Abstract
Good's syndrome (GS) is a rare combination of thymoma and hypogammaglobulinemia, resulting in immunodeficiency. Patients with GS are highly susceptible to bacterial infection, particularly encapsulated bacterial infection in upper and lower respiratory tracts. Good's syndrome patients with moderate-to- severe infection are often hospitalized. Clinical features of GS patients remain to be characterized.
Patients with the discharge diagnosis of GS and simultaneous infection from Peking Union Medical College Hospital between January 2001 and July 2015 were retrospectively analyzed.
Among 14 hospitalized GS patients, 12 of them were admitted for severe infections. Mean patient age was 56.7 + 10.1 years. Average concentrations of serum IgG, IgA, and IgM were 2.3 + 1.9 g/L, 0.28 + 0.28 g/L, and 0.06 + 0.07 g/L, respectively. Respiratory and intestinal tracts were the most common sites for infection, which occurred in 7 and 4 patients, respectively. Pathogens identified in 10 patients included cytomegalovirus in 5 patients, Pneumocystis jirovecii, Clostridium difficile in 2 patients, Klebsiella pneumonia in 2 patients, and Streptococcus pneumonia and Hemophilus influenza in 1 patient. Ten patients were treated with antibiotics and immunoglobulin replacement. Only 1 patient who was on immunosuppressant therapy died from P. jirovecii pneumonia.
Infection was the most frequent cause for hospitalization of GS patients. Both respiratory and intestinal tracts were the most common sites of infection. Cytomegalovirus and P. jirovecii represented 2 common opportunistic pathogens isolated from hospitalized GS patients with infections.
INTRODUCTION
Good's syndrome (GS) was first described by Dr Robert Good in 1954.1 Good's syndrome is a rare combination of thymoma and hypogammaglobulinemia, and features with few or absent B lymphocytes, CD4 + T cell lymphopenia, and abnormal ratio of CD4 + : CD8 + T cell.2 The cause and pathogenesis of this rare disease are unknown. Thymectomy does not seem to effectively reverse the immunological deficiency.3
An increased risk for infections in GS patients has been demonstrated by many case reports and reviews,4–6 but most infections are mild and can be managed at the outpatient department. Good's syndrome patients, when with moderate-to-severe infections, have to be hospitalized, but most of the hospitalized cases were described in a form of case report. The largest series of reported GS patients who were hospitalized for infections, only included 5 patients.5 Another study by Malphettes included 21 GS cases, but the paper did not state whether these patients were hospitalized for infections.7 This study presented a series of 12 GS patients hospitalized for infections in our hospital, and the clinical characteristics of this rare disease were illustrated.
METHODS
The medical files of patients who were hospitalized at Peking Union Medical College Hospital from January 2001 to July 2015 were searched for the discharge diagnosis of “Good's syndrome.” Only GS patients with infections were included in this retrospective study. The following data were retrieved for analysis: age, sex, clinical symptoms, laboratory findings, sites of infection, treatment, and outcomes. Informed consent was waived because of the retrospective nature of this study. The study was approved by the Ethical Committee of Peking Union Medical College Hospital.
Good's syndrome is defined as following: (1) the presence of thymoma, confirmed by chest computed tomography and/or pathology; and (2) hypogammaglobulinemia, defined as serum immunoglobulin G (IgG) < 5 g/L, and/or immunoglobulin A (IgA) < 0.7 g/L, and/or immunoglobulin M (IgM) < 0.4 g/L.
An infection was diagnosed when clinical manifestations indicated infection (fever, cough, sputum, diarrhea, etc) and corresponding pathogens were identified. The infection was also diagnosed if clinical manifestations for infections were unequivocal and the treatment aiming at the infection was effective even no pathogen was isolated.
Quantitative data were expressed as mean value ± standard deviation, and qualitative results were described as percentage.
RESULTS
A total of 14 patients with Good's syndrome were initially identified during this period. Two patients were excluded because of no infection associated with their hospitalization, and 12 patients were eligible for this study. All of 12 patients were hospitalized for moderate to severe infections. The clinical characteristics are summarized in Table 1. Female patients accounted to three-fourths of 12 patients, and the patient age was ranged from 38 to 70 years (mean age 56.7 + 10.1 years).
TABLE 1.
Clinical Characteristics of Good's Syndrome Patients Hospitalized for Infections
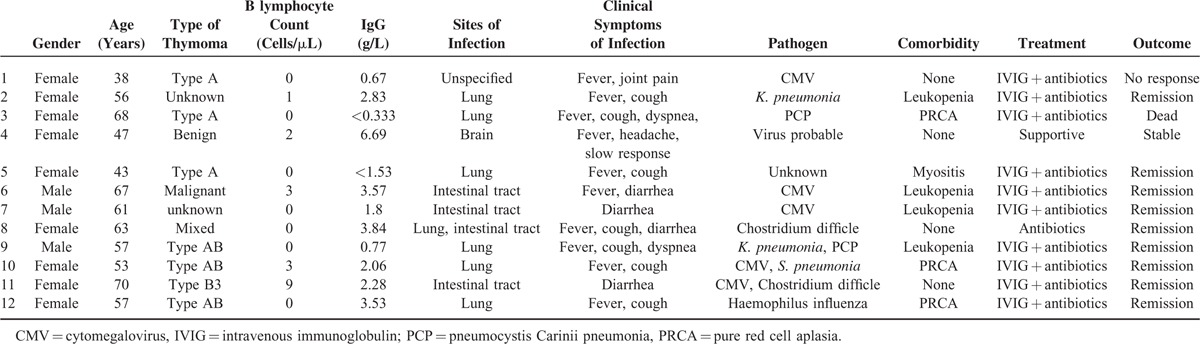
The thymoma was histologically confirmed in all patients and resected in 10 of them. The histological classification of thymoma was available for 7 patients: 3 with type AB, 3 patients with type A, and 1 patient with type B3. B lymphocyte was significantly decreased in all patients, ranging from 0 to 9 cells/μL (1.5 + 2.6 cells/μL). CD4 + T lymphocyte was also decreased (350.3 + 150.7 cells/μL), and the ratio of CD4 + : CD8 + T lymphocyte was inversed in all patients (0.49 + 0.16). Serum IgG, IgA, and IgM concentrations were all decreased, and the mean concentrations were 2.3 + 1.9 g/L, 0.28 + 0.28 g/L, and 0.06 + 0.07 g/L, respectively.
Respiratory and intestinal tracts were the most common sites of infection, which occurred in 7 and 4 patients, respectively. Co-infection of the respiratory and intestinal tracts was noted in 1 patient. Encephalitis was diagnosed in 1 patient, and the site of infection in 1 patient was not specified. Clinical symptoms were associated with the sites of infection. In patients with pulmonary infection, fever and cough were the most common clinical symptoms (7/7), whereas diarrhea occurred in all patients with intestinal tract infection (4/4). In addition, noninfection-related diarrhea was noted in 3 patients. Pathogens were identified in 10 patients including Cytomegalovirus (CMV) in 5 patients, P. jirovecii and C. difficile in 2 patients, K. pneumonia in 2 patients, and S. pneumonia and H. influenza in 1 patient. Eleven patients survived the infections, and only 1 died from severe pneumonia caused by P. jirovecii.
DISCUSSION
To the best of our knowledge, this study presented the largest series of GS patients who were complicated by infections in a single center. We revealed several interesting findings: (1) infections were the most frequent cause for hospitalization of GS patients; (2) respiratory and intestinal tracts were the most common sites of infection in hospitalized GS patients; (3) opportunistic pathogens including CMV and P. jirovecii were commonly detected in hospitalized GS patients with infections.
Both our study and the literature showed that GS had a peak incidence of infection in the 5th and 6th decade.5,8 A systemic review demonstrated that 83.3% of GS patients caught infections.5 In this study, the infections were identified in 85.7% of the hospitalized GS patients, suggesting that infection was the most frequent cause for hospitalization of GS patients. This finding implies that GS should be suspected in a thymoma patient with recurrent infections.9
It has been reported that upper and lower respiratory tracts were the most common sites of infection in GS patients.8 Upper respiratory tract infection and superficial fungal infection were precluded in this study because they generally would not be hospitalized. Pulmonary infection was noted in 58.3% of patients in this study. However, in GS patients who were regularly followed for a long period of time, as high as 85.7% of patients had at least 1 episode of pneumonia.10
A systemic review showed that diarrhea occurred in 31.8% of GS patients, but only 11.4% of patients were infectious.5 A higher percentage (58.3%) of patients with diarrhea was detected in this study, and 33.3% of them had infectious diarrhea, demonstrating that the intestinal tract is another frequent site of infection in GS patients.
Bacteria, especially encapsulated bacteria including S. pneumonia, H. influenza, K. pneumonia, are the most important pathogens in infected GS patients. Similar pathogens were identified in our study. Recurrent bacterial infection in GS patients most likely reflects the IgG deficiency, the part of problems with GS.
Different from common variable immunodeficiency (CVID),6,7 cell-mediated immunodeficiency is a common manifestation in GS patients. CD4 + T lymphocyte count was decreased in all patients and was <400 cells/μL in 58.3% of patients. Cell-mediated immunodeficiency explains why GS has a poorer prognosis than CVID.11,12 Although pathogenic bacterium is the most common pathogen in all GS patients,5 opportunistic pathogens associated with cell-mediated immunodeficiency including CMV (41.7%) and P. jirovecii (16.7%), frequently caused opportunistic infections in hospitalized GS patients in this study. CMV often appeared to cause intestinal infection, and P. jirovecii led to pulmonary infection.
Thymoma features with autoimmunity, and 32.7% to >50% of patients with thymoma exhibited autoimmune manifestations.13,14 Pure red cell aplasia (PRCA) was the most common autoimmune complication associated with GS5 as shown by this cohort. Leukopenia is also a common finding in GS patients,15 as was detected in 33.3% of patients in this study. Although myasthenia gravis (MG) is a common comorbidity of thymoma, it is relatively rare in GS patients. In fact, none of our GS patients exhibited MG symptoms, which was consistent with another GS series.10
Thymectomy is usually recommended in all patients with thymoma to prevent locally invasive growth and metastasis of tumor cells.16,17 It usually favorably impacts associated conditions such as PRCA and MG. However, the thymectomy is usually ineffective in improving immunodeficiency in GS patients, and it might worsen hypogammaglobulinemia in rare cases.18 Immunoglobulin replacement has been used to maintain appropriate serum IgG concentration to reduce infection.5,8,19 In this study, 83.3% of GS patients were infected and they received immunoglobulin replacement therapy in addition to antibiotics, resulting in clearance of infections in the majority of patients. Therefore, we recommend immunoglobulin replacement as standard therapy in all hospitalized GS patients with infections. The only patient who died in this study was an old woman who developed PRCA. She was on corticosteroid and immunosuppressant therapy when she caught pulmonary infection with P. jirovecii.20 In our opinion, the immunosuppressive drugs contributed more to her death than GS itself.
Because GS is a rare disease, the value of this study is limited by relatively small number of enrolled patients. Additionally, there are no definite criteria for hospitalization of GS patients, which may have resulted in biased findings by this study. Nonetheless, this study excluded many mild or less important infections, which highlighted important infections in GS patients that should be managed timely and carefully.
In conclusion, infections are a common manifestation in GS patients and are the most frequent cause for hospitalization. Beside the pathogenic bacteria, opportunistic pathogens including CMV and P. jirovecii represent frequent causes for infection. We recommend a combination of antibiotics with immunoglobulin replacement as standard therapy for hospitalized GS patients with infections.
Footnotes
Abbreviations: CMV = cytomegalovirus, CVID = common variable immunodeficiency, GS = Good's syndrome, MG = myasthenia gravis, PRCA = pure red cell aplasia.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Good RA. Absence of plasma cells from bone marrow and lymph nodes following antigenic stimulation in patients with a gamma globulinemia. Revue d’hematologie 1954; 9 (3 bis):502–503. [PubMed] [Google Scholar]
- 2.Kelleher P, Misbah SA. What is Good's syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol 2003; 56:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Souadjian JV, Enriquez P, Silverstein MN, et al. The spectrum of diseases associated with thymoma. Coincidence or syndrome? Arch Int Med 1974; 134:374–379. [PubMed] [Google Scholar]
- 4.Gafni J, Michaeli D, Heller H. Idiopathic acquired agammaglobulinemia associated with thymoma. Report of two cases and review of the literature. N Engl J Med 1960; 263:536–541. [DOI] [PubMed] [Google Scholar]
- 5.Kelesidis T, Yang O. Good's syndrome remains a mystery after 55 years: a systematic review of the scientific evidence. Clin Immunol 2010; 135:347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gathmann B, Mahlaoui N, Gerard L, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol 2014; 134:116–126. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999; 92:34–48. [DOI] [PubMed] [Google Scholar]
- 8.Tarr PE, Sneller MC, Mechanic LJ, et al. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine 2001; 80:123–133. [DOI] [PubMed] [Google Scholar]
- 9.Qu J, Lu X, Gao Q, et al. Good Syndrome, a rare cause of refractory chronic diarrhea and recurrent pneumonia in a Chinese patient after thymectomy. Clin Vaccine Immunol 2013; 20:1097–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malphettes M, Gerard L, Galicier L, et al. Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis 2015; 61:e13–19. [DOI] [PubMed] [Google Scholar]
- 11.Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med 1993; 86:31–42. [PubMed] [Google Scholar]
- 12.Agarwal S, Cunningham-Rundles C. Thymoma and immunodeficiency (Good syndrome): a report of 2 unusual cases and review of the literature. Ann Allergy Asthma Immunol 2007; 98:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holbro A, Jauch A, Lardinois D, et al. High prevalence of infections and autoimmunity in patients with thymoma. Human Immunol 2012; 73:287–290. [DOI] [PubMed] [Google Scholar]
- 14.Gadalla SM, Rajan A, Pfeiffer R, et al. A population-based assessment of mortality and morbidity patterns among patients with thymoma. International journal of cancer. J Int du Cancer 2011; 128:2688–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joven MH, Palalay MP, Sonido CY. Case report and literature review on Good's syndrome, a form of acquired immunodeficiency associated with thymomas. Hawai J Med Public Health 2013; 72:56–62. [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper JD. Current therapy for thymoma. Chest 1993; 103 (4 Suppl):334S–336S. [DOI] [PubMed] [Google Scholar]
- 17.Johnson SB, Eng TY, Giaccone G, et al. Thymoma: update for the new millennium. Oncologist 2001; 6:239–246. [DOI] [PubMed] [Google Scholar]
- 18.Ohuchi M, Inoue S, Hanaoka J, et al. Good syndrome coexisting with leukopenia. Ann Thorac Surg 2007; 84:2095–2097. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura A, Takiguchi Y, Tochigi N, et al. Durable hypogammaglobulinemia associated with thymoma (Good syndrome). Intern Med 2009; 48:1749–1752. [DOI] [PubMed] [Google Scholar]
- 20.Jian L, Bin D, Haiyun W. Fatal pneumocystis pneumonia with Good syndrome and pure red cell aplasia. Clin Infect Ddis 2004; 39:1740–1741. [DOI] [PubMed] [Google Scholar]


