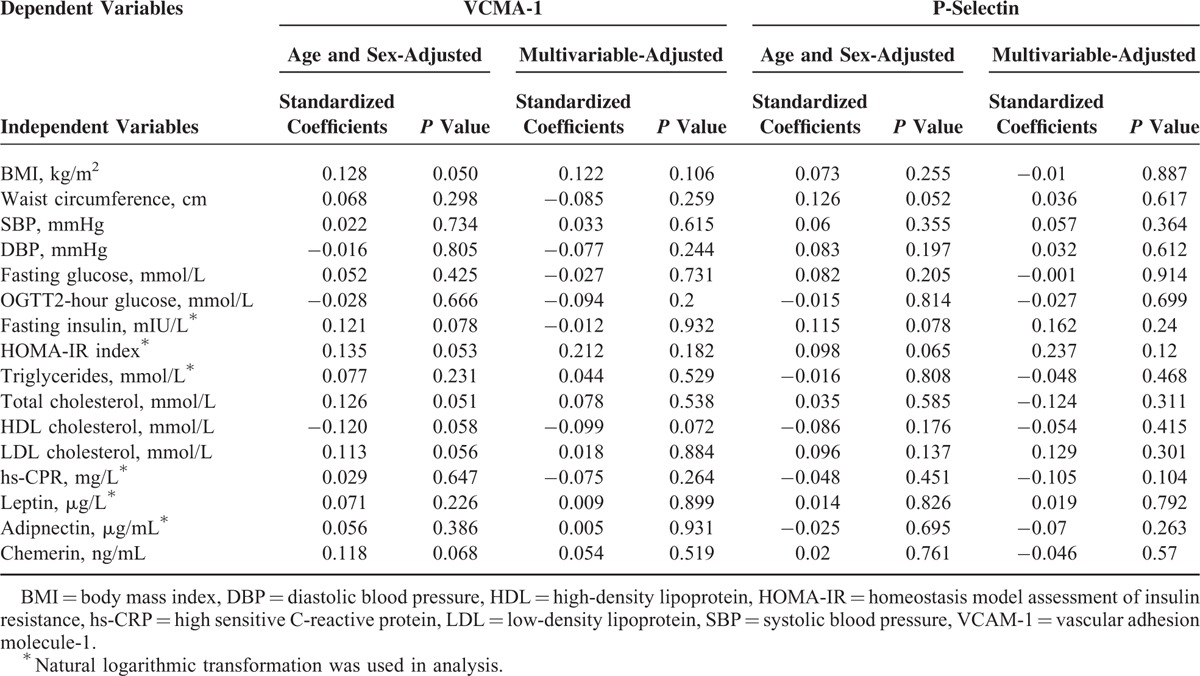
Multivariate regression analysis was performed to detect factors independently related to endothelial activation measured by ICAM-1, VCAM-1, P-selectin, and E-selectin. As given in Table 3, univariate analysis demonstrated age and sex-adjusted ICAM-1 was positively associated with BMI, waist circumference, fasting glucose, OGTT2-hour glucose, fast insulin, HOMA-IR, triglyceride, total cholesterol, LDL-cholesterol, hs-CRP, E-selectin, leptin, chemerin and negatively related to adiponectin in diabetic patients. The relationship of ICAM-1 with chemerin still persisted even after adjustment for age, sex, glucose homeostasis, lipid profiles, hs-CRP, and adipokines (β = 0.192, P = 0.017). Age and sex adjusted E-selectin was also significantly correlated to DBP, total cholesterol, hs-CRP, adiponectin, leptin, and chemerin, but the association between E-selectin and chemerin was lost in multivariate analysis. However, no correlation of plasma VCMA-1 and P-selectin concentrations with aforementioned variables was detected in our study (Table 4).
TABLE 3.
Multivariate Regression Analysis: Independent Predictors of Endothelial Activation in Type 2 Diabetic Patients
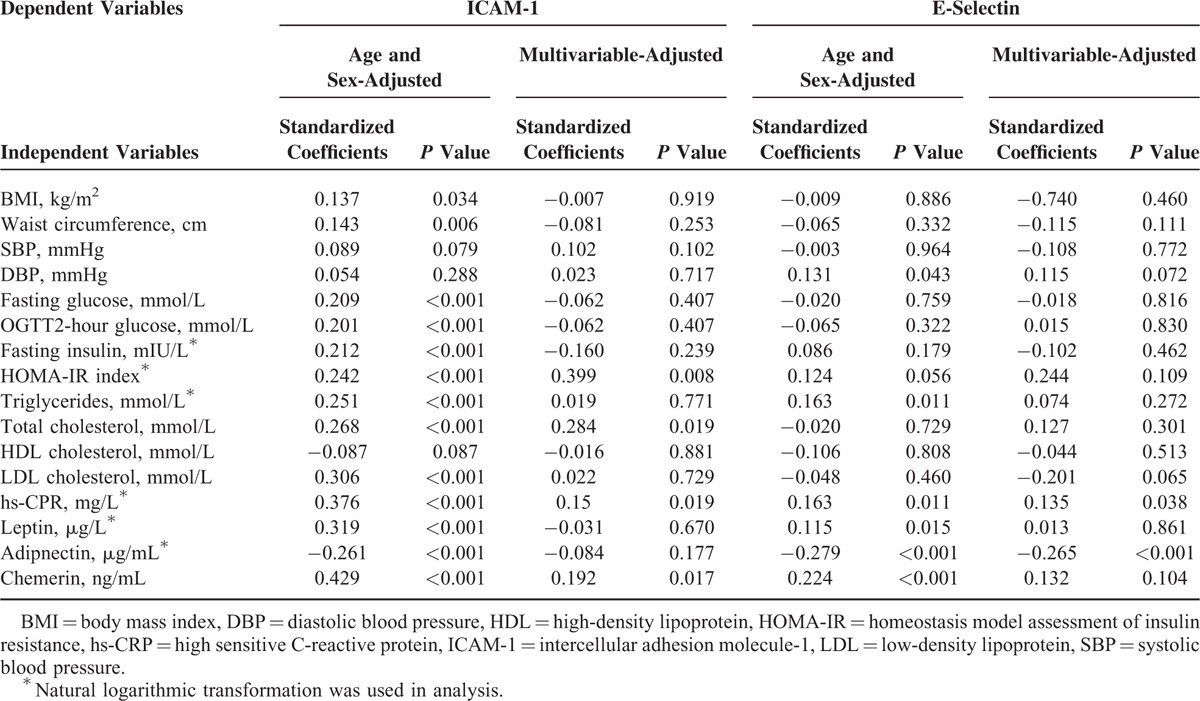
TABLE 4.
Multivariate Regression Analysis: Independent Predictors of Endothelial Activation in Type 2 Diabetic Patients
Logistic Regression Analysis for Predictors of Subclinical Atherosclerosis in Type 2 Diabetic Patients
The association between progression to atherosclerosis and chemerin levels was assessed using logistic regression analysis. Dichotomous outcome variables (men: FMD ≤ 6.3% and IMT ≥ 0.96 mm; women: FMD ≤ 7.1% and IMT ≥ 0.85 mm) were employed to assess whether chemerin impaired vasodilation and enhanced thickening of carotid vessel wall. The result of logistic regression analysis was shown in Table 5. Increasing concentrations of chemerin was associated with 1.13-fold increased risks of having endothelial dysfunction (OR = 1.131, 95% CI: 1.095–1.167, P < 0.01) and 1.17-fold increased risks of enhanced carotid vessel thickness (OR = 1.171, 95% CI: 1.114–1.231, P < 0.01) after adjusted for age and sex. These relations remained significant even after adjustment for metabolic variables (BMI, glucose homeostasis, and lipid profiles), adipokines, and adhesive molecules. (FMD: OR = 1.066, 95% CI: 1.012–1.142, P = 0.048; IMT: OR = 1.068, 95% CI: 1.021–1.148, P = 0.035).
TABLE 5.
OR of Serum Chemerin Level With Impaired Arterial Function and Structure by Multivariate Logistic Regression Analysis (FMD ≤ 6.5% and IMT ≥ 0.92 mm)
DISCUSSION
We present the first study to examine the relationship of circulating chemerin with endothelial cell activation, as well as with earlier atherosclerosis in patients with new diagnosed T2D. We found that plasma chemerin levels were associated with markers of endothelial activation including ICAM-1 and E-selectin in diabetic patients. Furthermore, the chemerin levels were significantly associated with early changes (both structural and functional) of the vasculature in diabetic patients. These findings indicate that diabetic patients with elevated chemerin levels are at an increased risk for cardiovascular complications, and chemerin plays an important role in atherogenesis from very early stages in diabetic patients.
Chemerin is an adipocyte-derived protein, which not only influences adipocyte differentiation and metabolism, but also has a role in adaptive and innate immunity. Therefore, chemerin may be a candidate protein in obesity-related disorders.23,24 In the current study, the plasma chemerin levels were found to be significantly increased in the diabetic group compared with NGT group, and it was also correlated positively with fasting glucose, fasting plasma insulin, OGTT2-hour glucose, HOMA-IR, total cholesterol, triglyceride, LDL-C, and plasma CRP levels in diabetic subjects. Furthermore, logistic regression analysis demonstrated that chemerin was independently associated with diabetes even after adjusted for age, sex, and metabolic risk factors. In line with our studies, Yang et al25 and El-Mesallamy et al15 had reported that circulating chemerin levels were elevated in type 2 diabetes and had been shown to correlate with insulin resistance independently of BMI and fasting insulin. Lee and coworkers26 showed that decrease in serum chemerin levels was associated with improved insulin sensitivity in overweight and obese T2D patients. Recently, Bobbert et al27 had published prospective data investigating the association between chemerin and the risk of T2D.27 A total of 440 subjects with normal glucose tolerance were enrolled in the study and mean follow-up of participants was 5.3 years. Their results showed that chemerin predicted the onset of T2D and prospective changes in fasting glucose and HbA1c were associated with chemerin. However, other studies by Bozaoglu et al12 and Takahashiet al28 did not find differences of chemerin levels between patients with T2D and controls. This difference between present studies might be related to the age of the patients, the adipose mass content, duration of diabetes, complications, and/or to race of the subjects. The mechanisms by which chemerin impacts glucose homoeostasis are still unclear, and experiment studies and large prospective investigation are required to clarify the relation between chemerin and diabetes incidence and eventually also the therapeutic potential of chemerin.
The diabetic state is hallmarked by abnormalities in a cluster of strongly interrelated clinical and metabolic factors that are likely to contribute to increased atherosclerosis susceptibility. Several studies suggested chemerin levels as predictor of advanced atherosclerotic lesions.16 However, the involvement of chemerin in very early stages of atherosclerosis was not examined so far. Furthermore, from a pathophysiological and potentially therapeutic point of view, it seems relevant to assess the role of chemerin on the early stage of atherosclerotic disease in a population-based sample.
Endothelial recruitment and monocytes adhesion are the earliest detectable events in the pathogenesis of atherosclerosis.29 The initial steps of endothelium/leukocyte interactions are considered to involve selectins (E-, P-, and L-selectin), and ICAM-1 and VCAM-1 are crucial for adhesion and migration of leukocytes into the subendothelium.30 So, the elevation of cell adhesive molecules is related to the higher morbidity,31 and these cytokines are considered as reliable biomarkers of endothelial activation and inflammation in diabetic group.32 We provided evidence that the plasma chemerin levels were strongly and independently correlated with measures of endothelial activation including ICAM-1 and E-selectin in diabetic patients, independent of metabolic parameters, and other adiopkines. Similar to our study, Landgraf et al33 reported that chemerin was the strongest predictor of ICAM-1 and E-selectin independent of BMI in obese children. Furthermore, on the cellular level, chemerin directly induced ICAM-1 and E-selectin expression in endothelial cells in vitro. Although some studies had shown the involvement of VCAM-1 and P-selectin in the development of atherosclerosis, we did not detect the association between circulating chemerin levels with soluble VCAM-1 and P-selectin, and these 2 molecules serum levels showed no significant differences between diabetic and NGT groups. ICAM-1 and E-selectin, not VCAM-1, have been shown to be independent early markers of atherosclerosis and incident coronary heart disease34 and appear to predict macrovascular disease within the diabetic population.35
Impaired FMD and enhanced carotid IMT are also established markers for early atherosclerosis36 and there are observations showing that carotid IMT and FMD can predict future cardiovascular events in diabetic patients.37 Toward this, we measured FMD and carotid IMT and our results showed that chemerin level was an independent predictor of impaired endothelial function and early structural changes of arteries in diabetic patients. To the best of our knowledge, this is the first study that earliest stages of atherosclerosis as assessed by decreased FMD and increased IMT are associated with hyper-chemerinemia in diabetic patients. However, the relationship between chemerin and early atherosclerosisis is controversial. A study in obese subjects by Yoo et al20 demonstrated chemerin was revealed not to be associated with carotid IMT. Therefore, further clinical data are required to establish the effect of chemerin on early atherogenesis in different population.
Accumulating evidence from preclinical and animal studies suggests chemerin has a causal role in vasculature and induces the early inflammatory reactions and recruitment of inflammatory cells to endothelium. In vitro, chemerin impaired endothelial dependent vascular relaxation by reducing NO production and decreasing NO-dependent cGMP signaling.38 Chemerin has also been shown to activate adhesion molecules and induce endothelial cells inflammation directly.33,39 Moreover, chemerin promotes the adhesion of macrophages to fibronectin and VCAM-1 by promoting clustering of the integrins which plays a central role of vascular remodeling.40 In addition, alteration in insulin sensitivity and glucose uptake by chemerin in adipocytes and skeletal muscle could contribute to development of atherosclerosis.41 Nevertheless, the exact pathophysiologic implication of chemerin in early atherosclerosis also remains to be clarified by further comprehensive investigations.
The present study has several potential limitations. First, our study population was relatively small and various nonsignificant associations would become statistically significant if larger sample sizes were adopted. Second, our data suggested that chemerin was involved in the pathophysiology of earlier atherosclerosis in T2D. But the observation data cannot prove any causal relationship, which need to be supported by further experiment studies. Third, our study was performed in newly diagnosed diabetic patients from outpatient center. These patients were younger and it is not representative for the general population.
In conclusion, our data demonstrate that in newly diagnosed T2D patients, plasma chemerin levels were independently associated with markers of endothelial activation including ICAM-1 and E-selectin. Moreover, the existence of impaired vascular function (FMD) and structure (IMT) with high serum chemerin concentrations was detectable in newly diagnosed T2D patients. These dynamic relationships between chemerin and atherosclerosis may give a meaningful contribution to the understanding of pathogenesis of atherosclerosis in diabetic patients. However, clinical evidence needed to be confirmed in large-scale prospective investigation, and animal studies are required to elucidate the role of chemerin in atherosclerosis and CVDs.
ACKNOWLEDGEMENTS
The authors thank Natural Science Foundation of China (30900697&81100568&81373605&81471018), Natural Science Foundation of Jiangsu Province (BK20131456), Postdoctoral Scientific Foundation of China (20100471843), and Postdoctoral Scientific Foundation of Jiangsu Province (1001027C) for the support. The authors also thank Professor Yuxiu Liu for his kind statistical consultation.
Footnotes
Abbreviations: BMI = body mass index, CVD = cardiovascular disease, DBP = diastolic blood pressure, FMD = flow-mediated dilatation, HOMA-IR = homeostasis model assessment of insulin resistance, hs-CRP = high sensitivity C-reactive protein, ICAM-1 = intercellular adhesion molecule-1, IMT = intima-media thickness, NGT = normal glucose tolerant, OR = odds ratio, T2D = type 2 diabetes mellitus, VCAM-1 = vascular adhesion molecule-1.
BL and MZ contributed equally to this study.
This study was supported by Natural Science Foundation of China (30900697&81100568&81373605&81471018), Natural Science Foundation of Jiangsu Province (BK20131456), Postdoctoral Scientific Foundation of China (20100471843), and Postdoctoral Scientific Foundation of Jiangsu Province (1001027C).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Seon CS, Min KW, Lee SY, et al. Cardiovascular risk assessment with vascular function, carotid atherosclerosis and the UKPDS risk engine in Korean patients with newly diagnosed type 2 diabetes. Diabetes Metab J 2011; 35:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239. [DOI] [PubMed] [Google Scholar]
- 3.Brohall G, Oden A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23:609–616. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002; 106:2085–2090. [DOI] [PubMed] [Google Scholar]
- 5.Henry RM, Ferreira I, Kostense PJ, et al. Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not; The Hoorn Study. Atherosclerosis 2004; 174:49–56. [DOI] [PubMed] [Google Scholar]
- 6.Su Y, Liu XM, Sun YM, et al. Endothelial dysfunction in impaired fasting glycemia, impaired glucose tolerance, and type 2 diabetes mellitus. Am J Cardiol 2008; 102:497–498. [DOI] [PubMed] [Google Scholar]
- 7.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348:383–393. [DOI] [PubMed] [Google Scholar]
- 8.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003; 46:760–765. [DOI] [PubMed] [Google Scholar]
- 9.Gomez Rosso L, Benitez MB, Fornari MC, et al. Alterations in cell adhesion molecules and other biomarkers of cardiovascular disease in patients with metabolic syndrome. Atherosclerosis 2008; 199:415–423. [DOI] [PubMed] [Google Scholar]
- 10.Rizzoni D, Muiesan ML, Porteri E, et al. Circulating adhesion molecules and carotid artery structural changes in patients with noninsulin-dependent diabetes mellitus. J Hum Hypertens 2003; 17:463–470. [DOI] [PubMed] [Google Scholar]
- 11.Ntaios G, Gatselis NK, Makaritsis K, et al. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis 2013; 227:216–221. [DOI] [PubMed] [Google Scholar]
- 12.Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007; 148:4687–4694. [DOI] [PubMed] [Google Scholar]
- 13.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab 2010; 21:660–667. [DOI] [PubMed] [Google Scholar]
- 14.Verrijn Stuart AA, Schipper HS, Tasdelen I, et al. Altered plasma adipokine levels and in vitro adipocyte differentiation in pediatric type 1 diabetes. J Clin Endocrinol Metab 2012; 97:463–472. [DOI] [PubMed] [Google Scholar]
- 15.El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med 2011; 28:1194–1200. [DOI] [PubMed] [Google Scholar]
- 16.Yan Q, Zhang Y, Hong J, et al. The association of serum chemerin level with risk of coronary artery disease in Chinese adults. Endocrine 2011; 41:281–288. [DOI] [PubMed] [Google Scholar]
- 17.Lehrke M, Becker A, Greif M, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol 2009; 161:339–344. [DOI] [PubMed] [Google Scholar]
- 18.Spiroglou SG, Kostopoulos CG, Varakis JN, et al. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb 2010; 17:115–130. [DOI] [PubMed] [Google Scholar]
- 19.Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev 2011; 22:331–338. [DOI] [PubMed] [Google Scholar]
- 20.Yoo HJ, Choi HY, Yang SJ, et al. Circulating chemerin level is independently correlated with arterial stiffness. J Atheroscler Thromb 2012; 19:59–66.discussion 67–58. [DOI] [PubMed] [Google Scholar]
- 21.Gu P, Cheng M, Hui X, et al. Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J Hypertens 2015; 33:1624–1632. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 23.Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 2007; 282:28175–28188. [DOI] [PubMed] [Google Scholar]
- 24.Zabel BA, Allen SJ, Kulig P, et al. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem 2005; 280:34661–34666. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, Yang G, Dong J, et al. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J Investig Med 2010; 58:883–886. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Lee SH, Ahn KY, et al. Effect of lifestyle modification on serum chemerin concentration and its association with insulin sensitivity in overweight and obese adults with type 2 diabetes. Clin Endocrinol (Oxf) 2014; 80:825–833. [DOI] [PubMed] [Google Scholar]
- 27.Bobbert T, Schwarz F, Fischer-Rosinsky A, et al. Chemerin and prediction of diabetes mellitus type 2. Clin Endocrinol (Oxf) 2015; 82:838–843. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M, Inomata S, Okimura Y, et al. Decreased serum chemerin levels in male Japanese patients with type 2 diabetes: sex dimorphism. Endocr J 2013; 60:37–44. [DOI] [PubMed] [Google Scholar]
- 29.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993; 362:801–809. [DOI] [PubMed] [Google Scholar]
- 30.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 1999; 79:181–213. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843. [DOI] [PubMed] [Google Scholar]
- 32.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003; 170:191–203. [DOI] [PubMed] [Google Scholar]
- 33.Landgraf K, Friebe D, Ullrich T, et al. Chemerin as a mediator between obesity and vascular inflammation in children. J Clin Endocrinol Metab 2012; 97:E556–E564. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997; 96:4219–4225. [DOI] [PubMed] [Google Scholar]
- 35.Jude EB, Douglas JT, Anderson SG, et al. Circulating cellular adhesion molecules ICAM-1, VCAM-1, P- and E-selectin in the prediction of cardiovascular disease in diabetes mellitus. Eur J Intern Med 2002; 13:185–189. [DOI] [PubMed] [Google Scholar]
- 36.Zhu W, Huang X, He J, et al. Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. Eur J Pediatr 2005; 164:337–344. [DOI] [PubMed] [Google Scholar]
- 37.Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol 2003; 41:661–665. [DOI] [PubMed] [Google Scholar]
- 38.Neves KB, Lobato NS, Lopes RA, et al. Chemerin reduces vascular nitric oxide/cGMP signalling in rat aorta: a link to vascular dysfunction in obesity? Clin Sci (Lond) 2014; 127:111–122. [DOI] [PubMed] [Google Scholar]
- 39.Yamawaki H. Vascular effects of novel adipocytokines: focus on vascular contractility and inflammatory responses. Biol Pharm Bull 2011; 34:307–310. [DOI] [PubMed] [Google Scholar]
- 40.Hart R, Greaves DR. Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J Immunol 2010; 185:3728–3739. [DOI] [PubMed] [Google Scholar]
- 41.Ernst MC, Haidl ID, Zuniga LA, et al. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 2012; 153:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]


