Abstract
Mannitol, an osmotic diuretic, is commonly used to treat patients with acute brain edema, but its use also increases the risk of developing acute kidney injury (AKI). In this study, we investigated the incidence and risk factors of mannitol-related AKI in acute stroke patients.
A total of 432 patients (ischemic stroke 62.3%) >20 years of age who were admitted to the neurocritical care center in a tertiary hospital and received mannitol treatment were enrolled in this study. Clinical parameters including the scores of National Institutes of Health Stroke Scale (NIHSS) at admission, vascular risk factors, laboratory data, and concurrent nephrotoxic medications were registered. Acute kidney injury was defined as an absolute elevation in the serum creatinine (Scr) level of ≥0.3 mg/dL from the baseline or a ≥50% increase in Scr.
The incidence of mannitol-related AKI was 6.5% (95% confidence interval, 4.5%–9.3%) in acute stroke patients, 6.3% in patients with ischemic stroke, and 6.7% in patients with intracerebral hemorrhage. Multivariate analysis revealed that diabetes, lower estimated glomerular filtration rate at baseline, higher initial NIHSS score, and concurrent use of diuretics increased the risk of mannitol-related AKI. When present, the combination of these elements displayed an area under the receiver operating characteristic curve of 0.839 (95% confidence interval, 0.770–0.909). In conclusion, mannitol-related AKI is not uncommon in the treatment of acute stroke patients, especially in those with vulnerable risk factors.
INTRODUCTION
Brain edema leading to increased intracranial pressure is one of the most devastating complications in patients with acute brain injury or stroke. It can lead to further neurological deterioration, a poor functional outcome, and death. Mannitol, an osmotic diuretic, has been used for decades to reduce intracranial pressure by increasing the osmotic gradient between blood and brain parenchyma, thereby promoting the transfer of water from brain tissue to the circulatory system.1 The efficacy of mannitol in reducing intracranial pressure is dose-dependent, with higher doses delivered by rapid bolus infusion producing long-lasting reductions in intracranial pressure.2,3 However, the use of mannitol also poses various risks that may preclude mannitol treatment, including acute kidney injury (AKI), electrolyte imbalance, volume overload, and rebound edema.1 Actually, mannitol may not be a direct nephrotoxic agent as it did show some renal protective benefits in specific indications. Dehydration and preexisting renal impairment may be the risk to aggravate the toxicity of mannitol therapy.4
Mannitol-related AKI has been investigated in only a few studies.5–7 Kim et al5 reported recently that a high infusion rate, advanced age, and impaired renal function are risk factors for mannitol-related AKI in patients with intracranial hemorrhage. However, the general clinical relevance of that study is limited considering the small patient number and the heterogeneity of intracranial bleeding among patients in the study, which included subdural hemorrhage, epidural hemorrhage, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage. As the prognostic factors for patients with traumatic brain injury, subdural hemorrhage, epidural hemorrhage, subarachnoid hemorrhage, and brain tumor may be different from those with ischemic stroke (IS) or ICH. In the present study, we aimed to focus specifically on the incidence and risk factors for mannitol-related AKI in acute stroke patients.
METHODS
Patients
This study was performed in the neurocritical care unit of a university hospital in Taiwan. The criteria for admission to the neurocritical care unit include patients with severe neurological deficit (eg, National Institute Health Stroke Scale [NIHSS] score higher than 8), stroke in evolution, IS patients receiving thrombolytic therapy or endovascular treatment, ICH patients receiving intensive blood pressure control, or medical conditions requiring intensive care.8
We conducted a retrospective review of consecutive patients >20 years of age who received mannitol therapy during January 1, 2010, to December 31, 2014, after admission to the neurocritical care unit due to IS, and nontraumatic ICH. Patients undergoing hemodialysis or peritoneal dialysis for end-stage renal disease, and those suffering from AKI before the initiation of mannitol therapy were excluded from analysis, for example, patients with a rapid change in the serum creatinine (Scr) level, which meets the Acute Kidney Injury Network diagnostic criteria before the initiation of mannitol therapy. Patients admitted for traumatic brain injury, subdural hemorrhage, epidural hemorrhage, subarachnoid hemorrhage, and brain tumor were also excluded. Mannitol (20% w/v solution; 100 mL/bottle; Taiwan) was administered via bolus intravenous infusion several times per day, the specific dosage and frequency determined by the healthcare practitioners according to the patient's clinical condition. The study protocol was approved by the National Taiwan University Hospital Research Ethics Committee (No. 201410029RINA). Inform consent for study participants was not obtained as this was a retrospective cohort study and we did no interventions except for standard poststroke treatment. Consent for publication was not obtained because our data could not be traced back to any identifiable individual.
Data Collection
We collected the following laboratory data and patient history for each individual enrolled in the study: age, gender, body height and weight, diagnosis, NIHSS score upon arrival, history of vascular risk factors (atrial fibrillation, coronary artery disease, congestive heart failure, chronic kidney disease, diabetes, hypertension, IS, transient ischemic attack, ICH, and myocardial infarction), Scr level within 3 days of admission and at the initiation and the end of mannitol treatment. Any major operation at the time of mannitol treatment was also recorded. We also collected information on the concurrent use of nephrotoxic medications during mannitol therapy, including aspirin, angiotensin-converting enzyme inhibitors, angiotensin receptor blocking agents, diuretics, nephrotoxic antimicrobial agents (ie, aminoglycosides, vancomycin, amphotericin B, acyclovir), nonsteroidal anti-inflammatory drugs, and nephrotoxic immunosuppressant agents (ie, cyclosporine, tacrolimus, sirolimus, and everolimus). Finally, we collected data on mannitol treatment: dosage, frequency, cumulative dose, and duration of therapy. To allow for interindividual comparisons, we divided the cumulative mannitol dose by body weight and duration of treatment (g/kg/day).
Study Outcomes
There were 2 study outcomes: (a) the incidence of mannitol-related AKI, and (b) the risk factors for mannitol-related AKI. Acute Kidney Injury Network diagnostic criteria were applied for AKI classification, which was defined as an absolute elevation in the Scr level of ≥ 0.3 mg/dL from baseline or an ≥ 50% increase in Scr.9 We calculated the estimated glomerular filtration rate (eGFR) by using the 4-variable Modification of Diet in Renal Disease equation: eGFR (mL/min/1.73 m2) = 175 × Scr−1.154 × age−0.203 × 1.212 (if the patient is black) × 0.742 (if female).10
Statistical Analysis
Descriptive statistics were used to obtain mean, median, standard deviation, and range. In univariate analysis, the baseline characteristics among patients with and without AKI were compared using Student's t test or the chi square test, as appropriate. Multivariate logistic regression was then used to identify the risk factors for mannitol-related AKI. Factors with P values < 0.05 in the univariate analysis were evaluated using multivariate regression. A receiver operating characteristic (ROC) curve was used to illustrate a predictive model of mannitol-related AKI in multivariate logistic regression. SPSS (version 12.0; SPSS Inc) was used for analysis, and values of P < 0.05 were considered statistically significant.
RESULTS
Incidence and Manifestations of Mannitol-Related AKI
During the study period, a total of 2518 patients were admitted to the neurocritical care unit, among whom 1748 (69.4%) were diagnosed with acute stroke and 1296 with IS (51.5%). Among the stroke patients, 542 (31.0%) received mannitol therapy. After excluding 110 patients with incomplete laboratory data, a total of 432 patients (IS: 269, 62.3%) were included for this analysis. Twenty-eight patients (13 males) developed AKI. The incidence of mannitol-related AKI was 6.5% (95% confidence interval [CI], 4.5–9.3%) among all stroke patients, 6.3% (95% CI, 4.0–10.0%) among IS patients, and 6.7% (95% CI, 3.8–11.9%) among ICH patients. There was no difference in the incidence of mannitol-related AKI between the IS and ICH groups (P = 0.84).
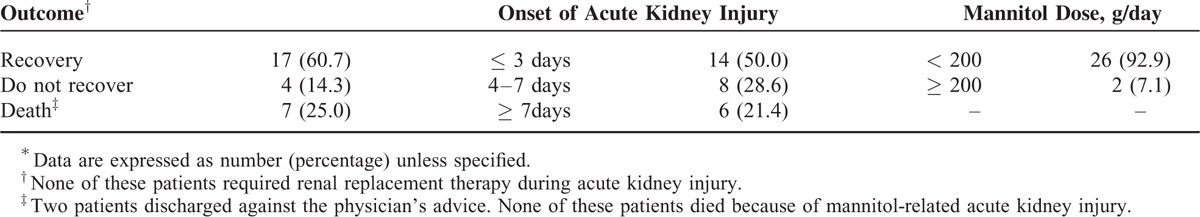
The onset, clinical manifestations, and outcomes of patients who developed mannitol-related AKI are summarized in Table 1. Seventy-eight percent of patients developed AKI within 1 week. Complete recovery with a return of eGFR to the baseline value was observed in 60.7% of patients. None of these patients required renal replacement therapy for AKI, and none of the mortality was related to AKI.
TABLE 1.
Outcome of Patients With Mannitol-Related Acute Kidney Injury∗
Risk Factors for Mannitol-Related AKI
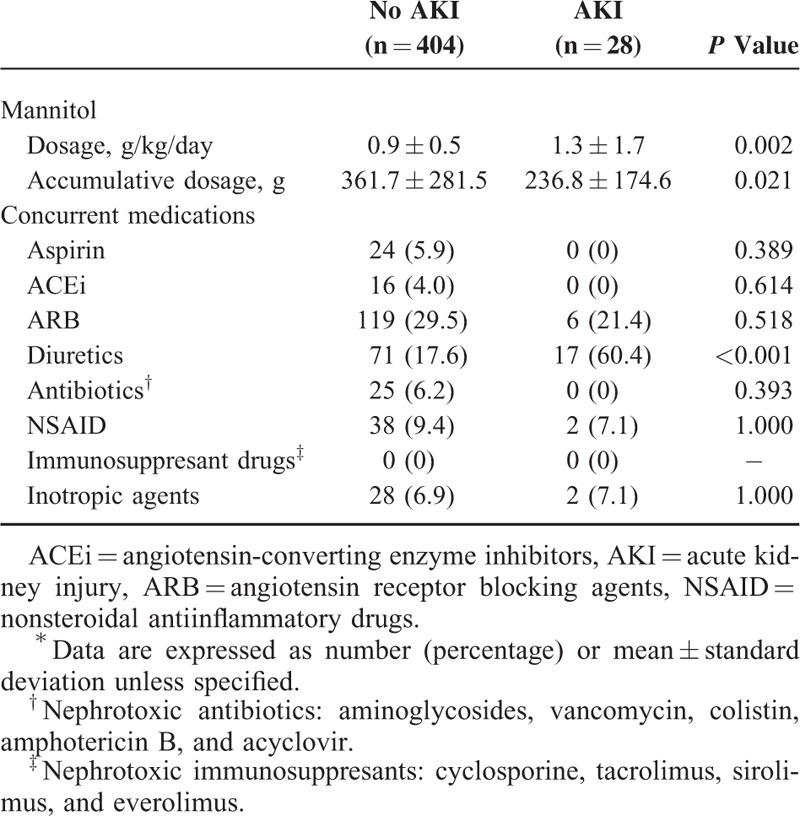
Table 2 lists the baseline characteristics for patient receiving mannitol therapy. Age, gender, and stroke type were generally comparable between patients with and without AKI. Univariate analysis showed that patients with AKI had a significantly greater prevalence of diabetes and chronic kidney disease and fewer previous IS or transient ischemic attacks than those without AKI (Table 2). Acute kidney injury patients had significantly higher initial NIHSS, and analysis of laboratory data revealed that AKI patients had significantly lower hemoglobin at admission, and lower eGFR values upon arrival and at the initiation and completion of mannitol therapy compared to non-AKI patients (Table 3). Patients with AKI received significantly higher mannitol doses (g/kg/day) and a lower cumulative mannitol dose (g) and were more often prescribed concomitant diuretics (Table 4).
TABLE 2.
Characteristics of Patients Who Was Prescribed Mannitol∗
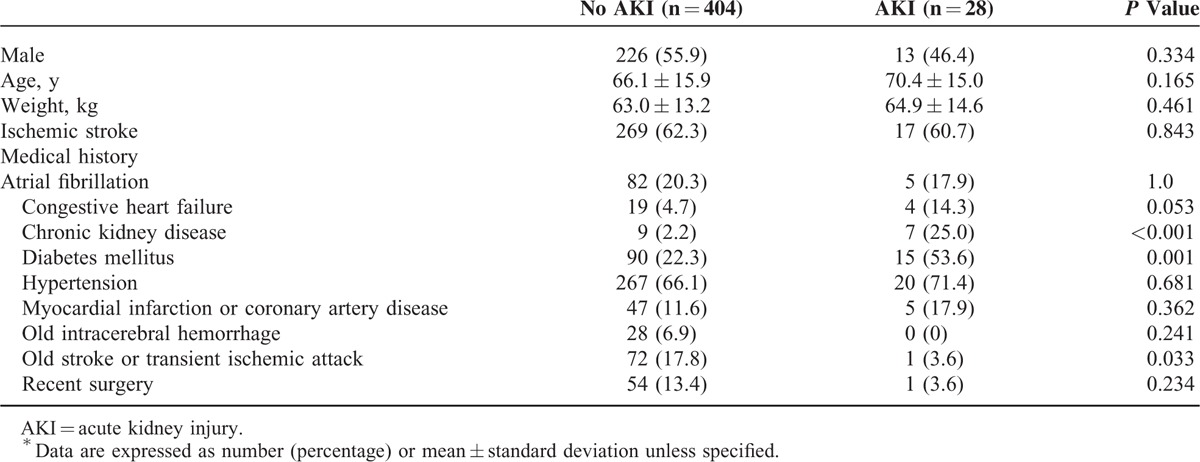
TABLE 3.
Associated Data of Patients Who Was Prescribed Mannitol∗
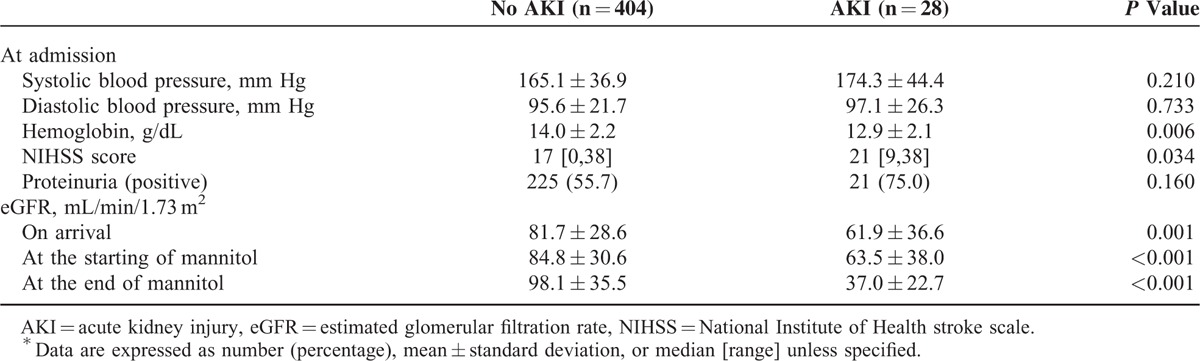
TABLE 4.
Information About Mannitol and Concurrent Medications∗
The results of multivariate logistic regression analysis are summarized in Table 5. Higher initial NIHSS score, lower eGFR on arrival, diabetes, and the concomitant use of diuretics significantly increased the risk of mannitol-related AKI. Higher mannitol doses (g/kg/day) showed a trend for increasing the risk, but the effect was not significant (P = 0.053). Figure 1 shows the ROC curve for a predictive model of mannitol-related AKI based on the parameters of initial NIHSS score, eGFR on arrival, diabetes, and concomitant use of diuretics. The area under the ROC curve was 0.839 (95% CI, 0.770–0.909). Furthermore, subgroup analysis on different types of stroke showed that concomitant use of diuretics in both IS and in ICH patients were independently associated with mannitol-related AKI: IS, relative risk = 4.38, P = 0.008; ICH, relative risk = 50.4, P < 0.001; and diabetes mellitus in ICH patients, relative risk = 14.04, P = 0.019.
TABLE 5.
Multivariate Regression for Mannitol-Related Acute Kidney Injury
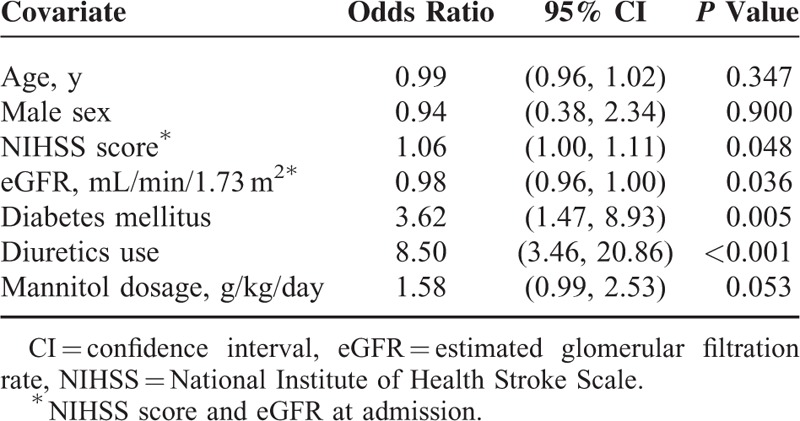
FIGURE 1.
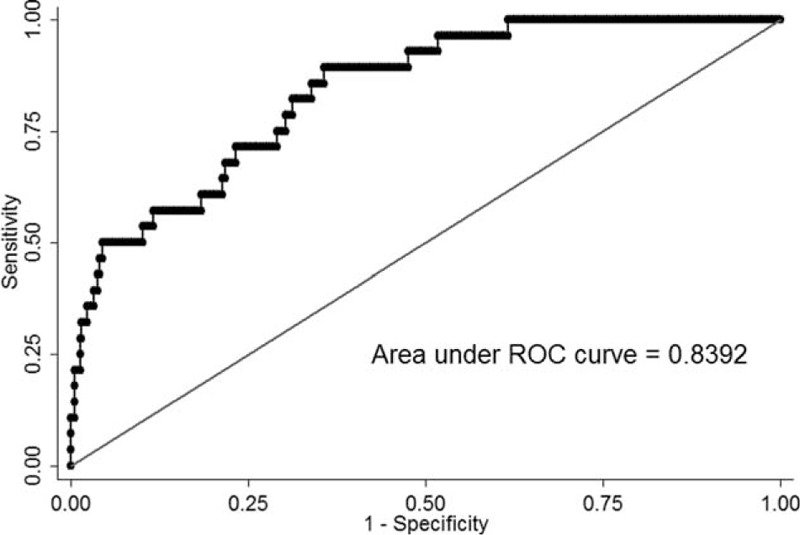
Receiver operating characteristic curve for prediction of mannitol-related acute kidney injury.
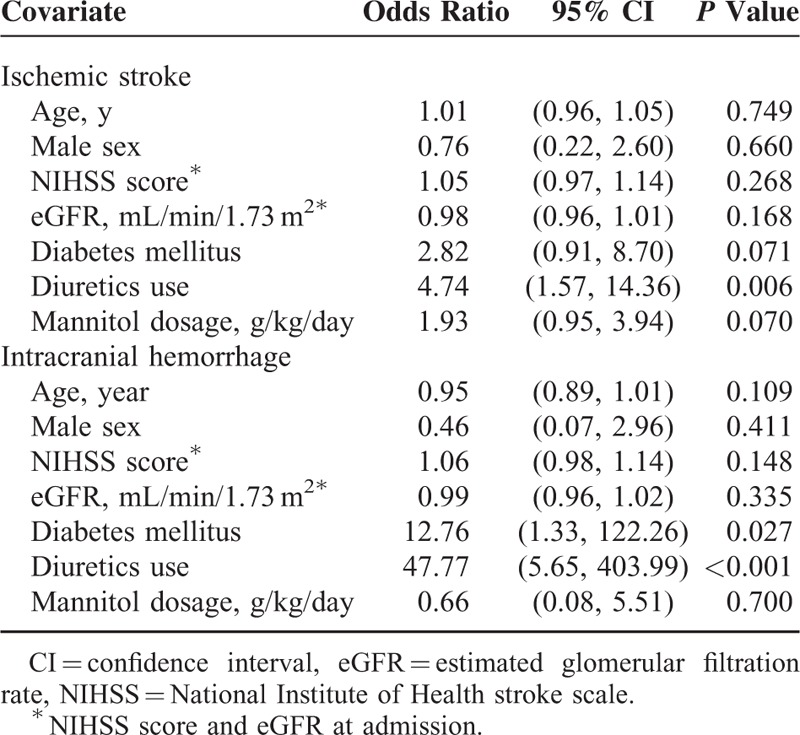
In subgroup analysis, diabetes remained a significant risk factor for mannitol-related AKI in both IS and ICH patients. Concomitant use of diuretics significantly increased the risk for mannitol-related AKI in ICH patients (Table 6).
TABLE 6.
Multivariate Regression for Mannitol-Related Acute Kidney Injury: Subgroup. Analysis Among Patients With Ischemic Stroke and Intracerebral Hemorrhage
DISCUSSION
This study was the first of its kind to investigate the incidence and risk factors for mannitol-related AKI exclusively in acute stroke patients. The results revealed 2 important findings. First, the incidence of mannitol-related AKI was 6.5% and did not differ significantly between patients with IS and ICH. Second, we identified diabetes, reduced baseline eGFR, higher initial NIHSS score, and concurrent use of diuretics as independent risk factors for mannitol-related AKI.
Previous studies have reported the incidence of mannitol-related AKI ranges from 10.5% to 20.3% among different patient cohorts.5,7,11 However, our analysis revealed a lower occurrence (6.5%), despite the high proportion of elderly individuals in our patient cohort (age ≥70 years, 46.8%). There may be several factors contributing to the relatively low incidence of mannitol-related AKI in this study. First, as the harmful effect of mannitol is reported to be dose-related, daily doses of ≥200 g or a cumulative doses of >1100 g should be discouraged.12 Most of our patients received mannitol treatment with doses below the aforementioned 200-g daily dose (1.3%) as well as the 1100-g cumulative dose (2.4%). Second, most mannitol-related AKI occurred in patients who received mannitol to treat brain edema or increased intraocular pressure.13 Whether there is difference in the incidence of mannitol-related AKI between patients with various etiologies such as acute stroke, head injury, or whether it was affected by the severity of brain edema needs to be clarified.
The pathogenesis of mannitol-related AKI is not fully understood. Proposed mechanisms include (a) renal vasoconstriction produced by a high dose/concentration of mannitol; (b) profound diuresis and natriuresis; and (c) isomeric tubular vacuolization or tubular cell swelling, that is, so-called osmotic nephropathy.13–15 Furthermore, patients with preexisting risk factors such as advanced age, underlying kidney disease, and concomitant use of nephrotoxic agents have been shown to be predisposed to the development of drug-related osmotic nephropathy.12,16 Our results were similar in this regard. Reduced baseline eGFR and concurrent use of diuretics were independent risk factors for mannitol-related AKI. Mannitol is freely filtered through glomerular filtration with limited reabsorption.13 In renal impairment, the half-life of mannitol can increase from 70 to 100 min to >36 h.13,14 As a result, it is possible for mannitol to accumulate, leading to harmful effects. Furthermore, mannitol induces a marked reduction in water and salt reabsorption along the entire renal tubule. The increase in water and salt delivery from the proximal tubule actually leads to enhancement of sodium reabsorption at Loop of Henle, distal tubule, and collecting duct.13 Nevertheless, concomitant use of diuretics abolishes the compensatory effect and causes profound diuresis, volume depletion, and, more devastating, AKI.12,14 The results for subgroup analysis were similar between patients with IS and ICH, indicating the important role of diuretics in the development of mannitol-related AKI.
Acute kidney injury is not an uncommon complication after stroke. Furthermore, a high NIHSS score was previously reported to be a risk factor for AKI after stroke.17 We excluded patients who developed AKI before mannitol treatment, and still found the association between stroke severity and development of mannitol toxicity. On the other hand, the association between diabetes and nephrotic complications has been well established. Moreover, diabetic patients have been reported to be at a higher risk of AKI.18 Renal hemodynamics in diabetic patients appears to be different from that in nondiabetic individuals. The increase in renal blood flow after mannitol administration is accompanied by a redistribution of renal blood flow, which leads to reduced oxygen delivery to the medulla and predisposes patients to ischemic renal injury.19,20 In support of these points, our result showed that a high NIHSS score and the presence of diabetes were 2 independent risk factors for mannitol-related AKI.
Regarding the clinical course, more than half of our patients developed AKI within 1 week of mannitol therapy,14,21 and ∼60% of them recovered to the baseline renal function after discontinuation of mannitol. Early interruption of mannitol treatment may be the main cause of lower accumulative mannitol dose in the AKI group. Management of mannitol-related AKI includes discontinuing mannitol treatment and starting dialysis if clinically indicated. This adverse reaction usually responds to prompt removal of mannitol.13 Although mannitol-related AKI can be transient, the association between AKI after stroke and poor clinical outcome has been mentioned in several studies.17,22 Physicians should be aware that a patient's underlying condition and commitment medications may exacerbate the risk of mannitol therapy.
We acknowledge the following limitations of this study. First, the decision to initiate mannitol therapy and the mannitol doses varied according to the physician's expertise. As patients with worse baseline renal function tended to receive lower doses of mannitol for shorter durations, and this may lead to an underestimation of the incidence of mannitol-related AKI. Second, this is a retrospective study, and patients with missing data were excluded from analysis. Third, outcome after acute stroke was not included in the present study. Actually, it is not appropriate attribute stroke outcome to mannitol-related AKI. Cormobidities, medications, and laboratory tests all affects stroke outcome.23,24 Fourth, the causative relationship between concurrent medications and the development of mannitol-related AKI could not be easily clarified. A further prospective study is necessary to validate the results of our findings.
CONCLUSIONS
Patient-specific factors including diabetes, underlying renal impairment, high initial NIHSS scores, and concurrent use of diuretics were associated with an increased risk of developing mannitol-related AKI. Physicians should pay close attention to these patients as the toxicity of mannitol therapy may be exaggerated.
Footnotes
Abbreviations: AKI = acute kidney injury, CI = confidence interval, eGFR = estimated glomerular filtration rate, ICH = intracerebral hemorrhage, IS = ischemic stroke, NIHSS = National Institutes of Health Stroke Scale, ROC curve = receiver operating characteristic curve, Scr = serum creatinine.
L-SY and T-SC equally contributed to this study.
This work was supported by a grant (A116) from National Taiwan University Hospital.
This article has not been published in part, or in full, in any form, and is not under consideration for any publication.
The authors declare that they have no competing interests.
REFERENCES
- 1.Fink ME. Osmotherapy for intracranial hypertension: mannitol versus hypertonic saline. Continuum (Minneap Minn) 2012; 18:640–654. [DOI] [PubMed] [Google Scholar]
- 2.Sorani MD, Manley GT. Dose-response relationship of mannitol and intracranial pressure: a metaanalysis. J Neurosurg 2008; 108:80–87. [DOI] [PubMed] [Google Scholar]
- 3.Sorani MD, Morabito D, Rosenthal G, et al. Characterizing the dose–response relationship between mannitol and intracranial pressure in traumatic brain injury patients using a high-frequency physiological data collection system. J Neurotrauma 2008; 25:291–298. [DOI] [PubMed] [Google Scholar]
- 4.Andrews PM, Cooper M, Verbesey J, et al. Mannitol infusion within 15 min of cross-clamp improves living donor kidney preservation. Transplantation 2014; 98:893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MY, Park JH, Kang NR, et al. Increased risk of acute kidney injury associated with higher infusion rate of mannitol in patients with intracranial hemorrhage. J Neurosurg 2014; 120:1340–1348. [DOI] [PubMed] [Google Scholar]
- 6.Fang L, You H, Chen B, et al. Mannitol is an independent risk factor of acute kidney injury after cerebral trauma: a case-control study. Ren Fail 2010; 32:673–679. [DOI] [PubMed] [Google Scholar]
- 7.Gondim Fde A, Aiyagari V, Shackleford A, et al. Osmolality not predictive of mannitol-induced acute renal insufficiency. J Neurosurg 2005; 103:444–447. [DOI] [PubMed] [Google Scholar]
- 8.Jeng JS, Huang SJ, Tang SC, et al. Predictors of survival and functional outcome in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci 2008; 270:60–66. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139:137–147. [DOI] [PubMed] [Google Scholar]
- 11.Chen CF, Liu XF, Meng XZ, et al. Comparative study of mannitol-induced acute kidney impairments in patients of different ages suffering from subarachnoid hemorrhage. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2007; 19:727–730. [PubMed] [Google Scholar]
- 12.Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 2008; 51:491–503. [DOI] [PubMed] [Google Scholar]
- 13.Better OS, Rubinstein I, Winaver JM, et al. Mannitol therapy revisited (1940–1997). Kidney Int 1997; 52:886–894. [DOI] [PubMed] [Google Scholar]
- 14.Dorman HR, Sondheimer JH, Cadnapaphornchai P. Mannitol-induced acute renal failure. Medicine (Baltimore) 1990; 69:153–159. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Perez AJ, Pazos B, Sobrado J, et al. Acute renal failure following massive mannitol infusion. Am J Nephrol 2002; 22:573–575. [DOI] [PubMed] [Google Scholar]
- 16.Bentley ML, Corwin HL, Dasta J. Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies. Crit Care Med 2010; 38:S169–S174. [DOI] [PubMed] [Google Scholar]
- 17.Tsagalis G, Akrivos T, Alevizaki M, et al. Long-term prognosis of acute kidney injury after first acute stroke. Clin J Am Soc Nephrol 2009; 4:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girman CJ, Kou TD, Brodovicz K, et al. Risk of acute renal failure in patients with Type 2 diabetes mellitus. Diabet Med 2012; 29:614–621. [DOI] [PubMed] [Google Scholar]
- 19.Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med 1994; 331:1416–1420. [DOI] [PubMed] [Google Scholar]
- 20.Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int 1994; 45:259–265. [DOI] [PubMed] [Google Scholar]
- 21.Nomani AZ, Nabi Z, Rashid H, et al. Osmotic nephrosis with mannitol: review article. Ren Fail 2014; 36:1169–1176. [DOI] [PubMed] [Google Scholar]
- 22.Covic A, Schiller A, Mardare NG, et al. The impact of acute kidney injury on short-term survival in an Eastern European population with stroke. Nephrol Dial Transplant 2008; 23:2228–2234. [DOI] [PubMed] [Google Scholar]
- 23.Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011; 151:318–322. [DOI] [PubMed] [Google Scholar]
- 24.Tuttolomondo A, Pedone C, Pinto A, et al. Predictors of outcome in acute ischemic cerebrovascular syndromes: The GIFA study. Int J Cardiol 2008; 125:391–396. [DOI] [PubMed] [Google Scholar]


