Abstract
The effect of hepatitis C virus (HCV) exposure on bone mineral density without advanced liver disease remains debated. Thus, we assessed the relation between HCV exposure and the risk of osteoporosis.
From 2000 to 2011, patients aged >20 years with HCV exposure were identified from the Longitudinal Health Insurance Database 2000. Of the 51,535 sampled patients, 41,228 and 10,307 patients were categorized as the comparison and the HCV exposure cohorts, respectively.
The overall incidence of osteoporosis in the HCV exposure cohort was higher than in the comparison cohort (8.27 vs 6.19 per 1000 person-years; crude hazard ratio = 1.33, 95% confidence interval = 1.20–1.47). The incidence of osteoporosis, higher in women than in men, increased with age and comorbidity of hypertension, hyperlipidemia, and heart failure. The risk of developing osteoporosis was significantly higher in the HCV exposure cohort than in the comparison cohort after adjusting for age, sex, diabetes, hypertension, hyperlipidemia, heart failure, stroke, and cirrhosis. However, the risk of osteoporosis contributed by HCV decreased with age and the presence of comorbidity. Furthermore, the risk of osteoporotic fracture did not differ significantly between patients exposed to HCV and the comparison cohorts.
HCV increases the risk of osteoporosis, but no detrimental effect on osteoporotic fracture was observed in this study. Furthermore, HCV may be less influential than other risk factors, such as hypertension, hyperlipidemia, and heart failure, in contributing to the development of osteoporosis.
INTRODUCTION
A negative balance between the formation and resorption of bone mass can lead to the development of osteoporosis. Moreover, osteoporosisis characterized by skeletal fragility resulting from reduced bone mass and disrupted bone microarchitecture. Osteoporosis increases the risk of fracture and thus has been considered a major public health concern.1 Traditionally, the development of osteoporosis is related to several risk factors, such as aging, immobility, hypertension, antihypertensive agents, hyperparathyroidism, menopause, diabetes mellitus, corticosteroid usage, low calcium intake, vitamin D deficiency, and genetic vulnerability.2
Osteoporotic fractures mainly consist of vertebral fracture and hip fracture. Vertebral fracture is the most common osteoporotic fracture, but only from one-third to one quarter of the patients with vertebral fracture can be clinically identified.3–5 Furthermore, it is reported that asymptomatic vertebral fractures are associated with future hip fracture by threefold and other nonvertebral fracture by twofold.6 Hip fracture is the second common osteoporotic fracture and it may incur substantial healthcare costs resulted from disability. Furthermore, the effect of hip fracture on mortality increase can adversely extend up to 10 years or more.3 The mortality following a hip fracture generally increases with the increment of age and is greater in men, but the sex difference declines after age 80.7 Although the rates of hip fracture have declined in the West, the rate is increasing in the developing world. It is estimated that >50% of hip fractures worldwide will occur in Asia by 2050.8 The functional outcome and increased mortality of osteoporotic fracture are heterogeneous and depend on age, activity of daily living prior to fracture, pre-fracture comorbidities, and the cognition.3,9,10
Hepatitis C virus infection (HCV) is a global health problem estimated to affect 170 million people worldwide.11 Hepatitis C virus infection is a hepatotropic virus that mainly causes inflammation and fibrosis of the liver. It is reported that ∼20% of HCV-infected patients will progress to liver cirrhosis.12 The late hepatic sequelae include chronic hepatitis, cirrhosis, and even hepatocellular carcinoma. However, HCV can also cause several extrahepatic manifestations, such as diabetes mellitus, rheumatic disorders, lymphoproliferative disease, cardiovascular events, and cognitive impairment.13 Although the role of osteoporosis as a sequence of cirrhosis or advanced liver disease has been thoroughly documented, the effect of HCV exposure on bone mineral density in the absence of advanced liver disease remains debated.14 Some scholars have proposed that chronic HCV infection without liver cirrhosis contributes to reduced bone mineral density, whereas other scholars have asserted the opposite.15–20
To assess the association between HCV exposure and subsequent development of osteoporosis, we conducted a nationwide population-based cohort study by analyzing data from a nationwide medical database, the National Health Insurance Research Database (NHIRD).
METHODS
Data Source
The National Health Insurance (NHI) program, initiated on March 1, 1995, provides comprehensive coverage for the medical care of Taiwan residents. At the end of 2014, 23.75 million people (∼99.9% of the population) were enrolled in the program.21 In cooperation with the Bureau of National Health Insurance (BNHI), the National Health Research Institutes established several data sets for public use, including the Longitudinal Health Insurance Database 2000 (LHID2000), a cohort data set comprising 1,000,000 randomly selected cases from the registry of NHI beneficiaries in 2000. To maintain confidentiality, personal information, such as patient identification numbers and sensitive personal data, are encrypted in the database, and International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes are used for disease classification. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH-104-REC2-115). The IRB also specifically waived the consent requirement.
Participants
From 2000 to 2011, patients aged 20 years and older with diagnosed HCV infection (ICD-9-CM codes 070.41, 070.44, 070.51, and 070.54) identified from the LHID2000 comprised the HCV infection cohort. The date for HCV exposure coding was designated as the index date. Patients with a history of osteoporosis (ICD-9-CM codes 733.0 and 733.1) and hepatitis B virus (HBV) infection (ICD-9-CM codes 070.20, 070.22, 070.30, and 070.32) diagnosed before the index date, those with missing information, and those younger than 20 years were excluded. Using 1:m case-control studies is to increase the power and to control possible confounding. Based on the statistical efficiency does not gain much when m > 4, we constructed a 1:4 matched cohort study. For each HCV case, 4 insurers with no history of HCV exposure, HBV infection, and osteoporosis were assigned to a comparison cohort and frequency matched with the HCV exposure cohorts according to age (every 5-year span), sex, and index year. Individuals were excluded from the comparison cohort using the same criteria used for the HCV exposure cohort.
Outcome and Comorbidities
The primary endpoint in this study was defined as the diagnosis of osteoporosis. Each participant was followed from the index date until the endpoint, withdrawal from the NHI program, or December 31, 2011. The baseline characteristics of participant comorbidities were also analyzed; the comorbidities included diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), heart failure (ICD-9-CM code 428), stroke (ICD-9-CM codes 430–438), obesity (ICD-9-CM code 278), and cirrhosis (ICD-9-CM codes 571.2, 571.5, and 571.6).
Statistical Analyses
The chi-square test and t test for categorical and continuous variables, respectively, were first used to compare the distributions of age, sex, and baseline comorbidities between the HCV exposure and the comparison cohorts. The incidence densities of osteoporosis were estimated in person-years for the various risk factors. To estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of osteoporosis, the univariate and multivariate Cox proportional hazards regression model was used. Multivariable models were simultaneously adjusted for age, sex, and the comorbidities of diabetes, hypertension, hyperlipidemia, heart failure, stroke, and cirrhosis. Kaplan–Meier estimates were plotted to illustrate the cumulative incidence of osteoporosis, and the log-rank test was performed to examine the difference between the HCV exposure and the comparison cohorts. All statistical analyses were performed using the SAS package (Version 9.4 for Windows; SAS institute, Inc, Cary, NC). A 2-sided p value < .05 was considered statistically significant.
RESULTS
Of the 51,535 sampled patients, 41,228 and 10,307 were categorized as the comparison and HCV exposure cohorts, respectively (Table 1). Most patients were aged ≥50 years (61.7%), and 54.6% of the patients were women. The mean age was 54.1 ± 15.3 years in the HCV exposure cohort and 53.7 ± 15.5 years in the comparison cohort. Regarding baseline characteristics, the HCV exposure cohort exhibited a higher prevalence of diabetes, hypertension, hyperlipidemia, heart failure, stroke, obesity, and cirrhosis than did the comparison cohort. During the mean follow-up periods of 5.44 and 6.01 years for the HCV exposure and comparison cohorts, respectively, the Kaplan–Meier curve revealed that the cumulative incidence of osteoporosis was higher in the HCV exposure cohort than in the comparison cohort (Figure 1, log-rank test P < 0.001).
TABLE 1.
Demographic Characteristics and Comorbidities in Cohorts With and Without HCV Exposure
FIGURE 1.
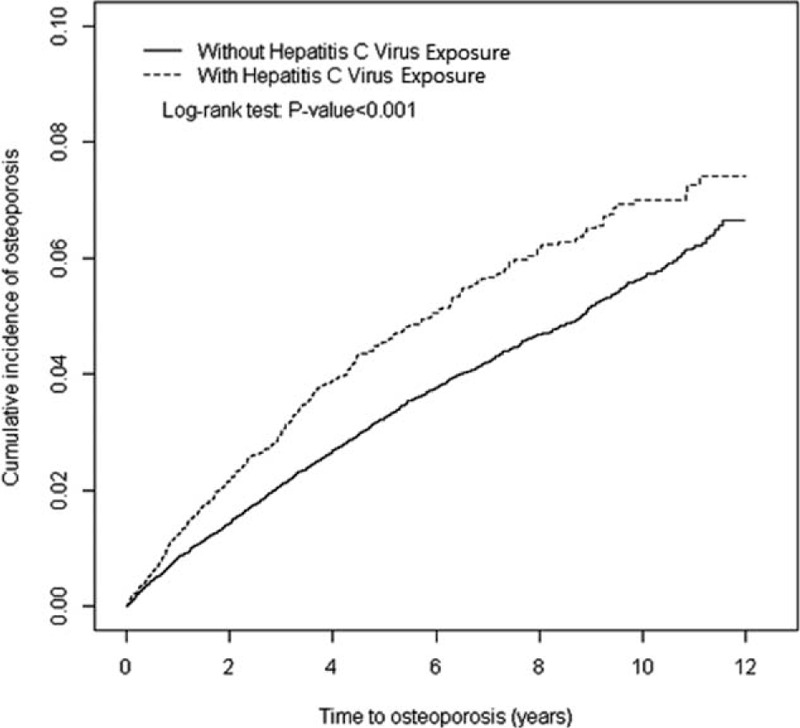
Cumulative incidence comparison of osteoporosis for patients with (dashed line) or without (solid line) HCV exposure.HCV = hepatitis C virus.
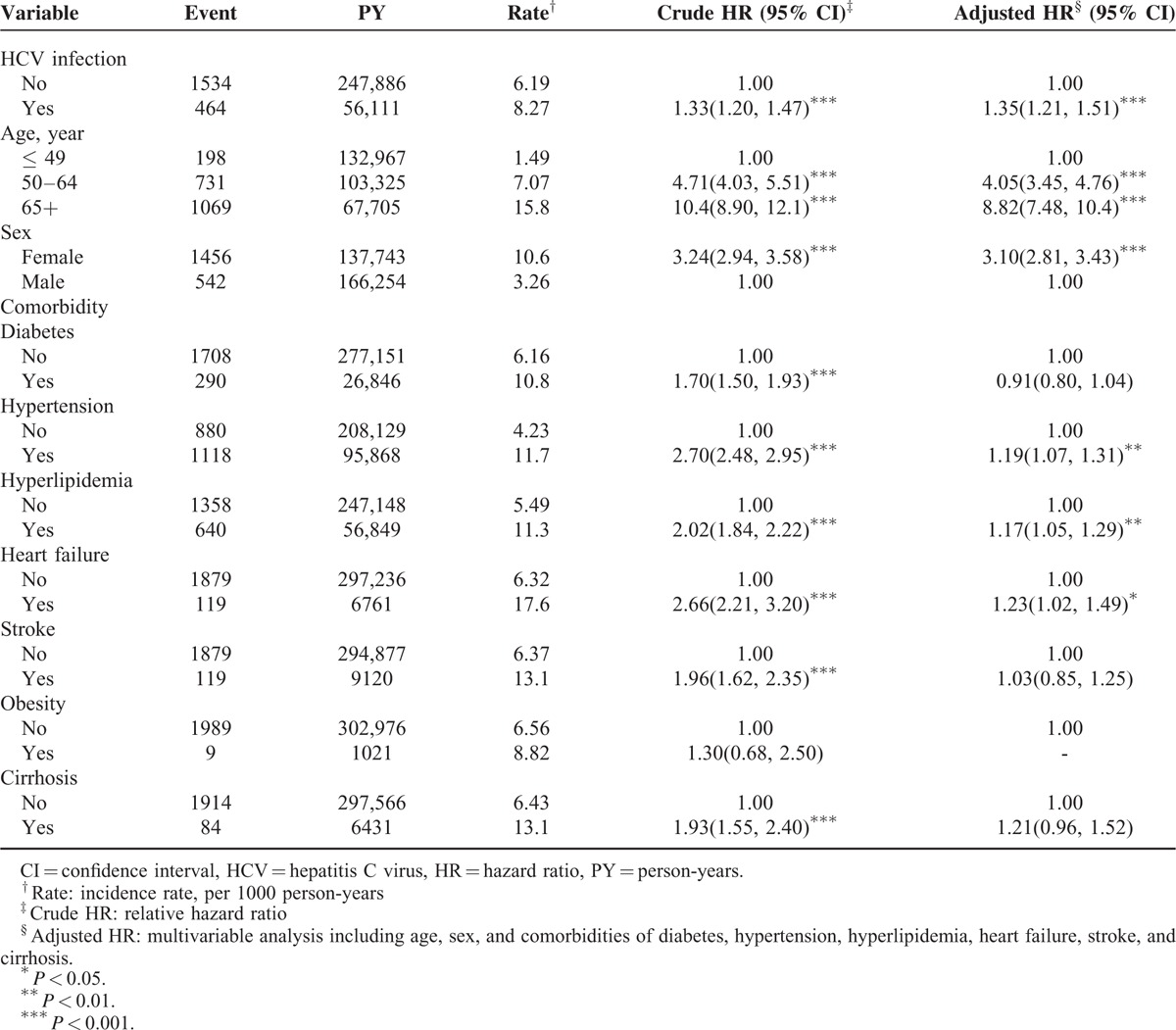
The overall incidence of osteoporosis in the HCV exposure cohort was higher than that in the comparison cohort (8.27 vs 6.19 per 1,000 person-years; crude HR = 1.33, 95% CI = 1.20–1.47) (Table 2). After we adjusted for factors such as age, sex, and comorbidities, namely diabetes, hypertension, hyperlipidemia, heart failure, stroke, and cirrhosis, the risk of developing osteoporosis was significantly higher in the HCV exposure cohort than in the comparison cohort (adjusted HR [aHR] = 1.35; 95% CI = 1.21–1.51). Compared with patients aged ≤49 years, the risk of developing osteoporosis was 4.05-fold higher in those aged 50 to 64 years (95% CI = 3.845–4.760) and 8.82-fold higher in those aged 65 years or older (95% CI = 7.48–10.40). In the multivariate model, the risk for osteoporosis was 3.10-fold higher for women than for men (95% CI = 2.81–3.43) and was higher for patients with the comorbidities of hypertension (aHR = 1.19, 95% CI = 1.07–1.31), hyperlipidemia (aHR = 1.17, 95% CI = 1.05–1.29), and heart failure (aHR = 1.23, 95% CI = 1.02–1.49).
TABLE 2.
The Incidence and Hazard ratio for Osteoporosis and Osteoporosis-Associated Risk Factor
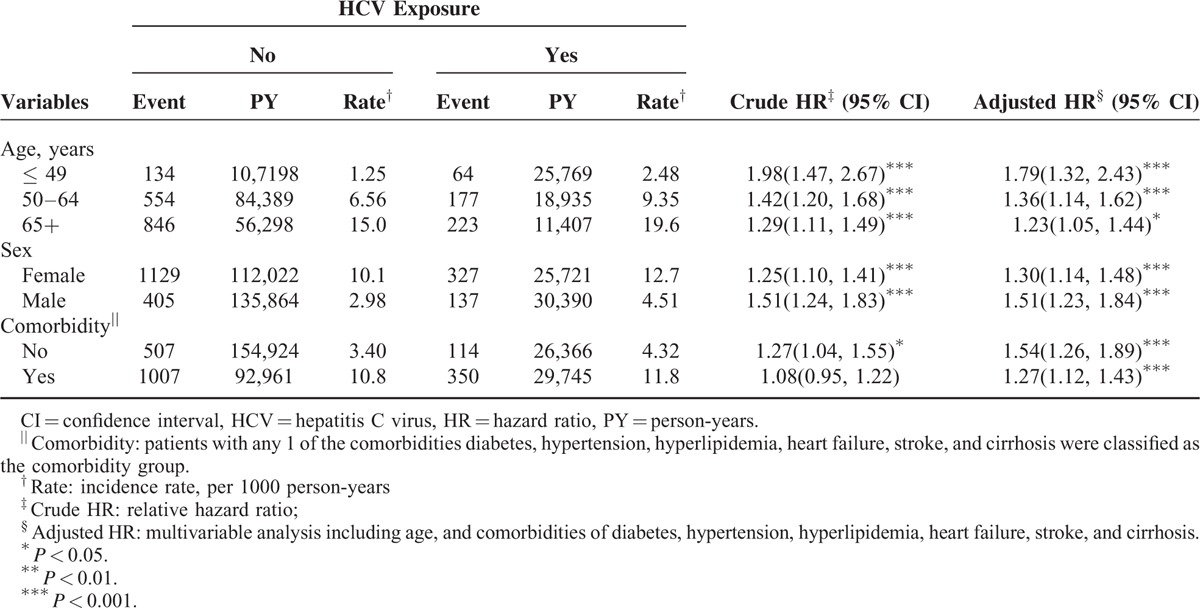
The incidence of osteoporosis increased with age, was higher in women than in men, and increased with comorbidity in both cohorts (Table 3). The overall risk of osteoporosis related to several variables including age, sex, and presence of comorbidities was compared in the HCV exposure cohort and the comparison cohort. The risk of osteoporosis in patients exposed to HCV in all stratifications was higher than that in the comparison cohorts. However, the risk of osteoporosis contributed by HCV decreased with age (aged ≤ 49: aHR = 1.79, 95% CI = 1.32–2.43; aged 50–64: aHR = 1.36, 95% CI = 1.14–1.62; aged ≥ 65: aHR = 1.23, 95% CI = 1.05–1.44) and the presence of comorbidity (no comorbidity: aHR = 1.54, 95% CI = 1.26–1.89; comorbidity: aHR = 1.27, 95% CI = 1.12–1.43).
TABLE 3.
Incidence of Osteoporosis by Age, Sex, and Comorbidity and Cox Model Measured Hazards Ratio for Patients With HCV Infection Compared Those Without HCV Exposure
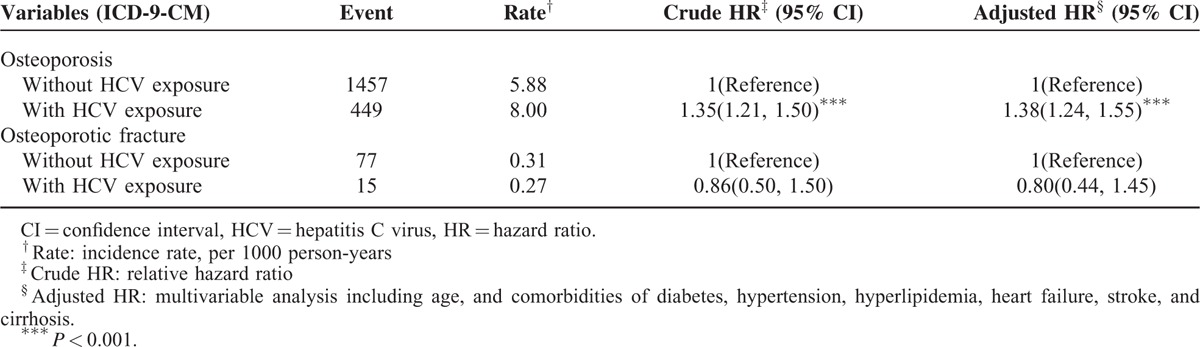
The patients exposed to HCV exhibited a 1.38-fold (95% CI = 1.24–1.55) higher risk of developing osteoporosis compared with the patients who were not exposed to HCV (Table 4). The risk of osteoporotic fracture did not differ significantly between patients exposed to HCV and the comparison cohorts (aHR = 0.80, 95% CI = 0.44–1.45).
TABLE 4.
Comparisons of Hazard Ratios Between Patients With and Without HCV Exposure for Different Outcomes Osteoporosis (or Osteoporotic Fracture)
DISCUSSION
Consistent with the literature proposing that HCV seroprevalence peaks after age 55 and that women are predisposed to HCV infection, our results revealed that most patients (61.7%) were aged ≥50 years and that 54.6% of the patients were women.22,23 The mean age in the HCV exposure cohort was 54.1 ± 15.3 years. Compared with patients who were not exposed to HCV, patients who were exposed to HCV tended to have more comorbidities, including diabetes, hypertension, hyperlipidemia, heart failure, stroke, obesity, and cirrhosis. Our results revealed that the HCV exposure cohort had more comorbidities; however, the risk of osteoporosis remained higher in the HCV exposure cohort after adjusting for age, sex, and the comorbidities of diabetes, hypertension, hyperlipidemia, heart failure, stroke, and cirrhosis. The mechanism affecting the pathophysiology that causes comorbidities in patients exposed to HCV may include HCV-associated insulin resistance and atherosclerosis.13 HCV may cause diabetes mellitus by directly interfering with insulin signaling and inducing insulin resistance in hepatocytes; HCV-infected hepatocytes can secrete mediators that induce extrahepatic insulin resistance, notably in the skeletal muscle. Hepatitis C virus may increase the risk of cardiovascular and cerebrovascular events through atherosclerosis induced by systemic inflammation, chronic endothelial injury, or direct infection of the arterial wall.24–26
Our study revealed that the osteoporosis incidence increased with age and was higher in women than in men. The association between osteoporosis and aging has been confirmed in the literature, and the gene expression of the rennin–angiotensin system independent of hypertension in the skeletal tissue may contribute to osteoporosis.27,28 Estrogen deficiency and aging are the major etiologies of primary osteoporosis. Estrogen can protect against osteoporosis by inhibiting osteoblast apoptosis and increasing osteoblast lifespan.29,30 The decreasing rate of bone mineral density is swifter in the early postmenopausal period, which typically begins after age 50, and women aged 40 to 59 years have the highest risk of developing osteoporosis.23,30,31 Consistent with our result that women are predisposed to osteoporosis, the US Preventive Services Task Force indicated that as many as 1 in 2 postmenopausal women and 1 in 5 men are at risk for osteoporosis-related fracture.32
Moreover, in the present study, osteoporosis was associated with hypertension, hyperlipidemia, and heart failure. Both osteoporosis and hypertension are common among the aging population and may share similar etiologies, such as low calcium intake and levels, vitamin D and vitamin K deficiency, high salt consumption, imbalanced nitric oxide levels, and antihypertensive agents that exert detrimental effects on the skeletal metabolism, strength, and density.33 Hyperlipidemia can reduce bone formation and promote bone loss by causing the products of lipid oxidation to accumulate in the subendothelial spaces of the vasculature and bone. Moreover, hyperlipidemia can induce secondary hyperparathyroidism, thereby impairing bone regeneration and mechanical strength.34 Heart failure and osteoporosis share several risk factors, such as aging, smoking, and postmenopausal and antihypertensive agents; heart failure can also accelerate bone loss by inducing hyperaldosteronism and secondary hyperparathyroidism.35 However, the effect of obesity on bone metabolism is controversial.36 Obesity is traditionally regarded as a protective factor for osteoporosis, conferring a positive mechanical loading on bone formation. Nevertheless, evidence supports the deteriorating effect of obesity on osteoporosis. For example, both osteoblasts and adipocytes are derived from a common mesenchymal stem cell and agents inhibiting adipogenesis-stimulated osteoblast differentiation and vice versa. Furthermore, the reduced bone formation caused by aging is usually accompanied by adipogenesis in bone marrow cavities. Moreover, elevated oxidative stress is common in people with obesity and osteoporosis.
To our knowledge, this is the first population-based study to assess the relation between HCV exposure and the incidences of osteoporosis and osteoporotic fracture.15–20 Our statistical analyses benefited from the use of a nationwide database and the 12-year observation of participants selected from a representative cohort comprising 1,000,000 residents covered by the NHI program. Hansen et al conducted a large-scale population-based study to explore the association between HCV exposure and all-site fracture, but omitted discussing osteoporosis development. They concluded that the risk of fracture equally increased in patients exposed to HCV (chronic or cleared infections), and the major determinants of fracture in such patients are lifestyle-related factors, such as alcohol and drug abuse, which substantially increases fracture risk.20 By contrast, our epidemiological study demonstrated that HCV exposure increases the risk of osteoporosis and the detrimental effect of HCV on osteoporotic fracture was not obvious.
Several pathogenic mechanisms are involved in bone mineral density loss in patients exposed to HCV.37 First, fibronectin can infiltrate the bone matrix to enhance matrix mineralization, reducing its production by the liver. However, oncofetal fibronectin increases and can directly inhibit osteoblast function. Second, insulin-like growth factor 1, involved in osteoblast differentiation and proliferation, is produced by the liver and is reduced in patients with chronic liver disease. Third, the receptor–activator ratio of nuclear factor kappa ligand and osteoprotegerin is higher in patients with chronic liver disease, enhancing bone resorption. Fourth, interleukin-6 is increased by chronic HCV infection and can activate osteoclasts to increase bone resorption. Fifth, hypogonadism in chronic liver disease can result in increased osteoclast activity. Finally, bilirubin elevation can inhibit in vitro osteoblast proliferation.38
By contrast, the association between HCV and osteoporosis may be due to shared risk factors since the prevalence of important osteoporosis risk factors was higher in the HCV exposed patients as compared to the patients without HCV exposure. However, it is reasonable to conclude that the increased risk of osteoporosis observed in HCV exposed patients was more likely to be due to the effect of their HCV status since the possible confounding effect of osteoporosis risk factors has been significantly minimized in our study. Moreover, the osteoporosis risk contributed by HCV decreased with the increment of age may be due to the absence or low prevalence of osteoporosis-associated comorbidities in the younger patients. The results of our subgroup analyses in which we excluded patients with important comorbidities at baseline also confirmed the validity of our results. This finding, coupled with the results of the subgroup analyses, affirms the possible causal association between HCV and osteoporosis, and suggested that HCV may be a possible risk factor for osteoporosis. Nevertheless, HCV may be less influential than other risk factors, such as hypertension, hyperlipidemia, and heart failure, in contributing to the development of osteoporosis.
The present study had some strength. First, the large-scale national database provided statistical benefits to our longitudinal study to evaluate the association between HCV and osteoporosis. Second, the recruited subjects were a stable population and ∼99% of the residents in Taiwan have been covered by the NHI program. Nevertheless, our study had several limitations. First, we could not ascertain the patients’ viremic status, such as the viral loads or the genotypes of the HCV. Nevertheless, all patients in our case cohort exhibited positive anti-HCV antibodies, which mean recent or past HCV exposure. Moreover, the effect of antiviral therapy on the stage of liver fibrosis or the grade of liver inflammation could not be ascertained in our study. Therefore, we could not prove the ameliorating effect of antiviral therapy on bone mineral density through the arrest of liver cirrhosis even though the association between osteoporotic fracture and cirrhosis has been thoroughly documented. Second, osteoporosis-associated lifestyle factors could not be investigated in this study. However, potential osteoporosis-associated comorbidities were confounded in our study. Third, validating the diagnosis of osteoporosis or osteoporotic fracture was difficult. However, to enhance the accuracy of diagnosis, we only recruited patients who received medical care for osteoporosis >3 separate visits. Moreover, the BNHI organizes regular audits performed by medical experts to ensure the accuracy of insurance claim codes in Taiwan. Finally, we cannot clarify the temporal association between HCV exposure and osteoporosis since the date of HCV exposure could not be ascertained.
In conclusion, this nationwide population-based cohort study concludes that HCV exposure increases the risk of developing subsequent osteoporosis, but no detrimental effect on osteoporotic fracture was observed. Furthermore, HCV may be less influential than other risk factors, such as hypertension, hyperlipidemia, and heart failure, in contributing to the development of osteoporosis.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, BNHI = Bureau of National Health Insurance, CI = confidence interval, HCV = hepatitis C virus, ICD-9-CM = International Classification of Disease, Ninth Revision, Clinical Modification, LHID2000 = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, SD = standard deviation.
Funding: this study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Raisz LG. Clinical practice. Screening for osteoporosis. N Engl J Med 2005; 353:164–171. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Melton LJ, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res 1994; 9:117–1141. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci 2013; 68:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper C, Atkinson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 1992; 7:221–227. [DOI] [PubMed] [Google Scholar]
- 5.Fink HA, Milavetz DL, Palermo L, et al. Fracture Intervention Trial Research Group. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 2005; 20:1216–1222. [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Arden NK, Palermo L, et al. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study on Osteoporotic Fractures Research Group. J Bone Miner Res 1999; 14:821–828. [DOI] [PubMed] [Google Scholar]
- 7.Haentjens P, Magaziner J, Colon-Eneric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010; 152:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper C, Cole ZA, Holroyd CR, et al. The IOF CSA Working Group on Fracture Epidemiology. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 2011; 22:1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Larramona G, Lucendo AJ, Gonzalez-Castillo S, et al. Hepatic osteodystrophy: an important matter for consideration in chronic liver disease. World J Gastroenterol 2011; 3:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliuc D, Nguyen ND, Milch VE, et al. Mortalty risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009; 301:513–521. [DOI] [PubMed] [Google Scholar]
- 11.WHO, Global. surveillance and control of hepatitis C: Report of a WHO consultation organized in collaboration with the viral hepatitis prevention Board, Antwerp, Belgium. J Viral Hepat, 1999, 6: 35–47. [PubMed] [Google Scholar]
- 12.Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002; 36:S35–S46. [DOI] [PubMed] [Google Scholar]
- 13.Negro F. Facts and fictions of HCV and comorbidities: steatosis, diabetes mellitus, and cardiovascular diseases. J Hepatol 2014; 61:S69–S78. [DOI] [PubMed] [Google Scholar]
- 14.Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology 2003; 125:941–966. [DOI] [PubMed] [Google Scholar]
- 15.Lin JC, Hsieh TY, Wu CC, et al. Association between chronic hepatitis C virus infection and bone mineral density. Calcif Tissue Int 2012; 91:423–429. [DOI] [PubMed] [Google Scholar]
- 16.Schiefke I, Fach A, Wiedmann M, et al. Rduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol 2005; 11:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanda KS, Ryan A, Murray BF, et al. Effect of chronic hepatitis C infection on bone disease in postmenopausal women. Clin Gastroenterol Hepatol 2009; 7:894–899. [DOI] [PubMed] [Google Scholar]
- 18.Pelazas-Gonzalez R, Gonzalez-Reimers E, Aleman-Valls MR, et al. Bone alterations in hepatitis C virus infected patients. Eur J Intern Med 2013; 24:92–96. [DOI] [PubMed] [Google Scholar]
- 19.Lo RV, III, Volk J, Newcomb CW, et al. Risk of hip fracture associated with hepatitis C infection and hepatitis C/HIV coinfection. Hepatology 2012; 56:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen AB, Omland LH, Krarup H, et al. Fracture risk in hepatitis C virus infected persons: results from the DANVIR cohort study. J Hepatol 2014; 61:15–21. [DOI] [PubMed] [Google Scholar]
- 21.Database NHIR. Taiwan, http://nhird.nhri.org.tw/en/Background.html (cited in 2015). [Google Scholar]
- 22.Li D, Long Y, Wang T, et al. Epidemiology of hepatitis C infection in highly endemic HBV areas in China. Plos ONE 2013; 8:e54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanafiah KM, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–1342. [DOI] [PubMed] [Google Scholar]
- 24.Vasselle C, Masini S, Bianchi F, et al. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart 2004; 90:565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alyan O, Kacmaz F, Ozdemir O, et al. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon Severity Score System. Circ J 2008; 72:1960–1965. [DOI] [PubMed] [Google Scholar]
- 26.Lee MH, Yang HI, Wang CH, et al. Hepatitis C infection and increased risk of cerebrovascular disease. Stroke 2010; 41:2894–2900. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Chai X, Li S, et al. Age-related changes in body composition and their relationship with bone mineral density decreasing rates in central south Chinese postmenopausal women. Endocrine 2013; 43:643–650. [DOI] [PubMed] [Google Scholar]
- 28.Gu SS, Zhang Y, Li XL, et al. Involvement of the skeletal rennin–angiotensin system in age-related osteoporosis of aging mice. Biosci Biotechnol Biochem 2012; 76:1367–1371. [DOI] [PubMed] [Google Scholar]
- 29.Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab 2012; 23:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svejme O, Ahlborg HG, Karlsson MK. Changes in forearm bone mass and bone size after menopause- a mean 24-year prospective study. J Musculoskelet Neuronal Interact 2012; 12:192–198. [PubMed] [Google Scholar]
- 31.Nohara T, Kamei T, Ohta A. Accelerated decrease in bone mineral density in women aged 52–57 years. Tohoku J Exp Med 2006; 210:341–347. [DOI] [PubMed] [Google Scholar]
- 32.US Preventive Services Task Force, et al. Screening for osteoporosis: US preventive services task force recommendation statement. Annals of internal medicine, 2011, 154.5: 356. [DOI] [PubMed] [Google Scholar]
- 33.Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif Tissue Int 2013; 92:217–227. [DOI] [PubMed] [Google Scholar]
- 34.Pirih F, Lu J, Ye F, et al. Adverse effects of hyperlipidemia on bone regeneration and strength. JBMR 2012; 27:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majumdar SR, Ezekowitz JA, Lix LM, et al. Heart failure is a clinically and densitometrically independent risk factor for osteoporotic fractures: population-based cohort study of 45,509 subjects. J Clin Endocrinol Metab 2012; 97:1179–1186. [DOI] [PubMed] [Google Scholar]
- 36.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res 2011; 6:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakchbandi IA. Osteoporosis and fractures in liver disease: relevance, pathogenesis and therapeutic implications. World J Gastroenterol 2014; 20:9427–9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janes CH, Dickson ER, Okazaki R, et al. Role of hyperbilirubinemia in the impairment of osteoblast proliferation asscoaited with cholestatic jaundice. J Clin Invest 1995; 95:2581–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]


